Abstract
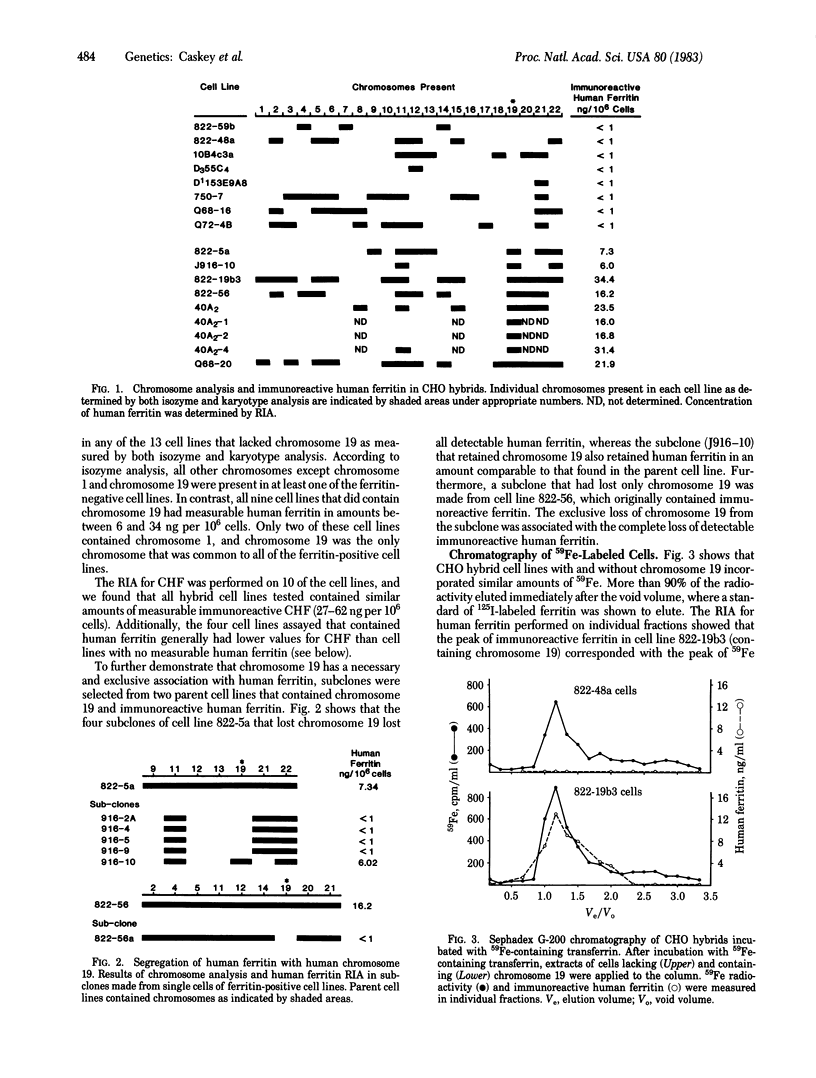
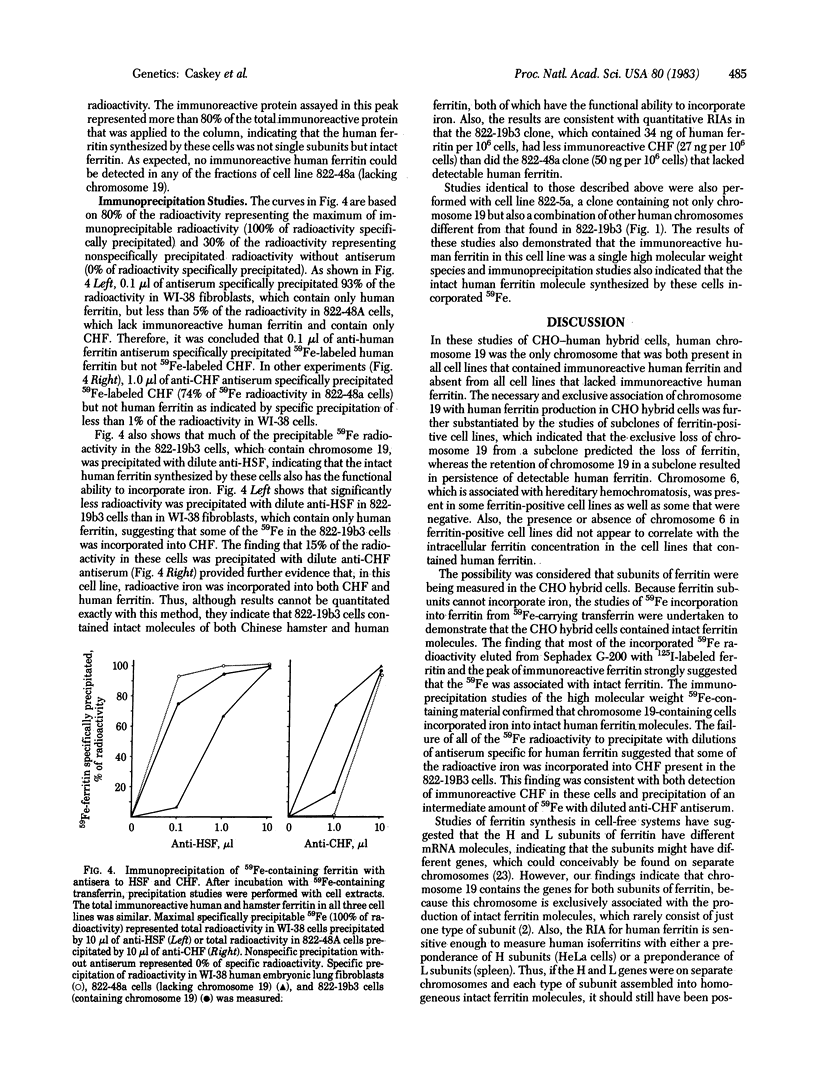
Ferritin is the intracellular iron storage protein. Tissue ferritin stores are markedly increased in hemochromatosis, a disease of iron overload that has been linked to chromosome 6. In order to provide further information concerning the genetics of ferritin synthesis and to determine if the structural gene for ferritin was on chromosome 6, studies were performed to identify the human chromosome that contains the ferritin gene. Ferritin immunoassays were performed on extracts of Chinese hamster ovary somatic cells that were hybridized with human lymphocytes and fibroblasts and contained various human chromosomes in different combinations. None of the 13 cell lines that lacked immunoreactive human ferritin contained chromosome 19, and all 9 of the cell lines that produced human ferritin contained chromosome 19. No other human chromosome shared this association with human ferritin. In studies of subclones of ferritin-positive cell lines, immunoreactive ferritin consistently segregated only with chromosome 19. Immunoprecipitation studies performed on cells that had been incubated with 59Fe-containing transferrin indicated that chromosome 19-containing cells incorporated iron into intact and functional molecules of human ferritin. The necessary and exclusive association of chromosome 19 with human ferritin indicates that a defect in the structural gene for human ferritin cannot account for the abnormalities of hemochromatosis. Moreover, this hamster-human hybrid cell system should prove useful in further studies of regulation of ferritin concentration and composition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Cartwright G. E., Edwards C. Q., Kravitz K., Skolnick M., Amos D. B., Johnson A., Buskjaer L. Hereditary hemochromatosis. Phenotypic expression of the disease. N Engl J Med. 1979 Jul 26;301(4):175–179. doi: 10.1056/NEJM197907263010402. [DOI] [PubMed] [Google Scholar]
- Clegg G. A., Fitton J. E., Harrison P. M., Treffry A. Ferritin: molecular structure and iron-storage mechanisms. Prog Biophys Mol Biol. 1980;36(2-3):56–86. [PubMed] [Google Scholar]
- Drysdale J. W. Ferritin phenotypes: structure and metabolism. Ciba Found Symp. 1976 Dec 7;(51):41–67. doi: 10.1002/9780470720325.ch3. [DOI] [PubMed] [Google Scholar]
- Frazier J. L., Caskey J. H., Yoffe M., Seligman P. A. Studies of the transferrin receptor on both human reticulocytes and nucleated human cells in culture: comparison of factors regulating receptor density. J Clin Invest. 1982 Apr;69(4):853–865. doi: 10.1172/JCI110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Sutherland R., Greaves M., Solomon E., Povey S. Expression of human transferrin receptor is controlled by a gene on chromosome 3: assignment using species specificity of a monoclonal antibody. Somatic Cell Genet. 1982 Mar;8(2):197–206. doi: 10.1007/BF01538677. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Halliday J. W., Mack U., Powell L. W. The kinetics of serum and tissue ferritins: relation to carbohydrate content. Br J Haematol. 1979 Aug;42(4):535–546. doi: 10.1111/j.1365-2141.1979.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Jones C., Kao F. T., Taylor R. T. Chromosomal assignment of the gene for folylpolyglutamate synthetase to human chromosome 9. Cytogenet Cell Genet. 1980;28(3):181–194. doi: 10.1159/000131529. [DOI] [PubMed] [Google Scholar]
- Jones C., Patterson D., Kao F. T. Assignment of the gene coding for phosphoribosylglycineamide formyltransferase to human chromosome 14. Somatic Cell Genet. 1981 Jul;7(4):399–409. doi: 10.1007/BF01542985. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Jones C., Puck T. T. Genetics of cell-surface antigens: regional mapping of three components of the human cell-surface antigen complex, AL, on chromosome 11. Somatic Cell Genet. 1977 Jul;3(4):421–429. doi: 10.1007/BF01542970. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Jones C., Puck T. T. Genetics of somatic mammalian cells: genetic, immunologic, and biochemical analysis with Chinese hamster cell hybrids containing selected human chromosomes. Proc Natl Acad Sci U S A. 1976 Jan;73(1):193–197. doi: 10.1073/pnas.73.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavoie D. J., Ishikawa K., Listowsky I. Correlations between subunit distribution, microheterogeneity, and iron content of human liver ferritin. Biochemistry. 1978 Dec 12;17(25):5448–5454. doi: 10.1021/bi00618a019. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Elliott R. W., Brown J. A., Shows T. B. Mapping of aminoacylase-1 and beta-galactosidase-A to homologous regions of human chromosome 3 and mouse chromosome 9 suggests location of additional genes. Am J Hum Genet. 1982 Mar;34(2):235–244. [PMC free article] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Shinjyo S., Abe H., Masuda M. Carbohydrate composition of horse spleen ferritin. Biochim Biophys Acta. 1975 Nov 10;411(1):165–167. doi: 10.1016/0304-4165(75)90295-0. [DOI] [PubMed] [Google Scholar]
- Simon M., Bourel M., Genetet B., Fauchet R. Idiopathic hemochromatosis. Demonstration of recessive transmission and early detection by family HLA typing. N Engl J Med. 1977 Nov 10;297(19):1017–1021. doi: 10.1056/NEJM197711102971901. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Drysdale J. Evidence for distinct mRNAs for ferritin subunits. Biochem Biophys Res Commun. 1981 Jan 30;98(2):507–511. doi: 10.1016/0006-291x(81)90869-x. [DOI] [PubMed] [Google Scholar]



