Abstract
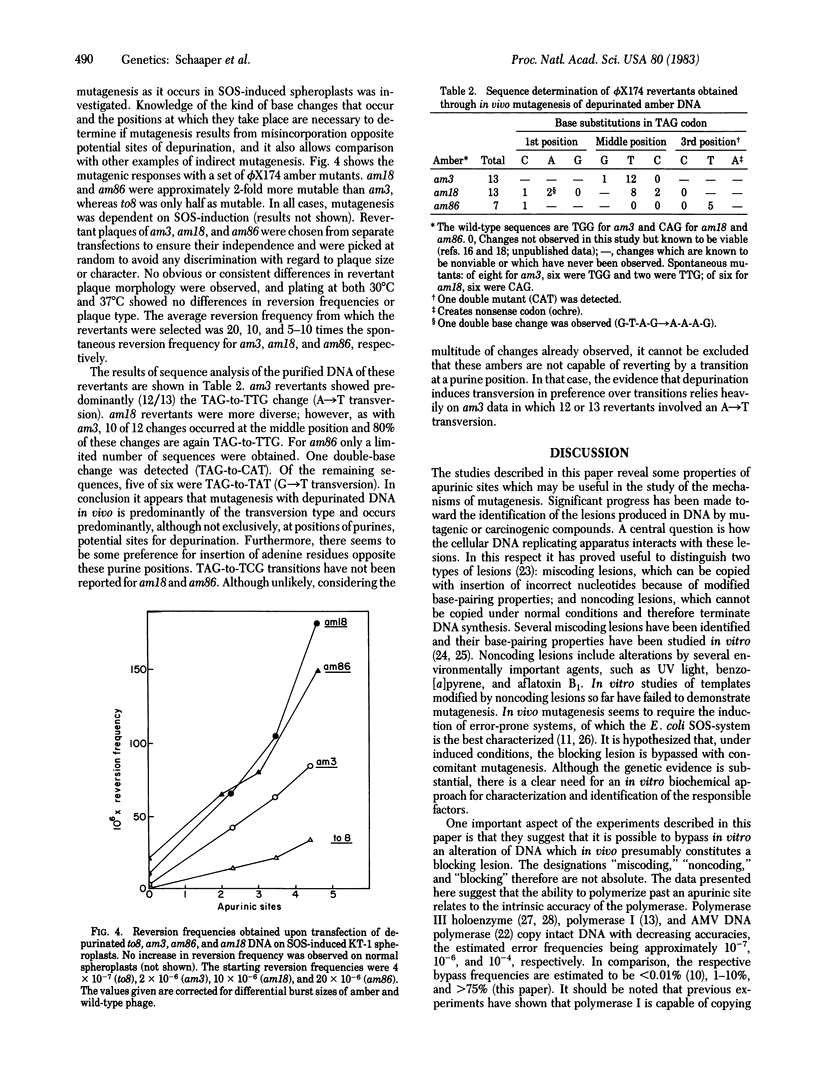
The mutagenic potential of apurinic sites in vivo has been studied by transfection of depurinated phi X174 DNA containing amber mutations into SOS-induced Escherichia coli spheroplasts. Mutagenicity is abolished by treatment of the depurinated DNA with an apurinic endonuclease from Hela cells, establishing the apurinic site as the mutagenic lesion. The frequency of copying apurinic sites in vitro was analyzed by measuring the extent of DNA synthesis using E. coli DNA polymerase I and avian myeloblastosis DNA polymerase. The inhibition of DNA synthesis by apurinic sites was less with avian myeloblastosis DNA polymerase, suggesting that this error-prone enzyme copies apurinic sites with greater frequency. Consistent with this conclusion is the observation that, upon transfection into (normal) spheroplasts, the reversion frequency of depurinated phi X174 am3 DNA copied with avian myeloblastosis virus DNA polymerase is much greater than that of the same DNA copied with E. coli DNA polymerase I. Sequence analysis of the DNA of 33 revertant phage produced by depurination indicates a preference for incorporation of deoxyadenosine opposite putative apurinic sites. The combined results indicate that mutagenesis resulting from apurinic sites is associated with bypass of these noncoding lesions during DNA synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Deutsch W. A., Linn S. DNA binding activity from cultured human fibrolasts that is specific for partially depurinated DNA and that inserts purines into apurinic sites. Proc Natl Acad Sci U S A. 1979 Jan;76(1):141–144. doi: 10.1073/pnas.76.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Gopinathan K. P., Weymouth L. A., Kunkel T. A., Loeb L. A. Mutagenesis in vitro by DNA polymerase from an RNA tumour virus. Nature. 1979 Apr 26;278(5707):857–859. doi: 10.1038/278857a0. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Mount D. W. Mechanisms of DNA replication and mutagenesis in ultraviolet-irradiated bacteria and mammalian cells. Prog Nucleic Acid Res Mol Biol. 1981;25:53–126. doi: 10.1016/s0079-6603(08)60483-3. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Kleber I., Benzinger R. Transfection of Escherichia coli spheroplasts. 3. Facilitation of transfection and stabilization of spheroplasts by different basic polymers. J Virol. 1973 Oct;12(4):741–747. doi: 10.1128/jvi.12.4.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houts G. E., Miyagi M., Ellis C., Beard D., Beard J. W. Reverse transcriptase from avian myeloblastosis virus. J Virol. 1979 Feb;29(2):517–522. doi: 10.1128/jvi.29.2.517-522.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane C. M., Linn S. Purification and characterization of an apurinic/apyrimidinic endonuclease from HeLa cells. J Biol Chem. 1981 Apr 10;256(7):3405–3414. [PubMed] [Google Scholar]
- Kuhnlein U., Tsang S. S., Edwards J. Characterization of DNA damages by filtration through nitrocellulose filters: a simple probe for DNA-modifying agents. Mutat Res. 1979 Jun;64(3):167–182. doi: 10.1016/0165-1161(79)90102-x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. The accuracy of Escherichia coli DNA polymerase I in copying natural DNA in vitro. J Biol Chem. 1980 Oct 25;255(20):9961–9966. [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Beckman R. A., Loeb L. A. On the fidelity of DNA replication. Effect of the next nucleotide on proofreading. J Biol Chem. 1981 Oct 10;256(19):9883–9889. [PubMed] [Google Scholar]
- Kunkel T. A., Shearman C. W., Loeb L. A. Mutagenesis in vitro by depurination of phiX174 dna. Nature. 1981 May 28;291(5813):349–351. doi: 10.1038/291349a0. [DOI] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Margison G. P., O'Connor P. J. Biological implications of the instability of the N-glycosidic bone of 3-methyldeoxyadenosine in DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):349–356. doi: 10.1016/0005-2787(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Radman M., Villani G., Boiteux S., Kinsella A. R., Glickman B. W., Spadari S. Replicational fidelity: mechanisms of mutation avoidance and mutation fixation. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):937–946. doi: 10.1101/sqb.1979.043.01.103. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Glickman B. W., Loeb L. A. Mutagenesis resulting from depurination is an SOS process. Mutat Res. 1982 Nov;106(1):1–9. doi: 10.1016/0027-5107(82)90186-5. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Glickman B. W., Loeb L. A. Role of depurination in mutagenesis by chemical carcinogens. Cancer Res. 1982 Sep;42(9):3480–3485. [PubMed] [Google Scholar]
- Schaaper R. M., Glickman B. W. Mutability of bacteriophage M13 by ultraviolet light: role of pyrimidine dimers. Mol Gen Genet. 1982;185(3):404–407. doi: 10.1007/BF00334131. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman C. W., Loeb L. A. Effects of depurination on the fidelity of DNA synthesis. J Mol Biol. 1979 Feb 25;128(2):197–218. doi: 10.1016/0022-2836(79)90126-8. [DOI] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Singer B., Fraenkel-Conrat H., Kuśmierek J. T. Preparation and template activities of polynucleotides containing O2- and O4-alkyluridine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1722–1726. doi: 10.1073/pnas.75.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]