Abstract
mtDNA polymorphism has been studied by restriction endonuclease site variation in Drosophila pseudoobscura and its sibling species D. persimilis. Eight enzymes have been used to study 54 isofemale strains from areas where the two species are sympatric and D. pseudoobscura is allopatric. Where sympatric, 75-80% of the strains have mitochondrial genomes found in both species. Where allopatric, D. pseudoobscura has diverged to the point where none of the strains have mtDNA in common with D. persimilis. The most likely explanation for this observation is that where sympatric the two species hybridize frequently enough to keep their mtDNA from diverging. However, hybridization has not prevented their nuclear genomes from diverging, perhaps due to selection against nuclear gene introgression contrasted with little or no selection against mtDNA introgression. These observations suggest that nuclear and cytoplasmic genomes have different evolutionary dynamics.
Full text
PDF
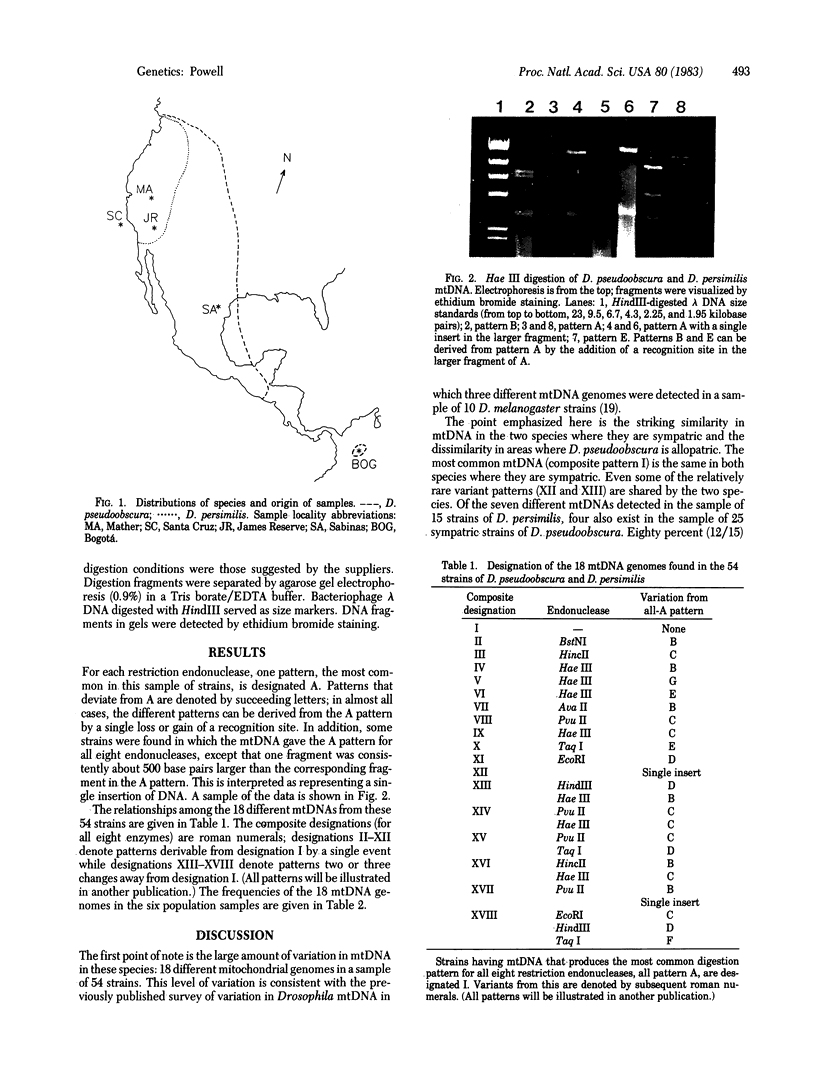


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. W., Ayala F. J., Michod R. E. Chromosomal and allozymic diagnosis of three species of Drosophila. Drosophila pseudoobscura, D. persimilis, and D. miranda. J Hered. 1977 Mar-Apr;68(2):71–74. doi: 10.1093/oxfordjournals.jhered.a108793. [DOI] [PubMed] [Google Scholar]
- Ayala F. J., Powell J. R. Allozymes as diagnostic characters of sibling species of Drosophila. Proc Natl Acad Sci U S A. 1972 May;69(5):1094–1096. doi: 10.1073/pnas.69.5.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky C. W., Jr Transmission genetics of mitochondria and chloroplasts. Annu Rev Genet. 1978;12:471–512. doi: 10.1146/annurev.ge.12.120178.002351. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A. Lack of genic similarity between two sibling species of drosophila as revealed by varied techniques. Genetics. 1976 Nov;84(3):593–607. doi: 10.1093/genetics/84.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Experiments on Sexual Isolation in Drosophila: X. Reproductive Isolation Between Drosophila Pseudoobscura and Drosophila Persimilis Under Natural and Under Laboratory Conditions. Proc Natl Acad Sci U S A. 1951 Dec;37(12):792–796. doi: 10.1073/pnas.37.12.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Genetic analysis of hybrid sterility within the species Drosophila pseudoobscura. Hereditas. 1974;77(1):81–88. doi: 10.1111/j.1601-5223.1974.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Studies on Hybrid Sterility. II. Localization of Sterility Factors in Drosophila Pseudoobscura Hybrids. Genetics. 1936 Mar;21(2):113–135. doi: 10.1093/genetics/21.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Intraspecific diversity of nucleotide sequences within the adenine + thymine-rich region of mitochondrial DNA molecules of Drosophila mauritiana, Drosophila melanogaster and Drosophila simulans. Nucleic Acids Res. 1980 Nov 25;8(22):5391–5410. doi: 10.1093/nar/8.22.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman R. A., Shade R. O., Shapira J. F., Avise J. C. The use of restriction endonucleases to measure mitochondrial DNA sequence relatedness in natural populations. III. Techniques and potential applications. J Mol Evol. 1981;17(4):214–226. doi: 10.1007/BF01732759. [DOI] [PubMed] [Google Scholar]
- Macknight R H. The Sex-Determining Mechanism of Drosophila Miranda. Genetics. 1939 Mar;24(2):180–201. doi: 10.1093/genetics/24.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S. Origin of reproductive isolation in the absence of apparent genic differentiation in a geographic isolate of Drosophila pseudoobscura. Genetics. 1972 Sep;72(1):143–155. doi: 10.1093/genetics/72.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. G., Thomas C. A., Jr Length polymorphisms, restriction site variation, and maternal inheritance of mitochondrial DNA of Drosophila melanogaster. Plasmid. 1980 Mar;3(2):109–115. doi: 10.1016/0147-619x(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Langley C. H. Inter- and intraspecific variation in restriction maps of Drosophila mitochondrial DNAs. Nature. 1979 Oct 25;281(5733):696–699. doi: 10.1038/281696a0. [DOI] [PubMed] [Google Scholar]