Abstract
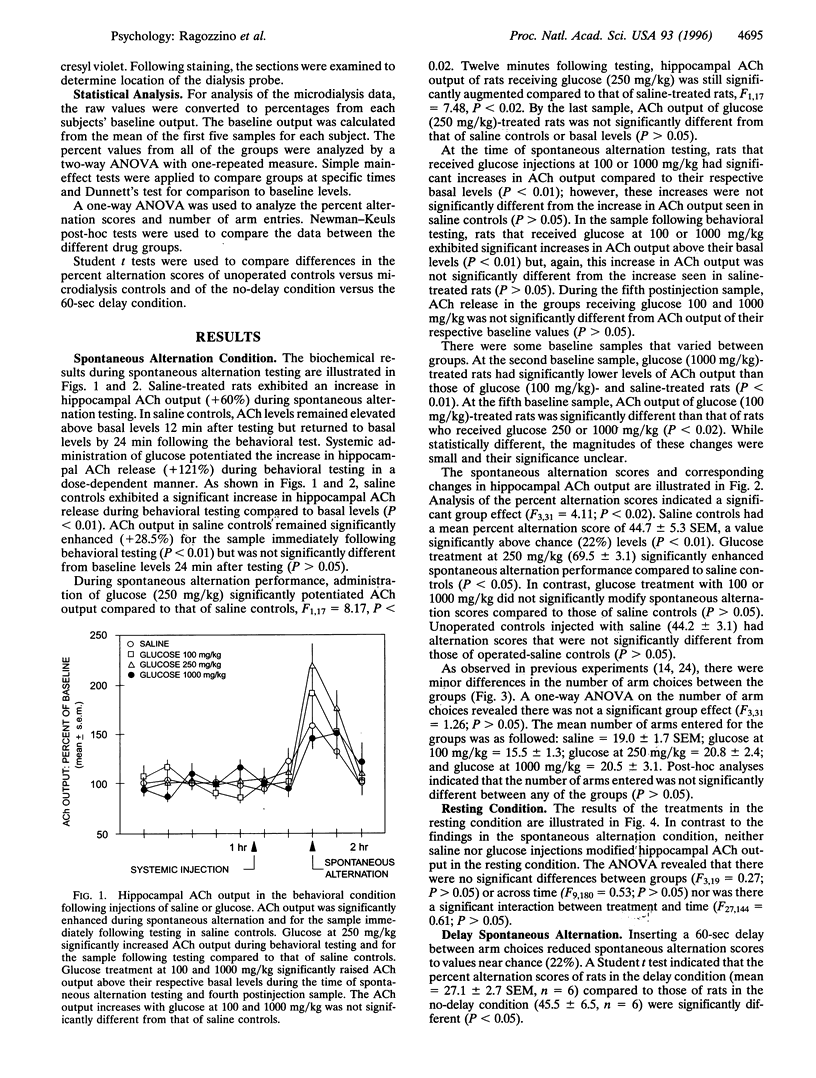
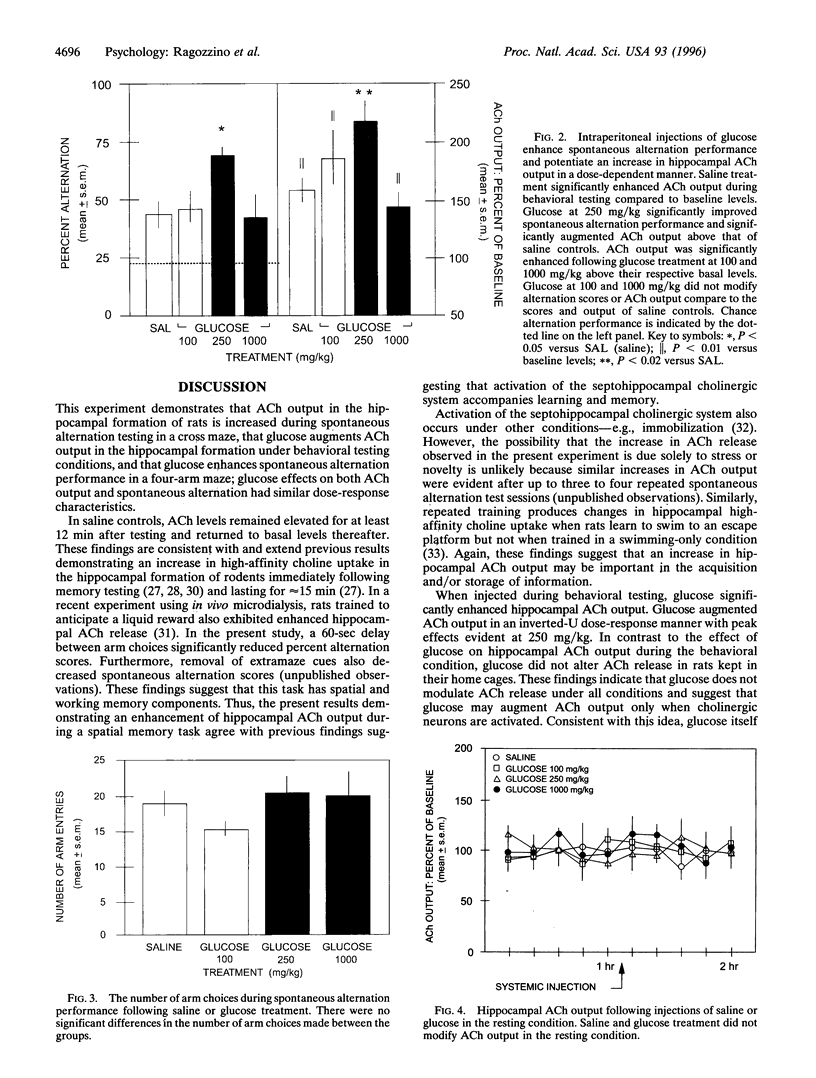
Several lines of evidence indicate that a modest increase in circulating glucose levels enhances memory. One mechanism underlying glucose effects on memory may be an increase in acetylcholine (ACh) release. The present experiment determined whether enhancement of spontaneous alternation performance by systemic glucose treatment is related to an increase in hippocampal ACh output. Samples of extracellular ACh were assessed at 12-min intervals using in vivo microdialysis with HPLC-EC. Twenty-four minutes after an intraperitoneal injection of saline or glucose (100, 250, or 1000 mg/kg), rats were tested in a four-arm cross maze for spontaneous alternation behavior combined with microdialysis collection. Glucose at 250 mg/kg, but not 100 or 1000 mg/kg, produced an increase in spontaneous alternation scores (69.5%) and ACh output (121.5% versus baseline) compared to alternation scores (44.7%) and ACh output (58.9% versus baseline) of saline controls. The glucose-induced increase in alternation scores and ACh output was not secondary to changes in locomotor activity. Saline and glucose (100-1000 mg/kg) treatment had no effect on hippocampal ACh output when rats remained in the holding chamber. These findings suggest that glucose may enhance memory by directly or indirectly increasing the release of ACh. The results also indicate that hippocampal ACh release is increased in rats performing a spatial task. Moreover, because glucose enhanced ACh output only during behavioral testing, circulating glucose may modulate ACh release only under conditions in which cholinergic cells are activated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bielarczyk H., Szutowicz A. Evidence for the regulatory function of synaptoplasmic acetyl-CoA in acetylcholine synthesis in nerve endings. Biochem J. 1989 Aug 15;262(1):377–380. doi: 10.1042/bj2620377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase D. A., Han Y. H., Dewey W. L. Effects of glucose and diabetes on binding of naloxone and dihydromorphine to opiate receptors in mouse brain. Diabetes. 1987 Oct;36(10):1173–1177. doi: 10.2337/diab.36.10.1173. [DOI] [PubMed] [Google Scholar]
- Day J., Damsma G., Fibiger H. C. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav. 1991 Apr;38(4):723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- Decker M. W., Pelleymounter M. A., Gallagher M. Effects of training on a spatial memory task on high affinity choline uptake in hippocampus and cortex in young adult and aged rats. J Neurosci. 1988 Jan;8(1):90–99. doi: 10.1523/JNEUROSCI.08-01-00090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin T. P., Messier C., de Boer P., Westerink B. H. Raised glucose levels enhance scopolamine-induced acetylcholine overflow from the hippocampus: an in vivo microdialysis study in the rat. Behav Brain Res. 1992 Aug 10;49(2):181–188. doi: 10.1016/s0166-4328(05)80163-9. [DOI] [PubMed] [Google Scholar]
- Fellows L. K., Boutelle M. G., Fillenz M. Extracellular brain glucose levels reflect local neuronal activity: a microdialysis study in awake, freely moving rats. J Neurochem. 1992 Dec;59(6):2141–2147. doi: 10.1111/j.1471-4159.1992.tb10105.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Blass J. P. Impaired synthesis of acetylcholine in brain accompanying mild hypoxia and hypoglycemia. J Neurochem. 1976 Jul;27(1):37–42. doi: 10.1111/j.1471-4159.1976.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Blass J. P., Jenden D. J. Measurement of acetylcholine turnover with glucose used as precursor: evidence for compartmentation of glucose metabolism in brain. J Neurochem. 1978 Jan;30(1):71–76. doi: 10.1111/j.1471-4159.1978.tb07036.x. [DOI] [PubMed] [Google Scholar]
- Gilad G. M. The stress-induced response of the septo-hippocampal cholinergic system. A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinology. 1987;12(3):167–184. doi: 10.1016/0306-4530(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Giovannini M. G., Camilli F., Mundula A., Pepeu G. Glutamatergic regulation of acetylcholine output in different brain regions: a microdialysis study in the rat. Neurochem Int. 1994 Jul;25(1):23–26. doi: 10.1016/0197-0186(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Givens B., Olton D. S. Local modulation of basal forebrain: effects on working and reference memory. J Neurosci. 1994 Jun;14(6):3578–3587. doi: 10.1523/JNEUROSCI.14-06-03578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P. E. Glucose modulation of memory storage processing. Behav Neural Biol. 1986 May;45(3):342–349. doi: 10.1016/s0163-1047(86)80022-x. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Gold P. E. The effects of training, epinephrine, and glucose injections on plasma glucose levels in rats. Behav Neural Biol. 1986 Sep;46(2):156–167. doi: 10.1016/s0163-1047(86)90640-0. [DOI] [PubMed] [Google Scholar]
- Inglis F. M., Day J. C., Fibiger H. C. Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience. 1994 Oct;62(4):1049–1056. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., da Cunha C., Rosat R., Jerusalinsky D., Ferreira M. B., Medina J. H. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992 Jul;58(1):16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Lapchak P. A., Araujo D. M., Collier B. Regulation of endogenous acetylcholine release from mammalian brain slices by opiate receptors: hippocampus, striatum and cerebral cortex of guinea-pig and rat. Neuroscience. 1989;31(2):313–325. doi: 10.1016/0306-4522(89)90376-x. [DOI] [PubMed] [Google Scholar]
- Manning C. A., Ragozzino M. E., Gold P. E. Glucose enhancement of memory in patients with probable senile dementia of the Alzheimer's type. Neurobiol Aging. 1993 Nov-Dec;14(6):523–528. doi: 10.1016/0197-4580(93)90034-9. [DOI] [PubMed] [Google Scholar]
- Marighetto A., Durkin T., Toumane A., Lebrun C., Jaffard R. Septal alpha-noradrenergic antagonism in vivo blocks the testing-induced activation of septo-hippocampal cholinergic neurones and produces a concomitant deficit in working memory performance of mice. Pharmacol Biochem Behav. 1989 Nov;34(3):553–558. doi: 10.1016/0091-3057(89)90557-1. [DOI] [PubMed] [Google Scholar]
- Marighetto A., Micheau J., Jaffard R. Effects of intraseptally injected glutamatergic drugs on hippocampal sodium-dependent high-affinity choline uptake in "naive" and "trained" mice. Pharmacol Biochem Behav. 1994 Nov;49(3):689–699. doi: 10.1016/0091-3057(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Meck W. H., Church R. M., Wenk G. L., Olton D. S. Nucleus basalis magnocellularis and medial septal area lesions differentially impair temporal memory. J Neurosci. 1987 Nov;7(11):3505–3511. doi: 10.1523/JNEUROSCI.07-11-03505.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck W. H. Selective adjustment of the speed of internal clock and memory processes. J Exp Psychol Anim Behav Process. 1983 Apr;9(2):171–201. [PubMed] [Google Scholar]
- Messier C., Durkin T., Mrabet O., Destrade C. Memory-improving action of glucose: indirect evidence for a facilitation of hippocampal acetylcholine synthesis. Behav Brain Res. 1990 Jul 9;39(2):135–143. doi: 10.1016/0166-4328(90)90100-s. [DOI] [PubMed] [Google Scholar]
- Messier C., White N. M. Memory improvement by glucose, fructose, and two glucose analogs: a possible effect on peripheral glucose transport. Behav Neural Biol. 1987 Jul;48(1):104–127. doi: 10.1016/s0163-1047(87)90634-0. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Ragozzino M. E., Arankowsky-Sandoval G., Gold P. E. Glucose attenuates the effect of combined muscarinic-nicotinic receptor blockade on spontaneous alternation. Eur J Pharmacol. 1994 Apr 11;256(1):31–36. doi: 10.1016/0014-2999(94)90612-2. [DOI] [PubMed] [Google Scholar]
- Ragozzino M. E., Gold P. E. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995 Oct;68(4):981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- Ragozzino M. E., Gold P. E. Task-dependent effects of intra-amygdala morphine injections: attenuation by intra-amygdala glucose injections. J Neurosci. 1994 Dec;14(12):7478–7485. doi: 10.1523/JNEUROSCI.14-12-07478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino M. E., Parker M. E., Gold P. E. Spontaneous alternation and inhibitory avoidance impairments with morphine injections into the medial septum. Attenuation by glucose administration. Brain Res. 1992 Dec 4;597(2):241–249. doi: 10.1016/0006-8993(92)91480-3. [DOI] [PubMed] [Google Scholar]
- Ragozzino M. E., Wenk G. L., Gold P. E. Glucose attenuates a morphine-induced decrease in hippocampal acetylcholine output: an in vivo microdialysis study in rats. Brain Res. 1994 Aug 29;655(1-2):77–82. doi: 10.1016/0006-8993(94)91599-7. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Atweh S., Kuhar M. J. Sodium-dependent high affinity choline uptake: a regulatory step in the synthesis of acetylcholine. J Neurochem. 1976 May;26(5):909–922. doi: 10.1111/j.1471-4159.1976.tb06472.x. [DOI] [PubMed] [Google Scholar]
- Steckler T., Keith A. B., Wiley R. G., Sahgal A. Cholinergic lesions by 192 IgG-saporin and short-term recognition memory: role of the septohippocampal projection. Neuroscience. 1995 May;66(1):101–114. doi: 10.1016/0306-4522(94)00603-3. [DOI] [PubMed] [Google Scholar]
- Stone W. S., Cottrill K. L., Walker D. L., Gold P. E. Blood glucose and brain function: interactions with CNS cholinergic systems. Behav Neural Biol. 1988 Nov;50(3):325–334. doi: 10.1016/s0163-1047(88)91018-7. [DOI] [PubMed] [Google Scholar]
- Stone W. S., Rudd R. J., Gold P. E. Glucose attenuation of atropine-induced deficits in paradoxical sleep and memory. Brain Res. 1995 Oct 2;694(1-2):133–138. doi: 10.1016/0006-8993(95)00810-d. [DOI] [PubMed] [Google Scholar]
- Stone W. S., Walser B., Gold S. D., Gold P. E. Scopolamine- and morphine-induced impairments of spontaneous alternation performance in mice: reversal with glucose and with cholinergic and adrenergic agonists. Behav Neurosci. 1991 Apr;105(2):264–271. doi: 10.1037//0735-7044.105.2.264. [DOI] [PubMed] [Google Scholar]
- Torres E. M., Perry T. A., Blockland A., Wilkinson L. S., Wiley R. G., Lappi D. A., Dunnet S. B. Behavioural, histochemical and biochemical consequences of selective immunolesions in discrete regions of the basal forebrain cholinergic system. Neuroscience. 1994 Nov;63(1):95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Tucek S., Cheng S. C. Provenance of the acetyl group of acetylcholine and compartmentation of acetyl-CoA and Krebs cycle intermediates in the brain in vivo. J Neurochem. 1974 Jun;22(6):893–914. doi: 10.1111/j.1471-4159.1974.tb04314.x. [DOI] [PubMed] [Google Scholar]
- Wenk G., Hepler D., Olton D. Behavior alters the uptake of [3H]choline into acetylcholinergic neurons of the nucleus basalis magnocellularis and medial septal area. Behav Brain Res. 1984 Aug;13(2):129–138. doi: 10.1016/0166-4328(84)90143-8. [DOI] [PubMed] [Google Scholar]