Abstract
Mechanisms modulating prostate cell fate determination remain unexplored. The leucine-rich repeat containing G-protein coupled receptors (LGRs) have been identified as important stem cell markers in various tissues. Here, we investigated the roles of Lgr4/Gpr48 in prostate stem cells and development. Lgr4 was ubiquitously expressed during early prostate development prior to lineage specification, with adult expression restricted to a few basal cells (principally Lin−Sca1+CD49f+). Lgr4−/− mice had compromised branching morphogenesis and delayed epithelial differentiation, leading to decreased prostate size and impaired luminal cell function. In vitro prostate sphere culture revealed that Lgr4−/− Lin−/Sca1+/CD49f+ (LSC) cells failed to generate p63low cells, indicating a differentiation deficiency. Furthermore, Lgr4 ablation arrested prostate stem cell (PSC) differentiation of in vivo kidney capsule prostate grafts, suggesting that Lgr4 modulates prostate stem cell properties independent of hormonal and mesenchymal effects. Analysis of neonatal prostates and prostate spheres revealed a decrease in Wnt, Sonic Hedgehog, and Notch1 expression in Lgr4−/− cells. Lgr4 loss blocked differentiation of prostate sphere p63hi cells to p63low. Treatment with exogenous Sonic Hedgehog partially restored the differentiation of p63hi cells in Lgr4−/− spheres. Taken together, our data revealed the roles of Lgr4 in early prostate development and in stem cell differentiation through regulation of the Wnt, Notch and Sonic Hedgehog signaling pathways.
Keywords: Lgr4, Gpr48, prostate stem cells, prostate development
Introduction
The delicate balance between stem cell differentiation and self-renewal drives major organ development, resulting in ordered layers of functional differentiated cells and residual stem cells responsible for renewal and repair 1, 2. Deregulation of these biological processes can result in developmental defects or cancer, yet many key regulators of stem cells remain unidentified or poorly understood. Mouse prostate development begins at E16.5-17.5 when stromal-epithelial interactions provide an asymmetric microenvironment for urogenital sinus epithelial stem cells to invade into the surrounding mesenchyme. Subsequently, prostate epithelial buds form ducts which elongate and branch out from the urethra and terminate in acini3. Neonatal prosate stem cells (PSCs) are located within the basal cell lineage and can differentiate into basal, luminal and neuroendocrine cells 4. In contrast, adult prostate epithelia are mainly maintained by respective stem/progenitors within the basal and luminal cell lineages 5. However, basal stem cells retain the capacity for multi-lineage differentiation in transplantation-based regeneration assays6, 7. Although EGF/EGFR, TGF-α/BMP, Wnt/β-catenin and Hedgehog signaling are implicated in prostate stem cell self-renewal and differentiation8, detailed mechanisms of PSC regulation are still largely unkown.
The leucine-rich repeat containing G-protein coupled receptor Lgr4 (also called Gpr48), together with its close family members Lgr5 and Lgr6, belongs to the glycoprotein hormone receptor family 9, 10. Lgr4 inactivation in mice leads to developmental deficiencies in multiple organs. Lgr4-null mice display embryonic growth retardation with 60% embryonic lethality11. Surviving Lgr4-knockout mice are infertile12 and exhibit significant renal hypoplasia13, 14, delayed osteoblast differentiation15, deficiencies in blood pressure and electrolyte homeostasis16, transient anemia during midgestation17, malformation of the eye anterior segment 18, 19, impaired mammary gland branching morphogenesis and mammary stem cells 20, 21, disrupted innate immunity 22, susceptibility to inflammatory bowel disease 23, and extensive reproductive system defects 12, 24-27. Recently, Lgr4 and Lgr5 have been shown to enhance Wnt signaling through association with R-spondin28, 29, partially explaining the similar phenotype found in Lgr4, Lgr5, and Wnt knock-out mouse models. Lgr5 has been identified as a stem cell marker in the small intestine30, stomach 31 and hair follicles32. Furthermore, Lgr5 is also proposed to be a cancer stem cell (CSC) marker in intestinal adenomas33. Lgr6 expression also marks hair follicle epidermal stem cells34. Although the function of Lgr4 in stem cells remains unclear, its connection with Wnt signaling together with the breadth of developmental deficiencies in multiple organs upon Lgr4 ablation strongly suggest that it could function as a key stem cell regulator similar to its family members Lgr5 and Lgr6. We therefore used a mouse model to investigate the role of Lgr4 in prostate development and prostate stem cell function.
Materials and Methods
Animals
All experiments using mice were performed in accordance with a protocol approved by the Texas A&M Health Science Center Institutional Animal Care and Use Committee. Lgr4 null mice were generated from an Lgr4 gene trap ES cell clone (LST020) purchased from Williams Skarnes (Bay Genomics) as previously described 18. Original Lgr4 null mice (129×C57/BL6 background) were backcrossed with male CD-1 mice from Charles River (Wilmington, MA) for 9 generations. Inbred CD-1 Lgr4 null mice were used in this study.
Primers used for genotyping Lgr4 null mice include, 5′-GGT CTT TGA GCA CCA GAG GAC ATC-3′ (pGT2TMPFS R), 5′-AAA AGC CAC ATT CAA ATC TTA GTA ACC-3′ (Lgr4 WT reverse), 5′-AAG CAC TTG ATG GTC AGA CTA CAT GC-3′ (Lgr4 WT forward).
Castration was performed on 8 week old male mice as described previously35. After castration, mice were regressed for 2 weeks prior to androgen re-administration. Androgen therapy was terminated after 2 weeks, at which time prostates were considered fully regenerated.
Histology, LacZ staining and immunostaining
Prostate glands were dissected and sectioned as described previously 35. Prostate weight, body weight and prostate branching points were quantified. Each group contained at least 3 animals and data are presented as mean ± S.E. Prostate immunohistochemistry (IHC) and immunofluorescence (IF) was performed on 5μm sections, using antibodies listed in Supplemental Methods, or sections were stained by Hematoxylin and Eosin.
Whole mount LacZ staining was carried out on 1, 2, 4 and 16 week old prostates, or P0 Lgr4+/+ and Lgr4+/− prostate spheres were collected, fixed and β-gal stained before embedding in HistoGel and sectioning. 5μm sections were counterstained with Eosin. Immunofluorescence images were taken at room temperature using a Zeiss Axiovert 200M with Zeiss EC Plan NeoFluar lenses equipped with a Zeiss LSM 510 camera and using the manufacturer's LSM 510 image acquisition software program.
In vitro prostate sphere forming assay
Isolation of 6-8 week-old prostate primary cells and sphere culture assay was performed as described36. After 7-10 days culture per generation, prostate spheres were passaged by digestion to a single cell suspension and 2×104 cells were seeded per well of 12 well plates.
Some experiments treated P1 prostate spheres with 0.25μg/ml recombinant mouse Sonic Hedgehog (R&D systems, Inc., Minneapolis, MN); or L-Wnt3a (ATCC, 10801 University Boulevard, Manassas, VA) conditioned medium mixed with PrEGM at 1:3 (L-cell conditioned medium as control), with or without 1ng/ml recombinant mouse R-spondin 3 (R&D systems, Inc., Minneapolis, MN). Bright field photomicrographs were taken using a Nikon Eclipse 80i microscope; images were taken using NIS-Elements BR3.2 software. Immunofluorescence photomicrographs were taken at room temperature using a Zeiss Axioskop with Zeiss EC Plan NeoFluar lenses equipped with an Olympus DP73 camera and images were taken using Cell Sens Dimensions software.
Flow Cytometry
Red blood cells in prostate primary cell suspensions were lysed in 2ml RBC lysis buffer (eBioscience, San Diego, CA) at room temperature for 5 minutes. Single-cell suspensions were then stained with 0.625μg/500μl APC-conjugated Anti-Human/Mouse CD49f (Integrin alpha 6) (eBioscience, San Diego, CA) and 1.25ug/500ul PE-conjugated Anti-Mouse Ly-6A/E (Sca-1) (eBioscience, San Diego, CA) in 1% BSA/PBS for 0.5-1 hour. Flow cytometry was performed using the BD FACS Canto and analyzed by CellQuest software. Cell sorting was conducted on a BD FACS Vantage (BD Biosciences, San Jose, CA).
In vivo prostate regeneration
Prostates from 6-8 week old mice were digested for 3 hours at 37°C in DMEM/F12/Collagenase/Hyaluronidase/FBS (StemCell technologies, Vancouver, Canada) followed by 1 hour digestion with 0.25% Trypsin-EDTA (Invitrogen, Carlsbad, CA) on ice. Cells were suspended in Dispase (Invitrogen, Carlsbad, CA, 5 mg/mL) and DNase I (Roche Applied Science, Indianapolis, IN, 1 mg/mL), and pipetted vigorously. Dissociated cells were passed through 70 μm cell strainers (BD Biosciences, San Jose, CA). 1×105 dissociated cells were mixed with equal numbers of mouse UGSM cells, resuspended in rat-tail collagen and setting solution (0.34 M NaOH: DMEM = 1:1.9, volume ratio) as previously described 37, and incubated at 37°C overnight in Bfs media (DMEM/5% FBS/5% Nu-serum/5 μg/ml insulin/10-8 M 5α dihydrotestosterone). The next day, collagen pellets were grafted under the kidney capsule of male NOD/SCID mice while 12.5 mg testosterone pellets were inserted subcutaneously; grafts were analyzed after two months.
RNA collection, cDNA synthesis and Quantitative PCR
Day 0 and 2 week-old prostate tissues were homogenized in 1ml Trizol LS reagent (Life Technology, Carlsbad, CA). P1 prostate spheres were disassociated into single cell suspensions and lysed in 1ml Trizol LS reagent. RNA was extracted using 200μl chloroform per 1ml Trizol lysate, and isopropanol precipitated.
0.5-1μg RNA was annealed with 1μg Oligo (dT) 15 Primer (Promega, Madison, WI) at 70°C and then subjected to cDNA synthesis using 200units/reaction M-MLV Reverse Transcriptase (Promega, Madison, WI) for 50 minutes at 42°C.
cDNA diluted 1:20-50 was used as templates for quantitative PCR. Real time quantitative PCR was done with GoTaq® qPCR Master Mix (Promega, Madison, WI). Quantitative PCR was performed on Mx3000P QPCR System (Agilent Technologies, Santa Clara CA) and analyzed with MxPro QPCR Software (Agilent Technologies, Santa Clara CA). Primers are listed in Supplemental Methods.
TOP-dGFP virus infection
Lentiviral-TOP-dGFP-reporter (Addgene, Cambridge MA) was used to produce Lentivirus. Virus production and infection was performed as described previously 38.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (CHIP) assays were done on P2 and P3 prostate spheres by using SimpleChIP Enzymatic Chromatin IP Kit (Cell signal, Danvers, MA) and following the included protocol.
Statistical Analysis
Analysis was performed using Student's t-test, with P<0.05 considered significant.
Results
Lgr4 is highly expressed in early prostate development and prostate progenitor cells
To study the Lgr4 expression pattern in mouse prostate, aCD4 trans-membrane domain fused β-galactodase gene in a trap vector was inserted into the first intron of the Lgr4 gene to generate an Lgr4 null allele 18. β-gal staining indicated ubiquitous expression of Lgr4 at the peak of branching morphogenesis in 1 week-old prostates (Figure 1A) and in 2 week-old prostates during epithelial differentiation (data not shown). After epithelial differentiation, Lgr4 is only expressed in cells adjacent to the basal membrane and the outer smooth muscle layer in 4 week-old (Figure 1A) and mature prostates (Figure 1B). Furthermore, co-staining of membrane localized β-gal and the nuclear-localized basal cell marker p63 in 8 week-old Lgr4+/− prostates revealed that Lgr4 is expressed in a subset of basal cells (Figure 1B). This matches the expression pattern in the adult human prostate (Figure 1C). To examine whether Lgr4 expression correlated with the PSC-enriched Lin−/Sca1+/CD49f+ (LSC) population, we FACS sorted primary prostate cells from 6-10 week old mice and assessed Lgr4 expression by qPCR. Lgr4 is highly expressed in PSC-enriched LSC cells (Figure 1D), suggesting that Lgr4 may play a role in regulating PSCs.
Figure 1. Lgr4 ablation compromised prostate development.

A) Whole mount prostates stained for LacZ were sectioned (7μm thickness) and counterstained with Eosin to show Lgr4 expression in 1-4 week-old prostates. Scale bar 25μm. B) In 8 week-old prostate, Lgr4 expression (shown by LacZ staining) is partially co-localized with p63 (shown by IHC). Representative photograph of section from anterior lobe; pattern is characteristic of all lobes. Arrows indicate co-localization of Lgr4 and p63. Scale bar 25μm. C) Lgr4 is expressed in basal epithelial cells in the human prostate. Lgr4 expression in adult human prostate was determined by immunohistochemistry. Representative photograph of N=5. Scale bar = 100 μm. D) Lgr4 mRNA levels in different populations of primary prostate cells were determined by qPCR. E). Reduced prostate sizeof Lgr4−/− mice when compared to their wild type littermates at indicated ages. Scale bar 5mm. F) Prostatic lobes were dissected from 6 week-old Lgr4−/− mice and their wild type littermates, and their ductal networks were microdisected. Scale bar 5mm. G) The branching points of each prostatic lobe from 6 week mice were quantified. Bar graph shows the mean ± S. E. from 3 pairs of mice, p≤0.02. AP, anterior prostate; VP, ventral prostate; DLP, dorsal-lateral prostate. H) Hematoxylin & Eosin staining of prostate sections at indicated ages. Scale bar 100μm.
Lgr4 regulates prostate development and branching morphogenesis
In order to investigate the role of Lgr4 in prostate development, Lgr4 null mouse prostates were compared to their wild type littermates at various developmental stages. As early as 2 weeks of age, Lgr4 ablation in mouse prostates results in a dramatically decreased prostate size (Figure 1E). In addition, deficient prostate branching morphogenesis was found in Lgr4−/− mice (Figure 1F). Branching points in 6 week-old Lgr4−/− prostates were decreased by 62%, 70% and 45% in anterior prostate (AP), ventral prostate (VP) and dorsal-lateral prostate (DLP), respectively (Figure 1G).
We next compared prostate histology at different stages of development (Figure 1H). Several deficiencies were noticeable in Lgr4−/− prostates in addition to the diminished gland size and branching morphogenesis. First, a significantly reduced luminal epithelial cell enfolding was found in Lgr4−/− AP beginning at 2 weeks (Figure 1H ii versus vi). By 4 weeks, wild type luminal cells aligned and formed a single layer, while Lgr4−/− luminal cells often aggregated as clusters, possibly due to impaired cell migration. Clustering persisted in Lgr4−/− prostates beyond 6 weeks into adulthood (Figure 1H, vii-viii, xv-xvi, xxiii-xxiv). Second, lumen secretion was greatly reduced in 6 week-old Lgr4−/− AP (Figure 1H iv& viii), implying a disruption of luminal cell function upon Lgr4 loss. Third, in 4-6 week old wild type prostates, columnar luminal cells showed normal nuclear versus cytoplasmic ratios, with nuclei evenly spaced along the luminal layer. In contrast, Lgr4−/− luminal cells lacked columnar morphology, had decreased cytoplasmic content and more crowded, condensed nuclei (Figure 1H, 3rd & 4th row). Finally, Lgr4−/− AP had thicker smooth muscle layers surrounding the prostatic glands from 2 weeks of age (Figure 1H vi-viii).
Lgr4 ablation regulates prostate epithelial cell proliferation and differentiation, and impairs luminal epithelial cell function
In order to uncover how Lgr4 interruption leads to attenuated prostate size, branching morphogenesis and luminal epithelial cell enfolding, we examined proliferation during prostate development (Figure 2A). In wild type prostate, epithelial cells rapidly proliferated during branching morphogenesis at 1 week. In 2 week-old prostates, the proliferation rate was reduced due to extensive epithelial cell differentiation. During puberty, when prostates enlarge and start to mature in response to androgen, epithelial proliferation elevated again (Figure 2A, 4 weeks). In the mature prostate, epithelial cell proliferation rate (% Ki67+) was low at 2.2% in 6 week old WT mice (Figure 2 A-B). In contrast, in 1 week-old Lgr4−/− prostate, Ki67 positive epithelial cells were reduced by 33% when compared to their wild type littermates, possibly leading to the diminished branching morphogenesis. A slight but not statistically significant decrease in epithelial cell proliferation was also found in Lgr4−/− prostates at 2 weeks, but levels of Ki67 positive proliferating cells became equivalent to wild type in 4 week-old prostates. Interestingly, Lgr4−/− prostates continued to display a high level of epithelial proliferation in 6 week-old prostates (16% compared to 2.2% in wild type), suggesting that Lgr4 inactivation deregulated prostate maturation (Figure 2 A-B). Together, these data imply that Lgr4 promotes cell proliferation and branching morphogenesis when it is ubiquitously expressed in early prostate development, and may affect prostate stem cell differentiation during later developmental stages.
Figure 2. Impaired proliferation, epithelial differentiation and function in Lgr4−/− prostate.

A) Ki67IHC shows proliferating cells in 1 to 6 week-old anterior prostate. Scale bar 50μm. B) Quantitation of Ki67 positive epithelial cells from 3 pairs of mice, 5 sections per prostate. Data expressed as mean ± S. E., * p≤0.001. C) CK8 IHC in anterior prostates at indicated ages. Photographs representative of CK8 pattern in all lobes. Scale bar 25μm.D) CK5 IHC in anterior prostates at indicated ages. Scale bar 25μm.E) Basal versus luminal epithelial cell ratios of 6 week-old anterior prostates were quantified. Bar graph expresses mean ± S. E. from 3 pairs of mice, 5 sections per prostate, p≤0.001. F) Probasin expression (green) in 4 week- and 6 week-old anterior prostate is shown by IF. Sections were counter stained with TO-PRO-3 (blue). Scale bar 100μm.G) Androgen receptor level in 2 week-old anterior prostates shown by IHC. Scale bar 50μm.
We next examined the consequences of Lgr4 loss on luminal and basal cell layers. In the wild-type mouse, cytokeratin 8 (CK8) expression peaked at 2 weeks, and gradually tapered off as intermediate cells differentiated into luminal cells with age. At 8 weeks, wild-type controls show a predominantly apical membrane localization of CK8, with localized cytoplasmic CK8+ regions (Figure 2C left panel). In contrast, at 2 weeks Lgr4−/− prostates lack strong CK8 staining, instead showing CK8 mainly localized to the apical membrane. CK8 expression gradually increased with age and remained high into adulthood (Figure 2C right panel). CK5 staining for basal cells shows that during basal cell differentiation wild type basal cells progress from a spherical to a flat laminar morphology (Figure 2D, left), whereas in Lgr4−/− prostates basal cells continued to show a spherical cell structure throughout development and adulthood (Figure 2D, right). The ratio of basal to luminal cells in wild type prostates decreased during puberty due to luminal cell expansion and stabilized at 1:5 after puberty. Lgr4−/− prostates, instead, retained a high basal to luminal cell ratio of 1:2 (Figure 2E). Finally, CK5 positive cells were also found in the luminal epithelial layer in 2-6 week-old Lgr4−/− prostates (Figure 2D), suggesting that CK5+/CK8+intermediate cell differentiation was arrested by Lgr4 inactivation. Together, these data reveal a crucial role for Lgr4 in cell fate determination.
Functional luminal epithelial cells express androgen receptor (AR) and secrete probasin. As shown in Figure 2F, probasin expression and secretion are dramatically reduced in Lgr4−/− anterior prostates at the age of 4 and 6 weeks. Furthermore, AR expression commenced at 2 weeks in wild type prostates, whereas very few luminal epithelial cells were AR positive in Lgr4−/− mice (Figure 2G), suggesting defective luminal epithelial cell functions in Lgr4−/− prostates.
Lgr4 ablation causes diminished epithelial differentiation in prostate progenitor cells without affecting their self-renewal
After completion of branching morphogenesis and differentiation, PSCs reside and slowly cycle in the prostate basal layer39; intriguingly, Lgr4 expression is restricted to a subset of basal cells (Figure 1A). Since Lgr4 is highly expressed in PSC-enriched LSCs (Figure 1C), and since Lgr4 loss affects epithelial cell differentiation (Figure 2), we hypothesized that Lgr4 may modulate PSC functions. In order to investigate PSC self-renewal and differentiation, we isolated prostate primary cells from 6-10 week-old Lgr4−/− mice and their wild type littermates and subjected them to sphere culture in vitro. To test PSC self-renewal in the absence of Lgr4, prostate spheres were passaged up to 4 generations. Lgr4 ablation did not alter sphere formation potency (Figure 3A), prostate sphere size (Figure 3B&D), or cell proliferation (Figure 3C). However, outer layer cell alignment was lost in Lgr4−/− prostate spheres (Figure 3E). These results indicate that Lgr4 does not affect prostate stem cell self-renewal in the sphere culture conditions tested.
Figure 3. Lgr4 ablation diminished prostate progenitor cell epithelial differentiation in prostate sphere culture without affecting their self-renewal abilities.
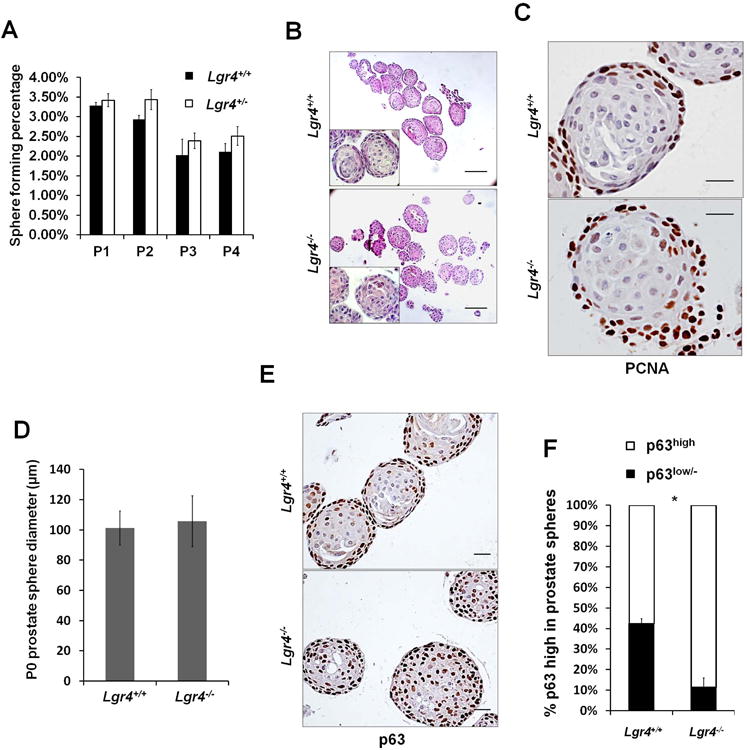
A) Number of prostate spheres from 3 pairs of mice in triplicate were counted for each generation. Prostate spheres were cultured for 4 generations. Graph shows the mean percentage of sphere forming cells ± S. E.. B) P0 prostate sphere morphology shown by Hematoxylin& Eosin staining. Scale bar 100μm. C) IHC of PCNA in P0 prostate spheres. Scale bar 20μm. D) Prostate sphere diameter was measured in at least 6 prostate spheres from 3 pairs of sphere samples. Bar graph shows the mean value ± S. D.E) p63 IHC of prostate spheres. Scale bar 20μm.F) p63high and p63low/− cells were counted from 8 prostate spheres. Bar graph shows the mean percentage ± S. E., p≤0.001.
In wild-type prostate spheres, undifferentiated p63high cells in the outer layer proliferate rapidly whereas inner layer cells undergo differentiation and lose p63 expression (Figure 3E, inner layer p63low/− cells). In accordance with the elevated basal cell numbers in vivo, Lgr4 disruption abrogated p63high cell differentiation into p63low/− cells in prostate spheres (Figure 3E, inner layer). p63low/−cell frequency was 42% in Lgr4+/+ versus 11% in Lgr4−/−(Figure 3F). These data strongly suggest a crucial role of Lgr4 in early PSC differentiation.
Lgr4 inactivation in prostate stem cells causes a malfunction in epithelial differentiation and impairs in vivo prostate graft regeneration
To examine Lgr4−/− PSC self-renewal and differentiation in vivo, and to eliminate possible hormonal effects and mesenchymal effects of Lgr4 loss, the kidney capsule regeneration assay was carried out using wild type urogenital sinus mesenchymal (UGSM) cells mixed with Lgr4+/+ or Lgr4−/− prostate primary cells. Two months after engraftment into the kidney capsule, both Lgr4+/+ and Lgr4−/− prostate primary cells were able to regenerate glandular structures (Figure 4A). However, Lgr4−/− graft weight was decreased by 70% and the number of glandular structures was significantly reduced (Figure 4A-B). Furthermore, the size and enfolding of glandular structures were compromised in Lgr4−/− grafts (Figure 4C). Examination of graft cell lineage commitment revealed the same patterns as described in early prostate development. In wild-type grafts, we found that luminal cells completely differentiated (indicated by low CK8 expression) and aligned in a single layer; also, only limited numbers of basal cells were present. In contrast, Lgr4−/− grafts had luminal cells that grouped in clusters and expressed high CK8 levels, as well as persistence of high numbers of basal cells (nearly two-fold compared to wild-type (Figure 4 D-E)). These data indicate that Lgr4 functions in a PSC-cell autonomous manner to modulate prostate branching morphogenesis and epithelial differentiation.
Figure 4. Lgr4 inactivation in prostate stem cells disrupted epithelial differentiation and impaired prostate graft regenerationin vivo.

A) Prostatic grafts after 2 months of regeneration in vivo. Scale bar 5mm. B) Prostatic graft wet weight. Data shows mean ± S. E. n=5, p≤0.002. C) Hematoxylin & Eosin staining of regenerated prostatic graft sections. Scale bar 200μm. D) Co-IF of CK5 (green) and CK8 (red) in regenerated prostatic grafts. Nuclei were counter stained with TO-PRO-3 (blue). Scale bar 20μm. E) CK8+ luminal epithelial cells and CK5+ basal epithelial cells were quantified from 5 pairs of regenerated prostatic grafts, 3 sections per graft. Bar graph shows mean ± S. E. basal versus luminal epithelial cell ratio. p≤0.001. F) CK5 (green) and CK8 (red) co-IF in adult mouse prostates after 8 days of androgen castration (left panel) and followed by 14 days of androgen replenishment (right panel). Nuclei were counter stained with TO-PRO-3 (blue). Scale bar 20μm.
To examine how Lgr4 deletion influences prostate regeneration, 6 week-old wild-type and Lgr4−/− mice were subjected to two weeks of androgen deprivation followed by 2 weeks of androgen replenishment. In both wild type and Lgr4−/− mice, castration resulted in prostate shrinkage and regression to a less differentiated state shown by high CK8 expression in luminal cells by 8 days after castration; also, basal cells shifted to aspherical morphology (Figure 4F, left panel). In wild type prostates, androgen replenishment induced luminal cell proliferation and gradual loss of CK8 expression, while basal cells proliferated and differentiated into a flat laminar structure (Figure 4F, right). After 14 days, wild type prostates fully regenerated and matured, while Lgr4−/−prostates maintained high luminal CK8 expression, spherical basal cells and a high basal cell compartment (Figure 4F, right panel), indicating a failure in epithelial differentiation and luminal cell expansion, respectively. Together, these data suggest that deletion of Lgr4 impairs prostate progenitor cell differentiation and prostate regeneration in response to androgen replenishment.
Lgr4 regulates prostate progenitor cells through Wnt/β-catenin signaling
Recently, several groups reported that R-spondins bind Lgr4 to enhance Wnt signaling 28, 29, 40. To determine whether Lgr4 regulates prostate stem cells through the Wnt/β-catenin pathway, we first examined the mRNA expression of Wnt pathway genes in cultured prostate spheres and in P1 prostate tissues. As shown in Figure 5A, the expression of Wnt target genes, including c-Myc and CD44, were decreased in Lgr4−/− prostate spheres without significant changes in canonical Wnt ligands and signaling modulators. In P1 Lgr4−/− prostates, the Wnt target genes Axin2 and c-Myc were also reduced (Figure 5B). Other Wnt signaling components were comparable between Lgr4−/− and Lgr4+/+ prostates, suggesting that the canonical Wnt signaling pathway remains intact while its activity is significantly decreased in Lgr4−/− prostates.
Figure 5. Lgr4 regulates PSC proliferation and differentiation through Wnt/β-catenin signaling.

Wnt/β-catein Signaling pathways in early prostate development were compared by qPCR between Lgr4+/+ and Lgr4−/− in P1 prostate spheres A) and in PD1 prostates B). Three pairs of prostate spheres or prostate samples were tested in these experiments. * p≤0.05.C) IF of β-catenin (green) and p63 (red) in P1 prostate spheres. Nuclei were counter stained with TO-PRO-3 (blue). Scale bar 20μm.D) Wnt/β-catenin activity indicated by GFP expression in wild-type and Lgr4−/− P1 prostate spheres treated with RSPO3 or control media (Mock). Scale bar 20μm. E) Prostate spheres treated 10 days with or without Wnt3a and/or RSPO3. Scale bar 20μm. F) Prostate sphere diameter was measured in at least 6 prostate spheres from 3 pairs of sphere samples. Bar graph shows the mean value ± S. E., *p≤0.05, ** p≤0.01. G) p63 (red) IF in prostate spheres treated with Wnt3a and/or RSPO3. Nuclei were counter stained with TOPRO-3 (blue). Scale bar 10μm.H) Quantification of p63 high cells in prostate spheres treated with or without Wnt3a and/or RSPO3. p63high and p63low/− cells were quantified from 6 prostate spheres. Bar graph shows the mean value ± S. E., *p≤0.05, ** p≤0.01.
To study the extent to which Lgr4 modulates Wnt/β-catenin activity, β-catenin localization was examined. Nuclear β-catenin was high in wild type prostate sphere p63low/− cells (Figure 5C, top), but was significantly reduced in Lgr4−/− prostate spheres (Figure 5C, bottom). Moreover, GFP expression was significantly higher in TOP-dGFP-infected Lgr4+/+ prostate spheres compared to Lgr4−/− spheres (Figure 5D, Mock), suggesting lower Wnt/β-catenin activity following Lgr4 ablation. Among R-spondin 1-4, R-spondin 3 was highly expressed around the mouse urogenital region during early prostate morphogenesis 41. Treatment of Lgr4+/+ prostate spheres with Rspo3 greatly enhanced GFP expression and activation of Wnt/β-catenin (Figure 5D, Rspo3). On the other hand, GFP expression in the inner layer of Lgr4−/− prostate spheres was unaffected by R-spo3, suggesting that Lgr4 is critical for R-spondin-mediated Wnt/β-catenin activation in prostate spheres (Figure 5D).
To examine the functional consequences of Lgr4 loss on R-spondin and Wnt responsiveness, we treated prostate spheres with R-spondin 3 or the canonical Wnt ligand Wnt3a. Wnt3a treatment alone increased the size of both wild-type and Lgr4−/− spheres, while R-spondin 3 only enlarged wild-type spheres (Figure 5E-F). Moreover, combined Wnt3a and R-spondin 3 treatments have synergistic effects on wild type prostate spheres but not on Lgr4−/− spheres (Figure 5E-F). These results support the inference that R-spondin 3-mediated enhancement of Wnt/β-catenin signaling in prostate stem cell spheres requires Lgr4.
Prostate stem cell differentiation in response to Wnt/β-catenin was then evaluated in wild-type and Lgr4−/− prostate spheres (Figure 5G-H). Consistent with a previous report 38, the p63high cell compartment was increased from 43% to 57% in wild-type spheres treated with Wnt3a, mainly by stimulation of p63high cell proliferation. In contrast, p63high cells decreased from 73% to 54% in response to Wnt3a treatment of Lgr4−/− spheres (Figure 5G-H), suggesting that activation of Wnt/β-catenin signaling partially rescued the epithelial differentiation defect observed in Lgr4−/− spheres. Moreover, adding R-spondin 3 to Wnt3a treatment promoted epithelial differentiation in wild type prostate spheres, reducing the p63high cell population from 57% to 35% (Figure 5G-H). This effect of R-spondin 3, again, was not observed in Lgr4−/− prostate spheres, confirming that R-spondin-mediated Wnt/β-catenin signaling requires Lgr4.
Key target genes regulated by Lgr4 in PSCs and early prostate development
In order to reveal the Lgr4down-stream target genes in PSCs and prostate development, we examined mRNA expression of the key genes and transcription factors critical for prostate development and morphogenesis using prostate spheres and postnatal day 1 (P1) prostates. Notch1, an important modulator of early prostate development, was decreased in Lgr4−/− prostate spheres (Figure 6A), and in P1 Lgr4−/− prostate (Figure 6B). Consistently, the Notch1 target gene Hes1 was also decreased in Lgr4−/− prostate spheres, as well as the Notch ligand Delta like in both prostate spheres and P1 prostates, suggesting that Notch signaling is regulated by Lgr4. Nuclear localized Notch1 was attenuated in Lgr4−/− prostate spheres (Figure 6C), providing further evidence for Lgr4 regulation of Notch1 activity.
Figure 6. Notch and Shh pathways are key targets regulated by Lgr4 in PSCs and early prostate development.

A) Notch1, Hes1, and Notch ligand expression in P1 prostate spheres. B) Notch1, Hes1, and Notch ligand expression in postnatal day 1 prostates. C) Notch1 (green) and p63 (red) co-IFin P1 prostate spheres. Nuclei were counter stained with TOPRO-3 (blue). Scale bar 20μm. D) Chromatin immuno-precipitation on Notch1 promoter in P1 prostate spheres. E) Notch1 mRNA level was assessed by qPCR in 4 pairs of prostate sphere samples treated with or without RSPO3. Bar graph shows the mean value ± S. E., *p≤0.05F) Shh expression in P1 prostate spheres. G) Shh signaling in postnatal day 1 prostates. H) p63 (red) IF in prostate spheres cultured with or without Shh treatment. Nuclei were counter stained with TOPRO-3 (blue). Scale bar 20μm. I) p63high and p63low/− cells were quantified from 6 prostate spheres treated with or without Shh. Bar graph shows the mean percentage ± S. E. of either population in prostate spheres, p≤0.02.
Lgr4 may either function as a typical Gαs coupled receptor signaling through PKA and CREB 15-18, or enhance Wnt signaling by binding to R-spondin28, 29. To understand the underlying molecular mechanisms downstream of Lgr4, we used rVista 2.0 (http://rvista.dcode.org) to analyze the potential transcriptional regulation of Notch1, one of the key genes regulated by Lgr4 in prostate stem cell spheres. Two CREB response elements (CRE) and one LEF1 binding site were conserved between the mouse and human 3.0kb proximal regions of Notch1 promoters. Chromatin immunoprecipitation (ChIP) of prostate spheres showed that the LEF1 binding element at -465∼-470bp was active in wild type spheres, but LEF1 binding was abrogated in Lgr4−/− prostate spheres (Figure 6D). The CRE at -951∼-956bp was inactive in both wild-type and Lgr4−/− prostate spheres, whereas CREB binding to the CRE at -1364∼-1369bp was comparable between Lgr4+/+ and Lgr4−/− (Figure 6D). Finally, upon R-spondin3 treatment, the Notch1 mRNA level was elevated in wild-type but not in Lgr4−/− spheres (Figure 6E). Together, these results suggest that Lgr4 modulates Notch1 expression through Wnt/β-catenin signaling but not via the cAMP-PKA-CREB pathway.
Sonic Hedgehog (Shh) expression was reduced by 77% in Lgr4−/− prostate spheres (Figure 6F) and by 41% in P1 Lgr4−/− prostates (Figure 6G). Its target genes in surrounding mesenchymal cells, including Gli1 and cMyc, were also down-regulated by 35% and 45%, respectively (Figure 6G). To further confirm Shh as a key target gene of Lgr4 in prostate spheres, we treated cultured prostate spheres with Shh. As shown in Figure 6H, Shh increased the p63low/− cell percentage in wild type spheres from 42% to 55%, indicating that Shh plays a positive role in promoting epithelial differentiation. The p63low/− cell compartment in Shh treated Lgr4−/− prostate spheres was increased from 11% in non-treated spheres to 44% following Shh treatment (Figure 6H-I), indicating that Shh treatment partially restored epithelial differentiation in Lgr4−/− prostate spheres.
Discussion
In this study, we elucidate the crucial role of Lgr4 as a modulator of PSCs and prostate development. Lgr4 is highly expressed in early prostate development and in PSC enriched LSC cells in adult prostates. Lgr4 inactivation attenuated branching morphogenesis by diminishing cell proliferation. Simultaneously, Lgr4 deletion interrupted PSC cell fate determination leading to arrested epithelial differentiation during prostate development. Moreover, Lgr4 associated with R-spondin to potentiate Wnt/β-catenin signaling in a temporal and spatial specific manner, thus modulating PSC proliferation and differentiation. Mechanistically, we found decreased expression of Wnt target gene, Notch1, and Shh in Lgr4−/− prostates, which are key regulators of stem cell activity and prostate branching morphogenesis. Furthermore, we provided evidence that Tcf/Lef mediated Lgr4 regulation of Notch expression. Taken together, our results indicate that Lgr4 plays a substantial role in prostate development and clarify a molecular mechanism by which Lgr4 regulates prostate stem cells.
Lgr4 activates multiple signaling pathways, including Gαs/cAMP/PKA/CREB 15-18, 42, HB-EGF mediated EGFR transactivation 19, 43, and potentiation of Wnt signaling through binding R-spondin28, 29. In prostate spheres, Wnt/β-catenin activity and target gene expression are correlated with Lgr4 expression. Interestingly, Wnt3a treatment induced modest Wnt/β-catenin activity that promoted p63high cell proliferation whereas R-spondin and Wnt3a co-treatment induced robust Wnt/β-catenin activation promoting both p63high cell proliferation and differentiation. Consequently, we speculate that modest Wnt/β-catenin activity is required for PSC self-renewal and maintenance, while R-spondin and Lgr4 together induced robust Wnt/β-catenin activity that is important for PSC differentiation and intermediate cell proliferation. Together, our data strongly indicate that Lgr4 modulates prostate progenitor cell proliferation and differentiation through Wnt/β-catenin signaling in a temporally and spatially specific manner.
In addition to well-known Wnt/β-catenin targets, we provided evidence that Notch1 is another direct target of Wnt/β-catenin signaling in Lgr4 expressing cells. Our results strongly resemble those of Wang et al. 44, where inducible Notch1 inactivation in the mouse prostate results in epithelial differentiation defects, including increased p63+ basal cells and CK8/CK14 positive intermediate cells. The Notch1 expression pattern also closely resembled that of Lgr4: high in the prostate epithelium during early branching morphogenesis and differentiation, followed by lower and basal layer restricted expression following differentiation45. Since Notch1 expression was down-regulated by Lgr4 inactivation (Figure 5 A-B), these data strongly suggest that Lgr4 affects prostate development in part through Notch1. Although a previous study reported that Wnt3a treatment inhibits Notch1 expression in prostate spheres, their inverse correlation was only found in p63high cells38. Here, we showed that R-spondin3 treatment elevated Notch1 expression in wild-type but not Lgr4−/− prostate spheres, and that Lgr4 regulates Notch1 expression through Wnt/β-catenin but not cAMP/CREB signaling. Therefore, we hypothesize that modest Wnt/β-catenin activity in p63high cells may inhibit Notch1 expression. Whereas in p63low/− cells, Lgr4 potentiates Wnt/β-catenin signaling and Notch1 expression, which coordinates prostate epithelial cell differentiation and transit-amplifying cell proliferation. This hypothesis is supported by a recent study showing that Notch signaling modulates PSCs in a cellular context-dependent manner, and that Notch signaling inhibits proliferation and promotes epithelial differentiation in basal cells but promotes proliferation in luminal cells 46.
Apart from effects mediated by Notch, two pathways are noteworthy as potential Lgr4 mediators in the prostate. Sonic Hedgehog (Shh) induces mesenchymal cell expression of Glis and thus modulates expression of various genes involved in epithelial differentiation47. Not only is Shh expression sharply reduced in Lgr4−/− prostates, we showed that Shh treatment promotes p63high cell differentiation into p63low/− cells in prostate spheres and partially rescues the Lgr4−/− sphere differentiation deficiency. Consequently, Lgr4 likely regulates prostate epithelial differentiation partially through controlling Shh expression. In addition, LGR4 and LGR5 potentiate Wnt/PCP signaling by binding to RSPO3, possibly by quenching the FZD inhibitor ZNRF3 40, 48, 49. Thus Lgr4 may also modulate PSC asymmetric division through Wnt/PCP signaling, and therefore regulate PSC invasion into the mesenchyme. Elucidating the relative importance of these signaling pathways in the prostate remains to be accomplished.
Castration resistant Nkx3.1+ progenitor cells (CARNs) residing in the luminal layer are responsible for luminal epithelial expansion and maturation during androgen induced prostate re-growth after castration 50. Lgr4 ablation impeded prostate regeneration in response to androgen. Interestingly, Nkx3.1 expression was reduced in Lgr4−/− prostates (data not shown). Hence, Lgr4 deletion affected luminal progenitor cell properties, contributing to impaired prostate regeneration. Alternatively, disruption of basal specific genes, such as Notch1, that are indispensable for androgen induced prostate re-growth51 may also contribute to diminished regeneration. Thus, Lgr4 inactivation may both disrupt luminal progenitors and also deregulate cross-talk between basal and luminal cells in prostate regeneration.
Finally, although Lgr4 has been extensively studied in mouse models, with defects in multiple organs upon Lgr4 loss, its relevance to human disease has been somewhat tenuous. The recent publication of a nonsense mutation in human LGR4 that is associated with low bone mineral density, electrolyte imbalance, hormonal dysregulation and increased skin and biliary tract cancer incidence 52 has reinforced the significance of Lgr4. Our results argue that investigation of a role for LGR4 in human prostate disease is highly warranted.
Supplementary Material
Figure 7. Lgr4 working model in PSCs.
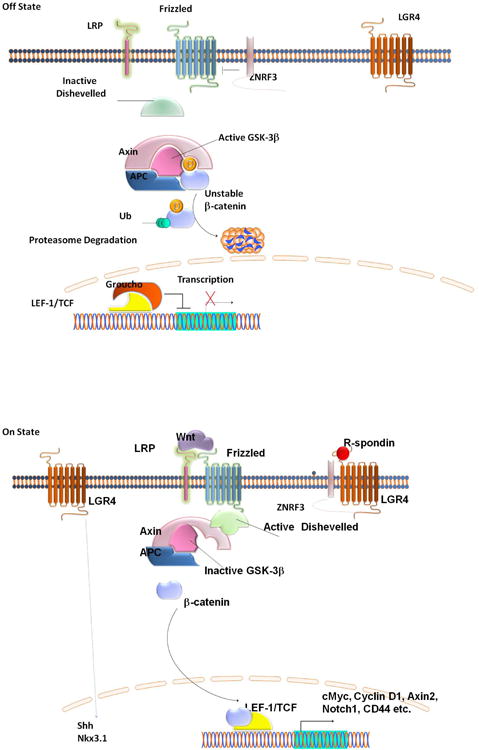
In the absence of R-spondin and Wnt (top), ZNRF3 ubiquitinates the Wnt receptor Frizzled and the co-receptor Lrp6 (Hao et al. 2012), leading to their proteasomal degradation; Dishevelled remains inactive, and active GSK-3β phosphorylates β-catenin leading to ubiquitination and degradation of β-catenin. Upon R-spondin binding to Lgr4 (bottom), ZNRF3 is inhibited, leading to increased membrane levels of Frizzled and LRP6. Addition of Wnt leads to activation of Frizzled, with consequent activation of Dishevelled, inhibition of GSK-3, and β-catenin accumulation, nuclear translocation, and association with LEF-1/TCF transcription factors. This results in the transcription of target genes including Notch 1. In addition, Lgr4 positively regulates Sonic Hedgehog (Shh) and Nkx3.1 gene expression through unknown mechanisms.
Acknowledgments
Human prostate sections were kindly provided by Dr. Michael Ittmann (Baylor College of Medicine, Houston, Texas, USA). This work was supported in part by the grants from National Basic Research Program of China (2012CB910402), from National Natural Science Foundation of China (30930055), and from DOD (PC093061 to M.L.) and NIH (R01CA106479 to M.L.). Weijia Luo and Melissa Rodriguez were supported by pre-doctoral research awards from the DOD (PC424681 to W.L. and PC094495 to M.R.).
Footnotes
Author contributions: Weijia Luo: Data collection, data analysis, manuscript writing
Melissa Rodriguez: Data collection
data analysis; Joseph Valdez: Data collection
Xinglei Zhu: Data Collection
Kunrong Tan: Data Collection
Dali Li: Data Collection, data analysis
Stefan Siwko: Data analysis, Manuscript writing
Li Xin: Data Analysis, Conception and design
Mingyao Liu: Conception and design, Financial support, Data analysis, Final approval of manuscript
The authors declare no conflicts of interest.
References
- 1.Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem cell reviews. 2008;4:193–201. doi: 10.1007/s12015-008-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matusik RJ, Jin RJ, Sun Q, et al. Prostate epithelial cell fate. Differentiation; research in biological diversity. 2008;76:682–698. doi: 10.1111/j.1432-0436.2008.00276.x. [DOI] [PubMed] [Google Scholar]
- 3.Marker PC. Does prostate cancer co-opt the developmental program? Differentiation; research in biological diversity. 2008;76:736–744. doi: 10.1111/j.1432-0436.2008.00297.x. [DOI] [PubMed] [Google Scholar]
- 4.Ousset M, Van Keymeulen A, Bouvencourt G, et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nature cell biology. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- 5.Choi N, Zhang B, Zhang L, et al. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer cell. 2012;21:253–265. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein AS, Lawson DA, Cheng D, et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson DA, Zong Y, Memarzadeh S, et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2610–2615. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mimeault M, Mehta PP, Hauke R, et al. Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocrine reviews. 2008;29:234–252. doi: 10.1210/er.2007-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu SY, Liang SG, Hsueh AJ. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Molecular endocrinology. 1998;12:1830–1845. doi: 10.1210/mend.12.12.0211. [DOI] [PubMed] [Google Scholar]
- 10.Hsu SY, Kudo M, Chen T, et al. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Molecular endocrinology. 2000;14:1257–1271. doi: 10.1210/mend.14.8.0510. [DOI] [PubMed] [Google Scholar]
- 11.Mazerbourg S, Bouley DM, Sudo S, et al. Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Molecular endocrinology. 2004;18:2241–2254. doi: 10.1210/me.2004-0133. [DOI] [PubMed] [Google Scholar]
- 12.Mendive F, Laurent P, Van Schoore G, et al. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Developmental biology. 2006;290:421–434. doi: 10.1016/j.ydbio.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Matsubara M, Matsuo T, et al. Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Experimental nephrology. 2006;104:e63–75. doi: 10.1159/000093999. [DOI] [PubMed] [Google Scholar]
- 14.Mohri Y, Oyama K, Akamatsu A, et al. Lgr4-deficient mice showed premature differentiation of ureteric bud with reduced expression of Wnt effector Lef1 and Gata3. Developmental dynamics: an official publication of the American Association of Anatomists. 2011;240:1626–1634. doi: 10.1002/dvdy.22651. [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Zhou W, Zhou X, et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136:2747–2756. doi: 10.1242/dev.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Li X, Ke Y, et al. GPR48 increases mineralocorticoid receptor gene expression. Journal of the American Society of Nephrology: JASN. 2012;23:281–293. doi: 10.1681/ASN.2011040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Luo J, Luo W, et al. Inactivation of G-protein-coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathway. The Journal of biological chemistry. 2008;283:36687–36697. doi: 10.1074/jbc.M800721200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng J, Luo J, Cheng X, et al. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6081–6086. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin C, Yin F, Lin M, et al. GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Investigative ophthalmology & visual science. 2008;49:4245–4253. doi: 10.1167/iovs.08-1860. [DOI] [PubMed] [Google Scholar]
- 20.Oyama K, Mohri Y, Sone M, et al. Conditional knockout of Lgr4 leads to impaired ductal elongation and branching morphogenesis in mouse mammary glands. Sexual development: genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation. 2011;5:205–212. doi: 10.1159/000329476. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Dong J, Li D, et al. Lgr4 Regulates Mammary Gland Development and Stem Cell Activity through the Pluripotency Transcription Factor Sox2. Stem cells. 2013 doi: 10.1002/stem.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du B, Luo W, Li R, et al. Lgr4/Gpr48 Negatively Regulates TLR2/4-associated Pattern Recognition and Innate Immunity by Targeting CD14 Expression. The Journal of biological chemistry. 2013;288:15131–15141. doi: 10.1074/jbc.M113.455535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Qian Y, Li L, et al. Lgr4 gene deficiency increases susceptibility and severity of dextran sodium sulfate-induced inflammatory bowel disease in mice. The Journal of biological chemistry. 2013;288:8794–8803. doi: 10.1074/jbc.M112.436204. discussion 8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian Y, Liu S, Guan Y, et al. Lgr4-mediated Wnt/beta-catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development. 2013;140:1751–1761. doi: 10.1242/dev.093641. [DOI] [PubMed] [Google Scholar]
- 25.Li XY, Lu Y, Sun HY, et al. G protein-coupled receptor 48 upregulates estrogen receptor alpha expression via cAMP/PKA signaling in the male reproductive tract. Development. 2010;137:151–157. doi: 10.1242/dev.040659. [DOI] [PubMed] [Google Scholar]
- 26.Hoshii T, Takeo T, Nakagata N, et al. LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biology of reproduction. 2007;76:303–313. doi: 10.1095/biolreprod.106.054619. [DOI] [PubMed] [Google Scholar]
- 27.Mohri Y, Umezu T, Hidema S, et al. Reduced fertility with impairment of early-stage embryos observed in mice lacking Lgr4 in epithelial tissues. Fertility and sterility. 2010;94:2878–2881. doi: 10.1016/j.fertnstert.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 28.de Lau W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 29.Carmon KS, Gong X, Lin Q, et al. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 31.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell stem cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Jaks V, Barker N, Kasper M, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature genetics. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 33.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 34.Snippert HJ, Haegebarth A, Kasper M, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Liu G, Zhang Y, et al. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134:723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- 36.Lawson DA, Xin L, Lukacs RU, et al. Isolation and functional characterization of murine prostate stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin L, Ide H, Kim Y, et al. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahi P, Seethammagari MR, Valdez JM, et al. Wnt and Notch pathways have interrelated opposing roles on prostate progenitor cell proliferation and differentiation. Stem cells. 2011;29:678–688. doi: 10.1002/stem.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. The Journal of clinical investigation. 2007;117:2044–2050. doi: 10.1172/JCI32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glinka A, Dolde C, Kirsch N, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO reports. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta V, Abler LL, Keil KP, et al. Atlas of Wnt and R-spondin gene expression in the developing male mouse lower urogenital tract. Developmental dynamics: an official publication of the American Association of Anatomists. 2011;240:2548–2560. doi: 10.1002/dvdy.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y, Kitagawa K, Shimada M, et al. Generation of a constitutively active mutant of human GPR48/LGR4, a G-protein-coupled receptor. [Hokkaido igaku zasshi] The Hokkaido journal of medical science. 2006;81:101–105. 107–109. [PubMed] [Google Scholar]
- 43.Wang Z, Jin C, Li H, et al. GPR48-Induced keratinocyte proliferation occurs through HB-EGF mediated EGFR transactivation. FEBS letters. 2010;584:4057–4062. doi: 10.1016/j.febslet.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Wang XD, Leow CC, Zha J, et al. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Developmental biology. 2006;290:66–80. doi: 10.1016/j.ydbio.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Shou J, Ross S, Koeppen H, et al. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer research. 2001;61:7291–7297. [PubMed] [Google Scholar]
- 46.Valdez JM, Zhang L, Su Q, et al. Notch and TGFbeta form a reciprocal positive regulatory loop that suppresses murine prostate basal stem/progenitor cell activity. Cell stem cell. 2012;11:676–688. doi: 10.1016/j.stem.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation; research in biological diversity. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao HX, Xie Y, Zhang Y, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 49.Koo BK, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Kruithof-de Julio M, Economides KD, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XD, Shou J, Wong P, et al. Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. The Journal of biological chemistry. 2004;279:24733–24744. doi: 10.1074/jbc.M401602200. [DOI] [PubMed] [Google Scholar]
- 52.Styrkarsdottir U, Thorleifsson G, Sulem P, et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature. 2013;497:517–520. doi: 10.1038/nature12124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


