Abstract
The advent of nanotechnology has reignited interest in the field of pharmaceutical science for the development of nanomedicine. Nanomedicinal formulations are nanometer-sized carrier materials designed for increasing the drug tissue bioavailability, thereby improving the treatment of systemically applied chemotherapeutic drugs. Nanomedicine is a new approach to deliver the pharmaceuticals through different routes of administration with safer and more effective therapies compared to conventional methods. To date, various kinds of nanomaterials have been developed over the years to make delivery systems more effective for the treatment of various diseases. Even though nanomaterials have significant advantages due to their unique nanoscale properties, there are still significant challenges in the improvement and development of nanoformulations with composites and other materials. Here in this review, we highlight the nanomedicinal formulations aiming to improve the balance between the efficacy and the toxicity of therapeutic interventions through different routes of administration and how to design nanomedicine for safer and more effective ways to improve the treatment quality. We also emphasize the environmental and health prospects of nanomaterials for human health care.
1 Introduction
In 1959, Richard Feynman proposed that the future of humans will be fully occupied by machines, small machines will be used to make further smaller machines, and these in turn, will be used to make even tinier machines, all the way down to the atomic level.1 Interestingly, with the advent of nanotechnology, the vision of Professor Feynman has been partially realized today. The advances in nanoscience have propelled innovations in a number of scientific disciplines, including pharmaceutical development for the treatment of various diseases. Nanotechnology has the potential to revolutionize medicine, and has already presented new regulatory challenges for scientific communities. Innovations are occurring rapidly, as demonstrated by the exponential increase in nanotechnology-related pharmaceutical patents over the past 20 years.2
The European Science Foundation (ESF) launched its forward vision on nanomedicine and provided the new definition: “Nanomedicine uses nano-sized tools for the diagnosis, prevention and treatment of disease and to gain increased understanding of the underlying complex patho-physiology of the disease. The ultimate goal is to improve the quality of human life”.
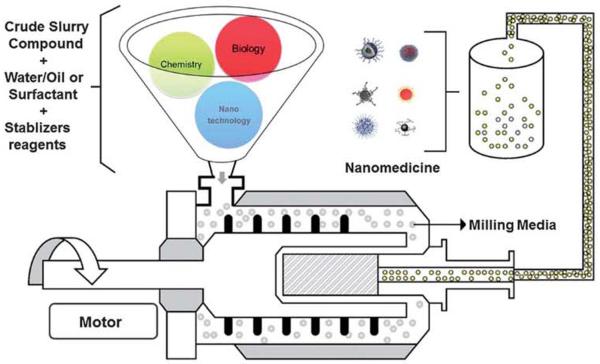
Nanomedicine encompasses the production and application of physical, chemical, and biological systems (Fig. 1) at scales, ranging from individual atoms or molecules to around 100 nanometers in diameter, as well as the integration of the resulting nanostructures into larger systems for real-time application (Box 1).
Fig. 1.
Schematic diagram for the future-nanorriedicine production system.
Over the past decades, nanomedicines have been translated into various commercial products, from the lab to the market. Many pharmaceutical companies are paying more attention to nanotechnology than before, in order to find new solutions for pharmaceutical innovation with lower cost, lower risks and much higher efficiency compared to traditional drug development.3 Currently, nanomedicine is dominated by nanoscale drug delivery systems.3 As we know, materials have unique properties at nanoscale dimensions that meet important medical needs and are already being used as the basis for new drug delivery formulation.4 Nanoscale particle/molecule based drug delivery formulations improve the bioavaliability and pharmacokinetics for better therapy, such as liposomes,5 nanosuspensions6 and polymeric nanoparticles.7 Some of the nanoparticles (NPs) are effective in improving safety and efficacy, and also decreasing the toxicity. For a better understanding of innovative pharmaceutical development based on nanoscale drug delivery formulation, we attempt to review the history and development of nanomedicine in the pharmaceutical field, and we also discuss about its advantages, delivery through different routes of administration and toxicological issues to develop more efficient nanoformulations for better health care.
2 History and development
The nanomedicines can be divided into three groups. The first group consists of the marketed nanomedicine products, which are already in routine clinical use, while the members of the second group of nanomedicines are mostly in the clinic stage.8 In 1965, researchers started to utilize nanotechnology in pharmaceutical research, they created a novel lipid vesicle for drug delivery, now known as a liposome.9 Later in 1976, the first control-released polymeric system for the delivery of macro-molecules was described,9 and in 1978, dendrimers were described as a delivery vehicle.10 Doxil11 (a doxorubicin carrying nano-drug) was the first Food and Drug Administration (FDA)-approved nanomedicine used in the treatment of cancer patients. In 1995, liposome injection was approved to treat a wide range of cancers like ovarian cancer, AIDS (acquired immunodeficiency syndrome)-related Kaposi's sarcoma and myeloma.12 All these early innovations provide valuable information to the researchers to design novel nanomedicines that would be helpful in treating multiple diseases. Long circulating PLGA–PEG NPs13 were first described in 1994 as biodegradable polymeric nanospheres for drug delivery. In 1996, the FDA approved Ferumoxide (iron oxide) to be applied for clinical use as MRI contrast agent.14 In addition to this, polymer coated iron oxide NFs have also been approved by the FDA as MRI contrast agents. In 2005, Abraxane15 (a protein based drug delivery system) was approved by the FDA. In 2007, targeted nanomedicines like Genexol–PM16 (polymeric micelle NPs), CALAA-0117 (a targeted cyclodextrin–polymer hybrid NP), BIND-01418 (targeted polymeric NPs) and SEL-06812 (integrated polymeric nanoparticle vaccines) for cancer therapy have already entered into human clinical study.
Finally, the third group consists of some nanoscale materials for drug delivery, such as gold (Au), silica nanoparticles, have been developed with more advanced nanotechniques, which may have the potential to help the conventional drugs to be more effective and cause adverse side effects. For example, mesoporous silica nanoparticles (MSNPs) can be used as an ideal drug delivery vehicle with its excellent drug loading capacity, easily synthesis, biocompatibility, but many details about MSNPs still remain unclear for clinical use. It is easy to conclude that the successful application of nanotechnology in drug delivery could draw the attention of scientists, governments and industrial investigators, which in turn is helpful for the development of nanomedicine and accelerates the speed of discovery of novel nanoscale drug formulations.
3 Advantages of nanomedicine over conventional medicine
In conventional medicine, treatment is based on the symptoms created by the disease and the adverse problems occurring at the tissue/organ level, (e.g. due to metabolic problems, or other infectious agents). At a certain stage, the disease may often be at an advanced and most dangerous stage, in such a condition that the symptoms become apparent in the patient and the clinician becomes confused over which way they can treat the patient to get rid of the disease.19 Conventional medicine and techniques have left many of the problems, which are hard to penetrate due to lack of information and appropriate techniques.
When the drug is being assessed for clinical use, both the therapeutic efficacy and toxicity should be taken into account. Some drugs are poorly soluble in water, but they can be dissolved in other organic solvents, which trigger severe allergic effects in the human body after administration.20 During cancer treatment, cisplatin and other drugs all have the drawback of high toxicity. One reason for this is the unspecific recognition and interaction with cells and organs.21 There are so many prevailing drawbacks, including: limited solubility, poor distribution within the body, a lack of selectivity, and unfavorable pharmacokinetics behavior can damage the healthy tissues.22
Due to the significant properties of materials at the nanoscale, nanomedicine shows different behavior from conventional medicine.19 Advancement of nanoformulation techniques has offered a new hope for incurable, severe diseases like cancer, human immunodeficiency virus (HIV)23–24 and for the treatment of some infectious pathogens.25 Based on nanoscale particles, nanomedicines represent many benefits over conventional drugs and here we have characterized the advantages of nanomedicine according to two aspects (Table 1). In addition, nanotechnology could give conventional materials or drugs additional functionality based on their unique properties at the nanoscale. For example, QDs have far brighter and more stable fluorescence than conventional fluorescent dyes for cell labeling. Gold nanorocls (AuNRs), nanoshells (NSs) and nanocages (NCs) have unique properties for imaging-guided cancer therapy.26–28
Table 1.
Advantages of nanomedicine over conventional medicine
| Advantages of nanomedicine according to physical aspects | Advantages of nanomedicine according to biological aspects |
|---|---|
| • Increase the aqueous solubility of pharmaceuticals | • Improve the bioavailability and biocompatibility of drugs, non-toxic, non-allergenic and non-irritating |
| • Protect the drugs from degradation | • Improve the active/passive target specificity in drug delivery |
| • Ability to encapsulate high drug content, e.g. polymeric nanoparticles | • Penetrate the cell membrane and biological barriers, e.g. Blood–brain barrier (BBB) |
| • Feasibility of carrying both lipophilic and hydrophilic drugs | • Enhance the permeability and retention (EPR) of tumors for accumulation of large molecules and small particles |
| • High surface activity and adsorption ration rate | • Prolong the circulation time of drugs towards the therapeutic window |
| • Increasing specificity towards disease | • Possibility to overcome resistance factors, e.g. P-glycoprotein and MDR factors |
| • Deliver drug for slow release to maintain the therapeutic dose | • Combined treatment of multiple diseases or theranostics application |
| • Produce a prolonged release of drugs to control systemic transportation | • It is possible to target individual pathogens or biomolecules according to the nature of the disease |
| • Stimuli release (pH, temperature, heat, light, radiation, magnetic field etc.) | |
| • Easily design and engineer to load different drugs for combination therapy |
However, current nanotechnology based drug molecules continue to dominate the pharmaceutical market despite conventional therapeutic and diagnostic agents, making a distinct niche for themselves. It offers an immense vision of enhanced, tailored treatment of disease, with monitoring, repairing, construction and control of human biological systems at the molecular level, using engineered nanodevices and nanostructures for human health care.
4 Techniques involve for the development and production of nanoscale drug formulation
Basically, there are two types of technique for the production of nanoscale formulations, one is top-down, and the other is bottom-up. The bottom-up techniques include chemical reactions and molecular assemblies, such as supercritical fluid techniques, precipitation, nanoemulsion, spray-drying, polymerization and synthesis. The top-down techniques include wet media milling and high-pressure homogenization. The resulting nanoparticles are referred to as nanosuspensions.6,29,30
Nanosuspensions are stabilized submicron colloidal dispersions of nanosized drug particles.30 Various technologies have been utilized in the development and production of nanosuspensions. As for top-down strategies, one important technique is high-pressure homogenization. The first method for high pressure homogenization was invented by employing a piston-gas homogenizer and processing in aqueous media at room temperature,31 and later in non-aqueous media (patent number: WO2001003670, 2001). J. Kluge and M. Mazzotti produced S-ketoprofen poly(lactic-co-glycolic acid) (PLGA) drug-polymer nanocomposites with CO2 assisted high pressure homogenization that greatly improved the bioavailability of the drug.32 Pereda et al. found that after treatment by high-pressure homogenization, the storage of milk fat was significantly improved.33
Other types of high-pressure homogenization, such as media milling29 and Nanoedge (US Patent 6018080, 2000), were also involved in the production of nanoscale drug formulations. Several pharmaceutical companies have developed a certain kind of processing standard operating procedures (SOPs) based on high pressure homogenization techniques, for example the Nanomorph™ from Abbott. Nanocrystal™ from Elan Nano-systems and Nanoedge from Baxter. Another important method to reduce the particle size in the industry is wet ball milling. Currently, there are more than 5 nanoscale drug formulations produced by this method in the market, and still some are in the developmental stage. One interesting point is that all of the FDA approved top-down-strategy nanoscale drug formulations are for oral delivery.34
As for the bottom-up strategy, apart from classical synthesis and polymerization, one powerful technique is the formation of nanoemulsions. As a nanoemulsion is itself a drug, it can serve as a template for the production of nanosuspensions.35,36 Solvent emulsification diffusion (SED) is the most commonly used method for the preparation of solid-lipid and polymeric nanoparticles.37 The ease and convenience in fabrication of nanoemulsions can precisely control drug loading and positioning (spatial placement), therefore, thousands of drugs and active pharmaceutical ingredients (APIs) have been extensively investigated and developed. For example, the nanosuspensions of ibuprofen, diclofenac and acyclovir are now available from the market (http://www.reportlinker.com/report/search/theme/ibuprofen).
Supercritical fluid techniques are also important, which have drawn great attention in the literature as well as the pharmaceutical industry in the early 2000s.38,39 SC (supercritical)-CO2 is commonly used as a supercritical fluid. Above its supercritical point, CO2 can serve as a solvent with low density, and this unique property was developed to increase nanoparticle production from a lab scale to pilot scale. One of the most popular supercritical fluid techniques is the rapid expansion of supercritical solution (RESS).40 RESS can produce nanoparticles with diameter less than 100 nm41 and free of solvents, which is quite promising for the fabrication of nanosuspensions. The combination of a supercritical fluid with an emulsion process offers greater benefit than the use of a single technique alone. For example, Porta et al. produced PLGA polymers loaded with 2 different nonsteroidal anti-inflammatory drugs (NSAIDs) by using supercritical CO2 fluid to extract drug molecules from the oily phase of an emulsion. The loading efficiency of this technique is 3 times greater com pared to common emulsion techniques.42
4.1 Pharmaceuticals importance of different formulation molecules
The rapid development of nanotechnology has been changing the face of the pharmaceutical industry, including the concept of new drug innovation and development as well as the designing of new techniques for drug formulation. The drug formulation studies mainly focus on preparation of a drug which is both stable and acceptable to the patient. Generally, the tablets and capsules improve the convenience and compliance for oral delivery, and the PEGylated adjuvants prolong circulation and reduce side effects such as allergies and toxicity.43,44 Various nanoparticles have been extensively studied for the development of “block-buster” drugs, based on proteins, polymers, liposomes, micelles, nano-emulsions, gold nanoparticles, etc.. Several formulations are in the pipeline, and few of them have FDA approval.45
4.2 Advantages of protein formulations
Proteins are naturally the first choice for developing nanoscale drug formulations because their safety, biocompatibility and ease of availability is superior. Most proteins are nanoscale macromolecules. The hydrophilic and hydrophobic sequences could be loaded with different drugs, thus serving as drug carriers. Albumin has been extensively explored because it is the most abundant protein in human serum and it is extremely stable during bioprocessing and pharmaceutical preparations compared to the other proteins.46 Most importantly, it can accumulate in tum or tissues and inflammation sites, thus would be preferred in the design of passive targeted drug delivery systems against cancer and inflammations. Abraxane was the first protein-based nanoscale drug formulation approved by the FDA in 2005 for breast cancer treatment, which has a superior antitumor efficacy over conventional paclitaxel and cremophor formulations.47 The global pharmaceutical sales in 2012 were 485 million USD (annual revenue in 2012), which is a real “block-buster” drug available for cancer patients. The success of Abraxane has led to other investigations into albumin-based drug formulations, such as rapamycin and docetaxel, both in phase-1 clinical trials.48 However, not all drugs are suitable for albumin-based drug formulation. Some drugs interact with album in and lose their activity such as camptothecin.49,50 These protein–drug interactions hindered the application of albumin as a general drug carrier, but still there is great potential for this strategy to develop new drug formulations for the market.
Another protein candidate called apoferritin, which is a large protein molecule with 24 subunits, a diameter around 10–12 nm and a 2 nm central cavity.51 Their cycle of assembly and disassembly can be controlled by tuning pH, which would be favorable for controlled drug loading and release. Zhen et al. encapsulated the anti-cancer drugs cisplatin and carboplatin into apoferritin at neutral pH and released the platinum drugs under acidic pH,52 with improved toxicity against cancerous cells. Kilic et al. developed a Dox loaded apoferritin delivery system and found that approximately 28 Dox molecules were encapsulated within 1 apoferritin molecules and the nanoformulation was stable for at least 48 h at neutral pH.51
4.3 Importance of liposomal formulations as drug carriers
In drug formulation development liposomes are the dominating nanocarrier systems. They are easily fabricated, modified, and most importantly they can be adopted to deliver nearly any biomolecules and drugs, such as small molecules, proteins, peptides, nucleotides, and so on. Meanwhile, the large production of liposomes is available for the pharmaceutical industry, which would definitely accelerate the transition from bench-side to bedside. However, one drawback of liposome technology is the “accelerated blood clearance” (ABC') effect.53 Usually one effective method to avoid the ABC effect is PEGlyted technology, which would significantly reduce the ABC effect and improve the biodistribution of liposome-formulated drugs. Another effective method is “active targeting”, that ls modifying the liposomes with targeting ligands that specifically deliver the liposome to its designed destination.54 Currently there are 21 liposome-based drug formulations which are in different developmental stages, and the FDA has approved 12 liposome-based drugs.5,55
4.4 Advantages of polymer formulations
Polymers are another major direction in nanoscale drug formulation development. Either natural polymers or synthetic polymers are extensively investigated in the development of new drug formulations, and the various resources of polymers encourage scientists as well as pharmaceutical companies to design more smart polymer-based drug delivery systems. With the help of highly flexible and tunable polymers, currently there are 12 candidates in various stages of clinical trials,55,56 and 1 has been approved by the FDA.45 For the formulation of polymer-based drug delivery systems, it is best to employ FDA approved biodegradable polymers such as PLA, PGA, PLGA, etc. There are two kinds of polymer-based formulations, one is polymer–drug composites, and the other is polymer–drug conjugates.57 However, with the bright outlook of polymer formulations, there is a concern about quality control in the pharmaceutical production.57 Most of the polymer-based formulations are multicomponent nanoparticles, such kinds of nanoparticles are likely to encounter technical difficulties during large-scale production, as well as the expensive instruments and materials. Unlike a fully developed large-scale liposome manufacture process which is easy to transfer and adopt, polymer based formulations tend to be more specific in large-scale production. The key to solving such problems may rely on the process of industrial standardization and the collaboration between pharmaceutical and research institutes.
4.5 Formulation of composite nanomaterials for medicinal application
Recently, the advances in material science and pioneering research have greatly broadened the scope for the development of new pharmaceutical formulations, and the emergence of “composite pharmaceutical formulations” is expected. Composite pharmaceutical formulations are combinations of more than one nanoscale drug formulation technique, which includes all of the available materials such as polymers, proteins, nucleotides (DNA, aptamers, siRNA, mRNA) and inorganic nanoparticles. The complexity for manufacturing such new formulations would definitely be beyond currently available pharmaceutical formulation techniques; therefore, there is no FDA approval for any formulations based on this concept. For pursuing the successful development of composite pharmaceutical formulations, one useful strategy is adopting the FDA approved materials in the design, such as polymer–liposorne and protein–liposome composites.58 This strategy could significantly reduce the risk of failure and unnecessary biosafety issues. Chan et al. developed the core–shell nanoparticle delivery system composed of a PLGA core, soybean lectin monolayer and polyethylene glycol (PEG) shell. The anti-cancer chemotherapeutic Docetaxel was loaded in PLGA core, protected by a lectin layer, and PEG shell was assembled onto the nanoparticle surface.59 This composite formulation greatly enhanced the therapeutic outcome of Docetaxel, which would be of benefit in clinical applications over the classical anti-cancer drugs. Another advantage of composite formulation is multifunctionality; therapy, imaging and diagnosis could be accomplished in one formulation.60,61 Howell et al. encapsulated MnO (manganese oxide) nanoparticles and doxorubicin in lipid micelles for lung cancer treatment and imaging. The designed nanoparticles could significantly accumulate within tumour tissues and indicate exact locations through MR1 imaging.62 In cancer therapy, one rapidly developing field is silence therapy based on the lipid–based polymer system for siRNA delivery, also known as siRNA–lipoplex platform technologies.63 This unique silence therapy based on properties of proprietary lipid components, which embed the siRNA molecules into lipid-bi-layer.64 The siRNA is combined with various developed lipid moieties containing cationic lipids, co-lipids (fusogenic) and PEGylated lipids, to form nanoparticles enabling functional delivery to various cell types upon systemic delivery.65 Interestingly, the first clinical trials of this class of therapy in age-related macular degeneration (AMD) involved injecting the RNAi drugs directly to the diseased tissue in the eye. This direct delivery ensured that the RNAi drugs reached their target intact.66,67 With the rapid development of composite pharmaceutical formulations, it is possible that the “smart drugs” would be accessible for the patients in the near future.
5 Nanomedicine as an emerging therapeutic approach for real solutions through different routes of administration
Conventional therapies have been used in the treatment of diseases for many years through different routes of administration. The existing treatment of severe diseases like cancer, highly pathogenic, infectious and also several other metabolic diseases are limited by general toxicity or low bioavailability of the drug or by both of these limitations. These treatments may be further improved as a new era of therapy, based on nanomedicine. As we know there are many potential advantages of using nanomedicine over conventional therapies as we described above, associating drugs with different nanocarriers or formulation systems is relevant to develop new effective and safe therapies.68 The benefit of using such delivery systems lies in the fact that a carrier can modify in vivo the fate of the d rug by changing the biodistribution profile of drugs and modulating the different control phase of therapeutic molecules to improve the delivery through different routes of administration (Fig. 2)69 In fact, combining our new knowledge of nanomaterials with an understanding of the cellular and molecular functions can be used to change the absorption, metabolism and elimination of a drug when it is administrated via different routes. Mere, we have emphasized the role of nanomaterial formulation through different and important routes of administration to develop better and safer therapies.
Fig. 2.
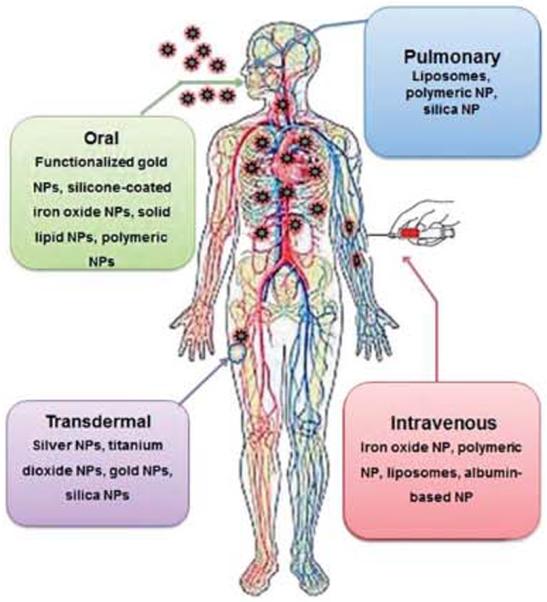
Important routes of nanopartide based drug administration.
5.1 Nanoparticle formulations for intravenous drug delivery
The primary purpose of intravenous (I.V.) medication is to initiate a rapid systemic response against the given medication. It is one of the fastest ways to deliver medication directly into a patient's blood stream. By this method, the drug is immediately available to the whole body. It is easier to control the actual amount of drug delivered to the body by using the I.V. method and it is easier to maintain drug level in the blood for therapeutic response (http://www.healthline.com/galecontent/intravenous-medication-administration).
Despite the various advantages of the I.V. route of administration, there still exists many problems for proper therapy:70
-
◆
The medication to be delivered would be destroyed by digestive enzymes.
-
◆
The drug is poorly absorbed by the tissue.
-
◆
It is painful or irritating and could result in speed shock when given to a patient.
-
◆
Onset of action is quick, facilitating a greater risk of addiction and overdose when injecting illicit drugs.
-
◆
Intravenous catheters are complicated to maintain drug interaction, because of incompatibilities.
-
◆
It is the most dangerous route of administration because it bypasses most of the body's natural defences (immune system), exposing the user to health problems such as hepatitis, abscesses, infections, and unclissolved particles or additives/contaminants.
-
◆
Errors in compounding of medication e.g. chemical phlebitis (clotting/deposition).
In contrast to microparticles with a diameter of more than 1 μm that cannot be administered via intravascular routes of administration, nanoparticles are small enough to allow intra-capillary passage followed by efficient cellular uptake.71 When nanoparticles are administered intravenously, they follow the same route as most other foreign particulates when enter into the body and are easily recognized by the body immune systems, and then will be cleared by phagocytes through circulation.72. Apart from the size and shape of the nanomaterials, their surface properties such as hydrophobicity determine the amount of adsorbed blood components, mainly with serum proteins (opsonins) and a process known as opsonization. This process in turn influences the in vivo fate of drugs and nanoparticles in reaching their target site.71,74 The binding of these opsonins onto the surface of nanomaterials, called opsonization, acts as a bridge between drugs, nanomaterials and phagocytes. The association of a drug to conventional carriers leads to modification of the drug distribution profile, as it is mainly delivered to mononuclear-phagocyte systems (MPS) such as the liver, spleen, lungs and bone marrow.74 After intravenous injection, surface unmodified nanoparticles or naked drugs and non-specific delivery systems are rapidly opsonized and found mostly in the liver. They are called `non-stealth' delivery system that are cleared by the macrophages of MPS rich organs.75 Hence, to increase the likelihood of the drug delivery and targeting, it is necessary to understand the distance between the site of the drug administration (site of injection) and the location of the disease in the body. At the same time, it is also necessary to minimize the opsonization and to prolong the circulation of nanoparticles in vivo. This can be achieved by using various design approaches with the advancement of nanoformulation techniques:
-
◆
Loading and protecting the drug inside different nanocarrier systems.
-
◆
Surface coating of nanoparticles with loaded drugs with hydrophilic polymers/surfactants.
-
◆
Applying the difference formulation with biodegradable copolymers with hydrophilic segments such as polyethylene glycol (PEG), polyethylene oxide, polyoxamer, poloxamine and so on.
-
◆
Preparation of `stealth' nanoparticles which can bypass the defense system.
-
◆
Engineering of nanomaterial that can bypass the capillary endothelial or escape from the blood compartment and reach to the target site.
Several studies have shown that PEG conformation at the nanoparticle surface is of utmost importance for the opsonin repelling function of the PEG layer.76 If the surface of the PEG is brush shaped, such configurations reduce the phagocytosis and complement activation, whereas PEG surfaces in a mushroom-like configuration were potent to the complement activators and favored phagocytosis.76–78 Therefore, by applying a certain methodology, it might be possible to overcome the above condition. Doxorubicin is a drug used to treat a wide range of cancers, but it damages the heart tissue. In 1995, an approved nanomedicine was Doxil, a doxorubicin-carrying drug that keeps the drug out of the heart. During the 1980s, researchers found that 100 nm particles cannot exit healthy blood vessels but can easily escape through the leaky tumor vasculature. Therefore, they loaded the doxorubicin into lipid bubbles about 100 nm in diameter, which can evade the immune system. Patients receiving Doxil have one-third congestive heart failure incidence of those given conventional doxorubicin. Again to improve the efficacy and decrease side effects of the drug delivery, companies such as BIND Biosciences in Cambridge, USA, use polymers to load the drug and also design how to control the drug release at the target site.79 The leading candidate BIND-014 a 100 nm polymer sphere loaded with docetaxel, a drug that can kill the proliferative cells. The polymer center was engineered to control the drug release and has two outer layers, a PEG layer to evade immune system and binding molecules to recognize the tumor. BIND-014's phase I clinical trial results look promising after intravenous injection.79
5.2 Nanoparticles for oral drug delivery system
Oral delivery is the most widely used, highly favorable, and readily accepted form of drug administration due to its convenience, the possibility of self-administration and improved patient compliance.88 Significant advances in biotechnology and biochemistry have led to the discovery of a large number of bioactive molecules and vaccines based on peptides and proteins. Currently greater than 60% of marketed drugs are oral products.89 However, because of their poor oral bioavailability88 most of the drugs administrated via the oral route lose their designed dosage regimen and have limited effects. The intestinal layer of human epithelium cells is highly absorptive and is composed of villi that increase the total absorptive surface area (300–400 m2) in the gastrointestinal (GI) tract.90–92 The mucus layers that protect the epithelial surfaces have been highlighted as significant barriers for drug and nanoparticle delivery. In some instances, over 90% of the administered drug is lost due to epithelial barriers of the GI tract and their susceptibility to intestinal degradation by digestive enzymes.91,93 However, despite having several advantages, oral drugs face several problems, particularly for peptides and proteins, and some other bimolecular drugs delivery:93
-
◆
Dose dumping, poor stability in the gastrointestinal tract, mucus layer and epithelial lining itself.
-
◆
Low solubility and/or bioavailability.
-
◆
The mucus barrier can prevent drug penetration and decrease the subsequent absorption rate of the drug.
-
◆
Proteolytic enzymes in the gut lumen like pepsin, trypsin and chymotrypsin, presence at the brush border the membrane.
-
◆
Bacterial gut flora.
All of the above factors reduce the potential for accurate dose adjustment of the final therapy. However, to overcome these limitations and barriers, the stability of nanocarriers offers a greater possibility of the drug to be successfully delivered through oral administration. The fate of the nanoparticles in the gastrointestinal tract has been investigated in number of studies, in general the uptake of nanoparticles occurs as follows:94
-
◆
Transcytosis of the drug via microfold cells (M-cells, a specific cell type in the intestinal epithelium).
-
◆
Intracellular uptake and transport via the epithelial cells lining the intestinal mucosa.
-
◆
Uptake via Peyer's patches.
Polymeric nanoparticle based formulation methods are being developed95 that encapsulate and protect (against enzymatic and hydrolytic degradation) drugs from damage and release them in a temporally or spatially controlled manner at the site of the disease.93,96–99 The surface of the nanoparticles can also be modified to enhance or reduce the bio-adhesion properties so the specific target cells should be recognized efficiently.93 There are several new approaches and techniques (Table 2), based upon the unique properties of nanoscale materials and the basic properties of the mucus layer, which have significantly improved the efficacy of pharmaceuticals products after oral administration.100–104 Recently, it has been observed that insulin-loaded nanoparticles have preserved insulin activity and produced blood glucose reduction in diabetic rats for up to 14 days following oral administration.105 Even after oral administration, drug loaded NPs still face several difficulties due to the presence of M-cells that have a tight junction of epithelial cells, which prevent the entry of foreign materials. Therefore targeting this region is necessary to cross the mucosal barrier for the efficient delivery of oral drugs.
Table 2.
Design of nanocarrier with adhesive molecules to improve oral administration
| Mucoadhesive nanopartide System | Nanoparticles/component | Advantages | Ref. |
|---|---|---|---|
| Mucoadhesive polymeric systems | Poly(lactic acid) (PLA), poly(sebacic acid) (PSA), poly(lactic-co-glycolic acid) (PLGA) and poly(acrylic acid) (PAA) | Improved bioavailability of particles, prolonged retention in the gut, improved blood glucose regulation | Suk et al. (2011)80 |
| Targeted mucoadhesive nanoparticle systems | Lectins, invasins, vitamin B12-derivatives, wheat germ agglutinin (WGA), bacterial enzymes and surface invasin protein (e.g. Salmonella and Shigella) | Particle uptake, increased binding efficiency, decreased rate of elimination, penetration of mucus barrier | Hussain et al. (1998),81 Salman et al. (2005)82 |
| Mucoadhesive pH-responsive systems | Acrylic-based polymers (methacrylic acid) (PMAA), polyethylacrylate (PMAA-PEA) and polymethacrylate (PMAA-PMA) | Retention of a collapsed state in the low pH of the stomach, penetration of the gastrointestinal (GI) tract, frequently localized drug molecules | Dai et al. (2000),83 Ganta et al. (2008)84 |
| Mucoadhesive lipid-based systems | Liposomes, mixed micelles, micro and nanoemulsions, and solid lipid nanoparticles | Increased solubility and bioavailability of the encapsulated drugs | Fricker et al. (2010)103 |
| Mucus penetrating systems | Component | Advantages | Ref. |
|---|---|---|---|
| Disrupting the mucus barrier | N-Acetyl-l-cysteine (NAC), a commonly used mucolytic, and some viscous molecules | Enhanced nanoparticle-based drug delivery, disruption of the barrier properties of the mucus lining | Carlstedt et al. (1998)85 |
| Particles that penetrate the mucus barrier | Virus particles, bacterial lysine, bacterial grown hydrophobic surface | Increased diffusion rate, penetration of the mucosal layer | Stuart et al. (2001),86 Paerregaard et al. (1991)87 |
5.2.1 Targeting of nanoparticles to epithelial cells in the gastrointestinal (GI) tract using ligands
To enhance the target specificity, it is necessary for the nanoparticles to interact with the enterocytes and M-cells of Payer's patches in the GI tract, because a large number of cell-specific carbohydrates is expressed on their cell surface, which serves as a binding site for the colloidal drug carrier containing specific ligands.106 Certain glycoproteins and lectins have the necessary properties to bind specifically on their cell receptor surface.107 In order to enhance oral peptide and vitamin B-12 absorption from the gut, different lectins (bean and tomato lectin) have been used.108 This study was carried out under physiological conditions via receptor-mediated endocytosis. For further studies, they also used various peptides (e.g. granulocyte colony stimulating factor, erythropoietin). The mucus membrane in the stomach secretes mucoprotein, which can bind specifically with the cobalamin.106 The mucoprotein reaches the ileum intact where transportation is facilitated by specific receptors.
5.2.2 Enhancing absorption using non-specific interactions in the gastrointestinal tract
In another approach, the absorption of macromolecules and certain materials in the gastrointestinal tract follows either a paracellular or endocytotic pathway. Nanoparticle absorption through the paracellular route utilizes 1% of the mucosal surface area. Macromolecules introduced via paracellular permeability can be increased by using polymeric materials such as chitosan, starch, or poly(acrylate).109,110 The absorption of nanoparticles via the endocytotic pathway is either by receptor-mediated endocytosis (i. e. active targeting) or by adsorptive endocytosis, where the ligands do not take part. This process is initiated by electrostatic forces (such as hydrogen bonding or hydrophobic interactions) between the nanomaterial and the cell surface.111 During the adsorptive endocytosis pathway, the size and surface properties of the material are important.112 If the nanoparticles are positive or uncharged, they have a greater affinity towards the adsorptive enterocytes. If the NPs are hydrophilic in nature and negatively charged, they have a higher affinity towards the adsorptive enterocytes and M-cells.113
5.3 Transdermal drug delivery (TDD) system using nanotechnology
Transdermal drug delivery system is the most promising route of drug administration and offers many advantages over oral, intravenous and others routes of administration. The skin of the human body is one of the most physical barriers for the drug delivery. The outermost layer of the skin is called the stratum corneum (SC), the viable epidermis is seen underneath the stratum corneum and deeper is dermis, which is 1–2 mm thick, and contains rich capillary blood vessels for systemic absorption of drugs just below the dermal–epidermal junction. The function of the stratum corneum is to protect the underlying tissues from infection, dehydration, chemicals and mechanical stress.114 Therefore, several approaches have been made to defeat this barrier and enhance the stratum corneum permeability. Such strategies include physical, biochemical, and chemical methods.115 Over the past decades, developing controlled drug delivery has become increasingly important in the pharmaceutical industry.
In the present approaches, different techniques have been developed to enhance transdermal delivery. Such techniques are physical enhancers (non-cavitational ultrasound, cavitational ultrasound, microdermabrasion, thermal ablation,58 iontophoresis, electroporation, magnetophoresis, microneedle), nanoscale-vesicle nanoparticulates (liposome, niosome, transfersome, microemulsion, solid lipid nanoparticle) dendrimer systems and chemical enhancers (sulphoxides, azones, glycols, alkanols, terpenes etc.) to penetrate the skin frequently and transport the drug through this barrier.116 Recently different types of nanocarriers (Fig. 3) have been used to design an important delivery system for topical transdermal drug delivery and also various nanoformulation drugs have been investigated in the pharmaceutical field (Table 3).
Fig. 3.
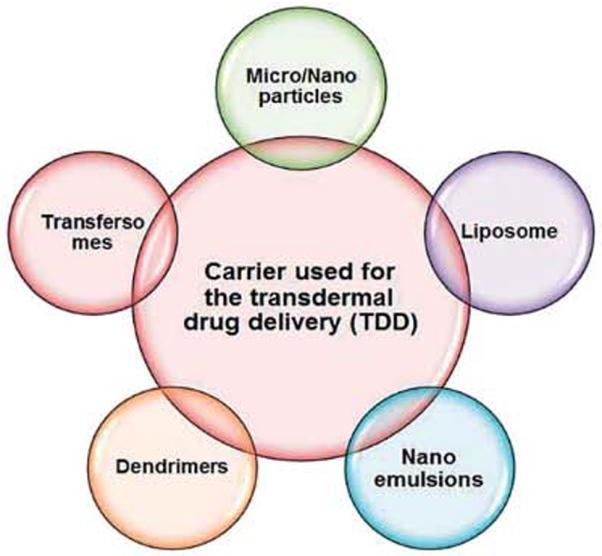
Nanoformulations used as transdermal drug delivery systems.
Table 3.
Examples of nanomedicine products on the market or under clinical trials with different routes of administration
| Route of administration | Product or drug | Formulation | Therapeutic use | Status | Company or references |
|---|---|---|---|---|---|
| Oral drug delivery | Gastromark (ferumoxsil) | Silicon-coated iron oxide NPs | GI imaging | Marketed | AMAG Pharmaceuticals |
| Rapamune (sirolimus) | Nanocrystal particles | Immunosuppressive | Marketed | Pfizer/Wyeth | |
| Emend (aprepitant) | Nanocrystal particles | Antiemetic | Marketed | Merck | |
| Tricor (fenofibrate) | Nanocrystal particles | Hypercholesterolemia | Marketed | Abbott | |
| Megace ES (megestrol) | Nanocrystal particles | Antianoretic | Marketed | Par Pharmaceuticals | |
| Triglide (fenofibrate) | Nanocrystal particles | Hypercholesterolemia | Marketed | ScielePharma | |
| Renagel (sevelamer) | Phosphate binding polymer | End stage renal failure | Marketed | Genzyme (Daiichi licensed) | |
| Welchol | Cholesterol binding polymer | Type 2 diabetes | Mariketed | Genzyme | |
| AMG223 | Phosphate binding polymer | Hyperphosphatemia in CKD patients on hemodialysis | Phase II | Amgen | |
| NKTR-118 | PEG-naloxone | Opioid-induced constipation | Phase II | Nektar | |
| SLIT cisplatin | Liposomes | Cancer—lung | phase II | Transave | |
| Pulmonary drug delivery | Budesonide | Nanoemulsion | Asthma | Reported | Jacobs et al. (2002)130 |
| Fluticasone | NPs | Asthma | Reported | Shekunov et al. (2005)131 | |
| Tobramycin | NPs | Infections | Reported | Joshi et al. (2003)132 | |
| Insulin | Liposomes | Diabetes | Reported | Carpenter et al. (2005)133 | |
| Leuprolide | Liposomes | Lung cancer | Reported | Khanna et al. (1997)134 | |
| Low molecular weight heparin | Dendrimer | Thrombosis | Reported | Zhang et al. (2001)135 | |
| Transdermal drug delivery | Lignocaine | Liposomes | Local anesthetic | Reported | Sharma et al. (1994)136 |
| Methotrexate | pH-sensitive liposomes | Cancer | Reported | Dubey et al. (2007)137 | |
| Indomethacin | Dendrimer | Anti-inflammatory | Reported | Chauhan et al. (2003)138 | |
| 5-Fluorouracil | Dendrimer | Cancer | Reported | Venuganti et al. (2008)139 | |
| Insulin | NPs | Diabetes | Reported | Huang et al. (2009)140 | |
| Celecoxib | NPs | Anti-inflammatory | Reported | Joshi et al. (2008)141 | |
| Triptolide | NPs | Anti-inflammatory, anticancer | Reported | Mei et al. (2003)142 | |
| Ocular drug delivery | GCV | Albumin NPs | Cytomegalovirus retinitis | Reported | Irache et al. (2005)143 |
| Oligonuleotides | Liposomes | Cancer | Reported | Bochot et al. (1998)144 | |
| Pilocarpine HCl | Liposomes | Miotic | Reported | Sahoo et al., (2008)145 | |
| Timolol maleate | Diocomes | Glaucoma | Reported | Vyas et al. (1998)146 | |
| Cyclopentolate | Niosomes | Mydriatic | Reported | Saettone et al. (1996)147 | |
| Acetazolamide | Liposomes | Diuretic | Reported | El-Gazayerly et al. (1997)148 | |
| Insulin | Liposomes | Diabetes | Reported | Sahoo et al. (2008)145 |
Recently various works in the literature have demonstrated the enhancement of drug penetration across the skin. Basically, two favored trends have been developed with nanoparticle delivery systems. First, the high drug loading capacity of the vehicle due to the small size nanoparticle system can reduce the interfacial tension and ensure excellent surface contact between the skin and vehicle over the entire application area. Secondly, the fluctuating structure of nanoemulsions or microemulsions (NE/ME), with ultralow interfacial tension and high oil and water content, makes it possible for both lipophilic and hydrophilic drugs from the relatively hydrophilic vehicle to penetrate the lipophilic stratum corneum and move towards the targeted site (Fig. 4).117
Fig. 4.
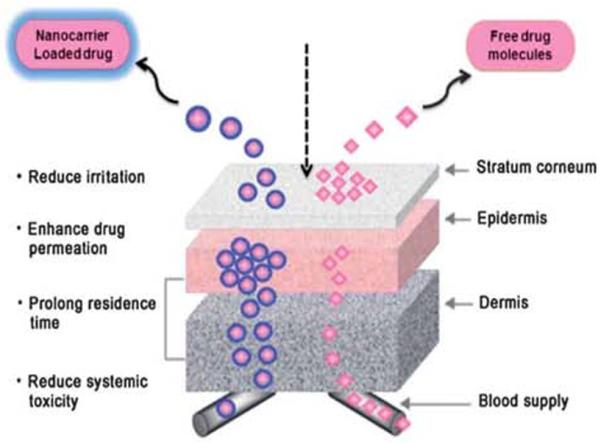
Systemic delivery of free drugs and nanocarrier systems Through the barriers of transderrnal drug delivery.
Kreilgaard et al. developed a nanoparticle based emulsion system, which is loaded with lipophilic (lidocaine) and hydrophilic (prilocaine hydrochloride) drug molecules and compared its flux to conventional methods. They found that the transdermal flux of lidocaine and prilocaine hydrochloride was increased up to 4 and 10 times, respectively, as compared to conventional method of drug formulation. This drastic enhancement was achieved due to concentration gradients created by high solubilization of the drugs and the increased thermodynamic activity of drug.117,118 However, researchers also demonstrated the possibility of an enhancement effect of different individual constituents, such as oil, surfactants and different nanosized particulates and nanocarrier based delivery systems (Table 3), to bypass the barrier of skin. The carriers then preclude excessive local drug clearance through the peripheral blood plexus for a better therapeutic effect.119
5.4 Nanotechnology for pulmonary drug delivery
There are several lung diseases which serve as prime candidates for inhalation therapy, such as asthma, emphysema, chronic obstructive pulmonary disease (COPD), cystic fibrosis, primary pulmonary hypertension, lung cancer, pneumonia, and some of the new born respiratory diseases.120 Since the drug avoids first-pass metabolism and is deposited at the disease site, it is advantageous in treating such diseases locally.
There are two major primary modes of pulmonary aerosol administration: nasal and oral inhalation.121 In respiratory system, the inhaled material deposits in different parts of lung regions mainly depending on the size and diameter of the particles and the overall inhalation process (Fig. 5). Discrimination between the deposition sites is realized by three different mechanisms: Brownian motion, sedimentation and impaction. According to Brownian motion, particles of a size smaller than 1 mm will deposit mainly in alveolar regions of the lung. Particles of size 1–5 mm are able to enter and sediment within the trachea-bronchial region whereas, the particles larger than 5 mm will mainly be deposited in the pharyngeal airways due to impaction (blockage of the digestive tract).
Fig. 5.
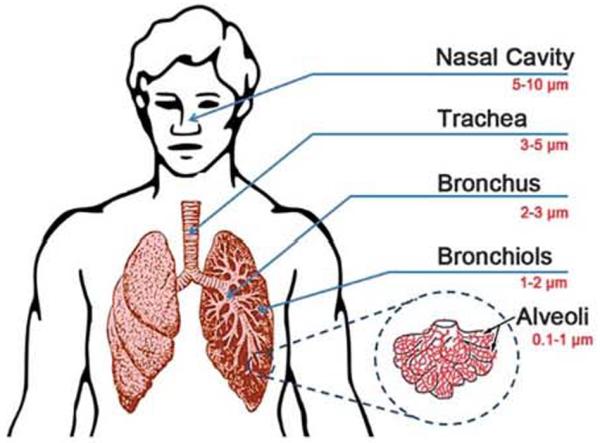
The image represents the regional deposition of particles within the lungs, depending on the particles size and the overall inhalation process.
Interestingly, ultrafine particles of 0.005–0.2 mm are efficiently deposited deep in the lungs, whereas most of the particles 0.2–1.0 mm in size are exhaled again. As mentioned before, the inhalation process (Fig. 6) or incorrectly entering the pulmonary path unsurprisingly results in reducing the therapeutic effects of the drug. However, most inhaled drugs are intended to reach the alveolar region, since the very thin epithelium (0.1–0.5 mm) and the huge alveolar surface (100–140 m2) offer superior conditions for drug absorption.122
Fig. 6.
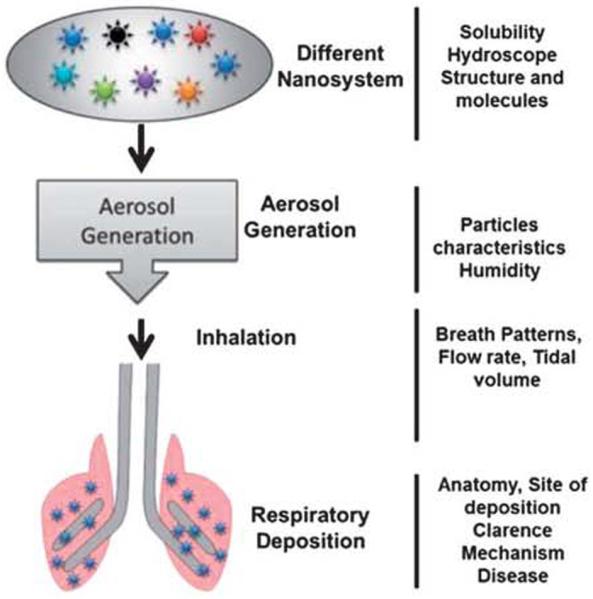
Different factors influence on the pulmonary delivery ot drug and nanoparticle sysiems
The advancement of nanotechnology has reignited interest in the treatment of lung disease because the lungs act as a major route of drug delivery for both systemic and local treatments. The large surface area of the lungs and the minimal barriers impeding access to the lung periphery make this organ a suitable portal for a variety of therapeutic interventions.122 Nanotechnology has provided new formulation options for both dispersed liquid droplet dosage forms (such as metered dose inhalers and nebulizers) and dry powder form ulations.123,124 There are various advantages in using nanocarrier systems in pulmonary11 drug delivery which are beneficial to the patient such as:
-
◆
The potential to achieve a relatively uniform distribution of drug dose among the alveoli.
-
◆
Enhanced solubility of the drug compared to its own aqueous solubility.
-
◆
The sustained-release of a drug which consequently reduces the dosing frequency.
-
◆
Suitability for delivery of macromolecules.
-
◆
Decreased incidence of side effects.
-
◆
Improved patient compliance.
-
◆
The potential for drug internalization by cells.
Recently, various drugs, pure molecules, proteins and peptides,83–86 polymeric nanoparticles, polyelectrolyte complexes, and drug-loaded liposomes have shown encouraging results for the delivery of drugs in the case of lung diseases.125–127 Several methods are also being developed to produce nanoparticles with properties suitable for improving access to the peripheral lung. Traditional techniques such as grinding, spray drying and with the help of more recent advances in supercritical fluid extraction procedure, precipitation, and solvent extraction methods have also been employed to produce more efficient nanoparticle formulations for the treatment of pulmonary diseases.128,129
Today, researchers have put forth a great effort for the development of precise pulmonary drug deliver technology through combination with nanotechnology both in terms of inhaler design and using advanced methodologies. Particle engineering for improving the diagnosis and therapy for heart, lung and blood diseases, and drug delivery represents an area of particular promise.149 Nanotechnology may provide novel treatments for a broad range of intractable pulmonary diseases, including bacterial biofllms, fungal infections, and tuberculosis and more promising targets for nanotechnology-based treatment for heart, lung and blood diseases through a pulmonary route of administration.149,150
5.5 Nanotechnology in ocular drug delivery
The eyes are the most readily accessible organs in our body. Drug delivery to eye tissues is particularly problematic and highly risky. The poor bioavailability of drugs from ocular dosage forms is mainly due to pre-corneal loss factors (e.g., tear dynamics, nonproductive absorption, transient residence time in the culdesac, and relative impermeability of the corneal epithelial membrane).151 Delivery of drugs to ocular region via nanotechnology based products satisfies three main objectives:152
-
◆
Enhancement of drug permeation.
-
◆
Controlled release of the drug.
-
◆
Targeting the drug to the diseased site.
The nanotechnological formulations include micro/nanoparticles, nanoemulsions, nanosuspensions, niosomes145,153 liposomes, cyclodextrins136 polymer scaffolds, solid lipid nanoparticles, light-sensitive nanocarrier systems, dendrimers etc. Treatment of posterior eye segment diseases, such as proliferative retinopathy or macular degeneration, remains dodgy, due to the present of the tight junctions of the blood–retinal barrier (BRB), but advanced nanotechnology systems have demonstrated successful outcomes.154,155
Recently, Mahmoud et al. have developed an efficient nano-based delivery system to achieve sustained therapeutic effects to treat ocular disease. In this study, they prepared chitosan nanoparticles using sulfobutylether β-cyclodextrin as a polyphonic cross linker to investigate the potential of this nanostructure for ocular drug delivery systems in albino rabbits. The result shows that approximately 50% drug release over an 8 hour period from its original amount shows zero order pharmacokinetics behavior. The in vivo study revealed that the prepared mucoadhesive nanoparticles have a better ability to sustain antifungal effects.156
In another work, Chaiyasan et al. developed self-assembled oppositely charged polymers, cationic chitosan (CS) and anionic dextran sulfate (DS), nanoparticles for drug delivery to the ocular surface. In this study, the mucoadhesive properties of the chitosan–dextran sulfate nanopartides (CDNs) was evaluated by imaging the retention of the fluorescein isothiocyanate-labeled CDNs on the cornea. The ex vivo experiment indicated that cationic, biocompatible mueoadhesive CDNs, loaded with a drug such as Rhodamine B (RhB) or Nile Red (NR), are able to release the drug analog at the ocular surface. The nanoparticles were stable to lysozymes and showed prolonged adherence to the corneal surface.157 Several other groups have also developed dendrimer based nanocarrier systems for the treatment of ocular disease.158
To incorporate anti-inflammatory drugs for ophthalmic applications, several nanoparticle based drug carriers are available in the market and some of them are in clinical trials (Table 3).
6 The environmental, health and safety issues related to nanoparticles
Several decades have passed, and the implementation of nanomedicine concepts in broad clinical application still remains puzzling due to the limited knowledge about safety and toxicity. Although one inherent property of NP based drug delivery formulations is decreased adverse side effects and reduced toxicity, NPs themselves may account for the adverse biological response.159,160 As more and more nanomaterials are used as drug delivery vehicles, we should first make sure they are safe to utilize as a drug delivery carriers for human medical application, and also pay more attention to the potential safety hazards and toxicity issues.
Because of their nanoscale size, NPs which are left in the environment after synthesis can be easily transported into the human body by inhalation and have some unknown influences upon health. Ultrafine particles in the air are considered as the main cause of respiratory disease, and the NPs may be harmful when directly exposed to human beings. One report says that, workers who are exposed to polystyrene fumes and polyacrylate NPs for several months are expected to be affected by certain diseases like pulmonary fibrosis, pleural effusions and granuloma formation.161 Sometimes the above behavior has also been observed when NPs are used in clinical situations. It may also cause some undesirable effects in human by the interaction with various components inside the body, like platelet aggregation or cardiovascular effects. In the United States of America (USA), some organizations have made efforts to establish some standard programs for the determination of health and environmental hazards caused by nanomaterials.162 The following are key reasons for toxicity of nanomaterials in human health applications.
Physicochemical properties (size, shape and surface charge)
NPs of <100 nm present ideal properties for drug delivery, which in turn to cause undesirable effects like crossing the blood–brain barrier, triggering immune reactions and damaging the membrane structure of human cells or organelles. The suface charge on the nanoparticles can cause organ accumulation and induce toxicity. Cationic lipids loaded with drugs, genes and siRNA used in laboratory research are not safe to be used in the clinic because of their dose dependent toxicity, pulmonary inflammation and potential hemolysis effects.163
High surface area to volume ratio
Due to their high surface area to volume ratio, nanoparticles can efficiently interact with biological systems, which leads to potential toxicity.163
Agglomeration and dispensability
Nanoparticles tend to agglomerate, due to their high surface to volume ratio. If agglomeration happens in the human body after drug administration, it will cause vascular or lymphatic blockage.165
Biodegradation and biocompatibility
In drug delivery system, if the nanocarriers are not degraded quickly, they may accumulate in cells or tissue, and lead to a chronic inflammatory response.166 Hence, excellent biodegradability must be one of the basic characteristics of NPs in drug delivery. Silver (Ag) NPs were reported to be toxic, due to the presence of residual CTAB from synthesis process.166 Chang et al. have reported silica NPs to be toxic at high concentration, because of the observation of cell viability reduction.167 Nanomaterials with poor biocompatibility would cause serious cell death and immunological effects.
Immunotoxicity
As we know, the immune system protects the body from possible harmful substances by recognizing and responding to antigens. When nanoparticles enter inside the human body as foreign agents, they may trigger a defense to protect the body. If a nanomaterial with immunotoxicity is applied in clinical use, it will cause a severe immune response, and show dangerous side effects. To measure nanoparticle-related toxicity in vitro, a plaque forming cell assay should be done.168,169
Genotoxicity
Some nanoparticles, such as gold NPs of <10 nm, can cross the cell nucleus membrane, and deliver loaded drugs or siRNA within the nucleus. At the same time, they can also cause gene mutation in the DNA helix. Assessment strategics for nanoparticle genotoxicity are limited, such as the salmonella reverse mutation assay, the micronucleus test, and the alkaline comet assay.170
Hemolysis and anticoagulation
Nanoparticles can cause hemolysis or coagulation phenomena when administrated by intravenous injection.168 Standard hemolysis and coagulation tests exist, and allow us to study these two properties of nanoparticles used in drug delivery.171,172 Early safety studies of the drug delivery nanoformulation are necessary to find and avoid the potential danger to the environment and toxicity to human health. Therefore, comprehensive studies of nanodrug formulation should be necessary, such as general toxicity assay, histocompatibility, complement activation, immunotoxicology, pharmacokinetics, toxicokinetics, and metabolic fate.8
Clearance and distribution
As mentioned above, nanoparticle based drug delivery formulation can target an active site, which can lead to a better biodistribution. Once the drug is released from the nanocarriers, there is no need for the nanocarriers to stay further in the human body. Therefore, it is necessary to study in detail about the mechanisms of how body clears the left over nanomaterials. The clearance rate of the nanoparticles is important to evaluate the toxicity effects.173 Moreover, the organ distribution should be studied to predict the potential hazards to the liver, kidney, spleen and so on.174,175
6.1 The toxicity of nanoparticles through different routes of administration
Nanoparticles can be used in drug delivery systems for clinical therapy, via various routes of administration such as oral, injection or transdermal patch. After administration, nanoparticles with varying size, shape, surface and composition can penetrate and transfer to the organs and cells by either diffusion or active intracellular transportation, or adsorb onto the surface of macromolecules,116,124 which could cause unknown interactions between the nanoparticles and biological systems, which decide the negative and positive aspects of materials for real therapeutic applications. In addition to this, the distribution, including regional deposition, retention, solubility, redistribution and translocation also need our attention as they all seriously affect the body. In the process of circulation, metabolism, accumulation and excretion pathways, nanoparticles with poor solubility or degradability would accumulate within the body and persist for a long time and cause undesirable adverse effects when higher concentrations are used. However, in most cases, the toxicity of the drug formulated nanocarriers is the due above mentioned factors but this is not the only region for the actual therapy. A number of cases and physiological factors (Fig. 7) of the host body decide the exact response of materials for real therapeutic applications.
Fig. 7.
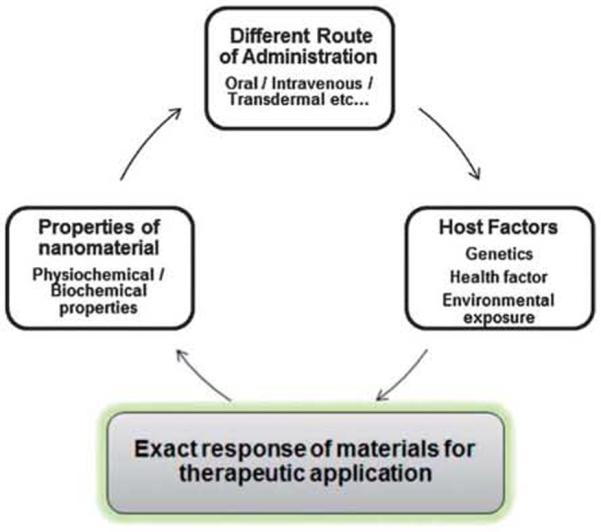
A simplified depiction of potential factors that may influence the effects of engineered nanoparticles on the health system.
7 What can we do for the reduction and assessment of toxicity through different modes of administration?
To improve the efficacy of in vivo drug delivery, certain basic rules have to be followed (Fig. 8) while designing nanocarriers. All types of nanocarriers must be formulated with biocompatible materials. For targeted drug delivery, the route of administration plays a major role. Before engineering the nanoparticles, suitable material selection has to be carried out for their in vivo compatibility and degradability.69 Next, scientists should think about which type of nanoparticles to synthesize, ranging from nanospheres to nanocapsules. The shape of the material greatly controls the loading and release properties of the drug. After synthesizing the material, the nanospheres occur as plain nanoparticles whereas nanocapsules appear like a vesicular structure. From the literature survey, it has been found that the shape of the nanoparticles is also an important factor, which greatly influences their fate in biological systems. Having chosen the type of the nanoparticles, the engineer has to decide which preparation process they wants to adopt, such as polymerization or some specific preparation procedure. The nature of the materials and the physicochemical properties of the drug-loading carrier directly influence their in vivo fate.176 Due to the submicron size of the nanoparticles, surface properties are also considered as an important parameter to control the in vivo fate of the drug carrier.177 The physicochemical properties of the nanocarrier are predicted obviously by the chemicals that are attached to their surface.
Fig. 8.
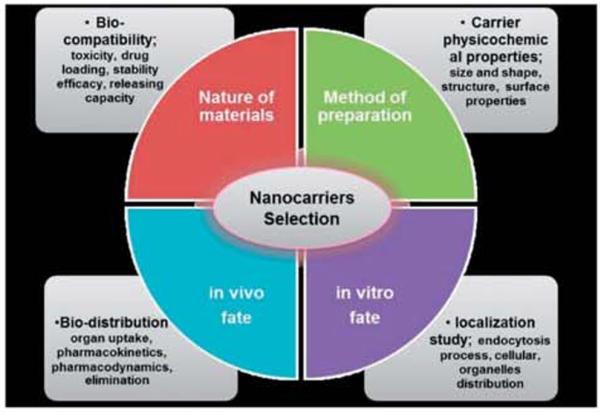
Different parameters for the selection of nanocarriers tor the formulation process.
Surface chemistry also contributes to define the type of interaction with components of the surrounding medium.69 However, the in vivo fate of the drug carrier is not only decided by their formulation, but the route of administration of the drug-loaded carrier is also considered. Hence, specific strategies of drug-carrier formulation have to be applied depending on the different routes of administration.
8 Nanomedicine in the market and industry
Nanotechnology has been hailed as the “next Industrial Revolution” (National Nanotechnology Initiative 2000) and promises to have substantial impacts on many areas of our lives.178 As expected, pharmaceutical companies are already investing in nanotechnology (Fig. 9). Nanomedicines include nano-based contrast agents and various drug delivery formulations. Currently, drug delivery formulation makes up about 75% of the total sales of nanomedicine.3 Here we just show the details about drug delivery formulation. About 23 nanoscale drug formulations are available in the market. Of which, three polymeric products have been sold for around $3.2 billion.3 Nanomedicine regulatory and standardisation methods have been initially built. Nowadays, the regulatory procedure of nanomedicine drug approval is similar to the conventional method. As time goes on, a new examination department may be needed for nanomedicine, with different requirements. Analysts have predicted that by 2014 the market for pharmaceutical applications of nanotechnology will be close to $18 billion annually.179 Another report indicates that the demand for medical products incorporating nanotechnology in the United States will increase by more than 17% per year to $53 billion in 2011. and $110 billion in 2016.180
Fig. 9.
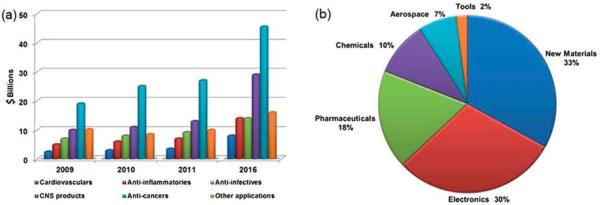
(a) Nanomedical global sales by therapeutic area, 2009–2016; (source: BCC Research) (b) projected contributions in the field of nanomedicine or pharmaceutical fields for human health care, according to the US economy, 2015 (source: US Science Foundation, 2003).
9 Conclusion and future prospects
After over-viewing the innovative pharmaceutical developments based on the unique properties of nanoscale drug delivery formulation, we can conclude that nanomedicine will become more important and much broader in clinical diagnosis, imaging and therapeutics, detection and treatment of various diseases. Indeed, the National Institute of Health (NIH) road-maps envisage that nanomedicine developed from nanoparticle based technology will begin to bring more medical benefits within the next 10 years. This dream includes the evolution of new nanoformulation materials like ocular implants and implanted transducers that can be used to restore lost vision and hearing, nanoscale polymer materials that mimic an artificial heart valve to treat cardiac problems and nanocomposites for bone scaffolds (Opportunities and risks of nanotechnologies, report in co-operation with the OECD International Futures Program). Furthermore, we already know that engineered nanoparticles with special structures and multifunctional nanodrug formulations for combined therapy will be the next step in the development.181,182 As we mentioned above, for better development of nanomedicine, the assessment of the toxicity of these drug carriers should be studied in detail through different routes of administration to avoid hazards to health and the environment issues.165 In order to achieve immense success in this field of nanomedicine. scientists with diverse backgrounds must unite as one to create amazing pharmaceutical formulations from different nanomaterials for clinical trials for better human health.
Box 1 Real applications of nanotechnology for health care.
An important area to focus on for bionanomedical applications has been investigated recently. We adopted all these concepts with the help of various literatures reports. This box summarizes the key points.
Drug delivery: nanoscale particles/molecules have been developed to target specific sites in the body, to improve the bioavailability and pharmacokinetics of therapeutics. Multifunctional targeted devices are capable of bypassing biological barriers to deliver multiple therapeutic agents directly to the cancer cells and also to tissues in the microenvironment.
Drug therapy: the nanostructure/molecules themselves have unique medical properties, which show therapeutic effects in biological systems. Such molecules differ from traditional small-molecule drugs. Examples include drugs based on fullerenes or dendrimers.
Imaging: nanoparticles, which are used particularly for MRI and ultrasound scans, provide an improved contrast and favorable biodistribution. For example, super paramagnetic iron oxide nanoparticles have been used as MRI contrast agents.
Repair and replacement: damaged tissues and organs are often replaced by artificial substitutes, and nanotechnology offers a range of new biocompatible coatings for the implants that improves their adhesion, durability and lifespan. New types of nanomaterials are being evaluated as implant coatings to improve interface properties for bone replacement and tissue regeneration. For example, nanopolymers can be used to coat devices in contact with blood (e.g. artificial hearts, catheters) to disperse clots or prevent their formation.
Hearing and vision: nanotechnology is being used to develop a potentially more powerful device to restore lost vision and hearing. One approach uses a miniature video camera attached to glasses to capture visual signals which are then transmitted to an array of electrodes placed in the eye. Another approach uses an array of electrodes at the tip of the device to solve hearing problems, with five times greater amplification than that of current devices.
Implants, surgery and coatings: nanotechnology has the capability to improve implant biocompatibility, either by coating implants with nanomaterials or by using nanomaterials as implant materials. They can also be used to create smaller, rechargeable batteries for use in active implants. The technology has also been applied to create corrosion free suture needles with improved strength and ductility. Nanoparticles have also been incorporated into fibers to be used for wound dressings and for dental cavity filling. The ultimate aim of using these novel technologies in implants, surgery or wound care is to heal the body quickly and efficiently without creating excessive pain or irritation.
Acknowledgements
This work was financially supported in part by grants from the State High-Tech Development Plan (2012AA020804), National Natural Science Foundation of China project (no. 30970784, and 81171455), National Key Basic Research Program of China (MOST 973 project 2009CB930200), National Distinguished Young Scholars grant (31225009) from the National Natural Science Foundation of China and Chinese Academy of Sciences (CAS) “Hundred Talents Program” (07165111ZX) and the CAS Knowledge Innovation Program. This work was also supported in part by NIH/NIMHD 8 G12 MD007597, and USAMRMC W81XWH-10-1-0767 grants.
Biographies

Anil Kumar is currently working as a Research Fellow in the research group of Prof. Xing-Jie Liang at the CAS Key Laboratory for Biological effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Chinese Academy of Sciences. Previously, he graduated form Bangalore University with major in Biotechnology, Chemistry and Zoology. He completed his Masters degree in Microbial Science and Technology from Amity University, Noida, India. His main research interest focuses on applied nanotechnology for the study of nano-drug delivery systems (gold nanomaterials for targeted drug delivery system) and to overcome drug resistance in cancer.

Fei Chen received his BS of Biopharmaceutics at Huazhong University of Science & Technology in 2012. Currently he is pursuing his master's degree under the guidance of Prof. Xing-Jie Liang at the National Center for Nanoscience and Technology (NCNST). His main research interest focuses on nanomalerial-based controllable drug delivery systems.

Anbu Mozhi received her Bachelor of advanced Zoology and Biotechnology in 2007 and obtained her M. Tech of Nanoscience arid Nanotechnology in 2010, both at the University of Madras, India, She is pursuing her PhD under the guidance of Prof. Xing-Jie Liang's lab at the National center for Nanoscience and Nanotechnology (NCNST) at Chinese academy of Science, China. Her research interest is focussed on design and development of stimuli-responsive polymer based nanomaterials for gene therapy and drug delivery.

Xu Zhang received in BS of Biology from Qufu Normal University. College of Life Science. Since 2008, he became a Ph.D. candidate at National Center for Nanoscience and Technology, Chinese Academy of Science in the group of Prof Xing-Jie Liang. His research interests include the design and applications of self-assembled peptides and peptide-based functiorial aggregation-induced emission moiecules.

Yuanyuan Zhao graduated in Applied Chemistry. She completed her M S. study in Physical Chemistry and obtained a Ph.D. in Polymeric Chemistry and Physics at Jilin University in 2010. Since 2011 she has been doing her Postdoctoral studies on design and application of nanoscale biomaterials in Prof. Xing-Jie Liang's group.

Dr Xing-Jie Liang received his Ph.D. at the National Key Laboratory of Bio-macromolecules, Institute o f Biophysics at CAS. He finished his postdoctoral studies at the Center for Cancer Research, NCI, NIH, and worked as a Research Fellow at the Surgical Neurology Branch, NINDS. He worked ort molecular imaging at the School of Medicine, Howard University before he became deputy director of the CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety NCNST. Developing drug delivery strategies for prevention/treatment of AIDS and cancers are current programs ongoing in Dr Liang's lab based on understanding the basic physiochemical and biological processes in nanomedicine.
Footnotes
Declaration of interest The authors do not have any conflicts of interest to declare.
Notes and references
- 1.Feynman RP. Engineer and Science. 1960;23:22–36. [Google Scholar]
- 2.Caruso F, Hyeon T, Rotello VM. Chem. Sac. Rev. 2012;41:2537–2538. doi: 10.1039/c2cs90005j. [DOI] [PubMed] [Google Scholar]
- 3.Wagner V, Dullaart A, Bock AK, Zweck A. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 4.Doane TL, Burda C. Chem. Soc. Rev. 2012;41:2885–2911. doi: 10.1039/c2cs15260f. [DOI] [PubMed] [Google Scholar]
- 5.Chang HI, Yeh MK. Int. J. Nanomed. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavhan SS, Petkar KC, Sawant KK. Crit. Rev. Ther. Drug Carrier Syst. 2011;28:447–488. doi: 10.1615/critrevtherdrugcarriersyst.v28.i5.20. [DOI] [PubMed] [Google Scholar]
- 7.Elsabahy M, Wooley KL. Chem. Soc. Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan R, Gaspar R. Mol. Pharmaceutics. 2011;8:2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 9.Bangham AD, Standish MM, Watkins JC. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 10.Buhleier E, Wehner W, Vogtle F. Synthesis. 1978;2:155–158. [Google Scholar]
- 11.Gabizon A, Shmeeda H, Barenholz Y. Clin. Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 14.Ros PR, Freeny PC, Harms SE, Seltzer SE, Davis PL, Chan TW, Stillman AE, Muroff LR, Runge VM, Nissenbaum MA, et al. Radiology. 1995;196:481–488. doi: 10.1148/radiology.196.2.7617864. [DOI] [PubMed] [Google Scholar]
- 15.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O'Shaughnessy J. J. Clin. Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 16.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, Kim NK, Bang YJ. Clin. Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 17.Davis ME. Mol. Pharmaceutics. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 18.Bourzac K. Nature. 2012;491:S58–S60. doi: 10.1038/491s58a. [DOI] [PubMed] [Google Scholar]
- 19.Furnham A, Lovett J. Journal of Applied Biobehavioral Research. 2001;6:39–63. [Google Scholar]
- 20.Montanez MI, Ruiz-Sanehez AJ, Perez-lnestrosa E. Curr. Opin. Allergy Clin. Immunol. 2010;10:297–302. doi: 10.1097/ACI.0b013e32833b1f17. [DOI] [PubMed] [Google Scholar]
- 21.Kim DK, Dobson J. J. Mater. Chem. 2009;19:6294–6307. [Google Scholar]
- 22.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Angew. Chem., Int. Ed. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisziewicz J, Toke ER. Nanomed.: Nanotechnoi., Biol. Med. 2013;9:28–38. doi: 10.1016/j.nano.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Boruah BM, Liang XJ. J. Nanomater. 2011;2011:1–17. [Google Scholar]
- 25.Armstead AL, Li B. Int. J. Nanomed. 2011;6:3281–3293. doi: 10.2147/IJN.S27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, He S, Cao W, Cai K, Liang XJ. Nanoscale. 2012;4:6135–6149. doi: 10.1039/c2nr31715j. [DOI] [PubMed] [Google Scholar]
- 27.Shibu ES, Hamada M, Murase N, Biju V. J. Photochem. Photobiol., C. 2013;15:53–72. [Google Scholar]
- 28.Kumar A, Zhang X, Liang XJ. Biotechnol. Adv. 2013;31:593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Van Eerdenbrugh B, Froyen L, Martens JA, Blaton N, Augustijns P, Brewster M, Van den Mooter G. Int. J. Pharm. 2007;338:198–206. doi: 10.1016/j.ijpharm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Rabinow BE. Nat. Rev. Drug Discovery. 2004;3:785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 31.Müller RH, Peters K. Int. J. Pharm. 1998;160:229–237. [Google Scholar]
- 32.Kluge J, Mazzotti M. Int. J. Pharm. 2012;436:394–402. doi: 10.1016/j.ijpharm.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 33.Pereda J, Ferragut V, Quevedo JM, Guamis B, Trujillo AJ. J. Agric. Food Chem. 2008;56:7125–7130. doi: 10.1021/jf800972m. [DOI] [PubMed] [Google Scholar]
- 34.Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Int. J. Pharm. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Patel VR, Agrawal YK. J. Adv. Pharm. Technol. Res. 2011;2:81–87. doi: 10.4103/2231-4040.82950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patravale VB, Date AA, Kulkarni RM. J. Pharm. Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 37.Mishra B, Patel BB, Tiwari S. Nanomed.: Nanotechnol., Biol. Med. 2010;6:9–24. doi: 10.1016/j.nano.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 38.York P. Pharm. Sci. Technol. Today. 1999;2:430–440. doi: 10.1016/s1461-5347(99)00209-6. [DOI] [PubMed] [Google Scholar]
- 39.Jung J, Perrut M. J. Supercrit. Fluids. 2001;20:179–219. [Google Scholar]
- 40.Lima AC, Sher P, Mano JF. Expert Opin. Drug Delivery. 2012;9:231–248. doi: 10.1517/17425247.2012.652614. [DOI] [PubMed] [Google Scholar]
- 41.Türk M. J. Supercrit. Fluids. 2009;47:537–545. [Google Scholar]
- 42.Della Porta G, Falco N, Reverchon E. J. Pharm. Sci. 2010;99:1484–1499. doi: 10.1002/jps.21920. [DOI] [PubMed] [Google Scholar]
- 43.Lee LS, Conover C, Shi C, Whitlow M, Filpula D. Bioconjugate Chem. 1999;10:973–981. doi: 10.1021/bc990076o. [DOI] [PubMed] [Google Scholar]
- 44.Veronese FM, Pasut G. Drug Discovery Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 45.Kim BY, Rutka JT, Chan WC. N. Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 46.Kratz F. J. Controlled Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P. Clin. Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 48.Hawkins MJ, Soon-Shiong P, Desai N. Adv. Drug Delivery Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 49.Burke TG, Munshi CB, Mi Z, Jiang Y. J. Pharm. Sci. 1995;84:518–519. doi: 10.1002/jps.2600840426. [DOI] [PubMed] [Google Scholar]
- 50.Burke TG, Mi Z. J. Med. Chem. 1994;37:40–46. doi: 10.1021/jm00027a005. [DOI] [PubMed] [Google Scholar]
- 51.Kilic MA, Ozlu E, Calis S. J. Biomed. Nanotechnol. 2012;8:508–514. doi: 10.1166/jbn.2012.1406. [DOI] [PubMed] [Google Scholar]
- 52.Zhen Y, Wang X, Diao H, Zhang J, Li H, Sun H, Guo Z. Chem. Commun. 2007:3453–3455. doi: 10.1039/b705326f. [DOI] [PubMed] [Google Scholar]
- 53.Woodle MC. Adv. Drug Delivery Rev. 1998;32:139–152. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 54.Sapra P, Allen TM. Cancer Res. 2002;62:7190–7194. [PubMed] [Google Scholar]
- 55.Wang AZ, Langer R, Farokhzad OC. Annu. Rev. Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 57.Davis ME, Chen ZG, Shin DM. Nat. Rev. Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 58.Prausnitz MR, Langer R. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, Farokhzad OC. Biomaterials. 2009;30:1627–1631. doi: 10.1016/j.biomaterials.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 60.Parveen S, Misra R, Sahoo SK. Nanomed.: Nanotechnol, Biol. Med. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Kelkar SS, Reineke TM. Bioconjugate Chem. 2011;22:1879–1903. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- 62.Howell M, Mallela J, Wang C, Ravi S, Dixit S, Garapati U, Mohapatra S. J. Controlled Release. 2013;167:210–218. doi: 10.1016/j.jconrel.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leng Q, Woodle MC, Lu PY, Mixson AJ. Drugs Future. 2009;34:721. doi: 10.1358/dof.2009.034.09.1413267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foged C. Curr. Top. Med. Chem. 2012;12:97–107. doi: 10.2174/156802612798919141. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Lu Z, Wientjes MG, Au JL. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, Reisen G, Lockridge JA, Short B, Guerciolini R, Nguyen QD, I. Sirna-027 Study. Am. J. Ophthalmol. 2010;150:33–39. e2. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Burnett JC, Rossi JJ, Tiemann K. Biotechnol. J. 2011;6:1130–1146. doi: 10.1002/biot.201100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Domb AJ, Tabata Y, Ravi Kumar MNV, Farber S. Nanoparticles for Pharmaceutical Applications. American Scientific Publishers; Stevenson Ranch, CA: 2007. [Google Scholar]
- 69.Vauthier C. Nanoformulation. 2012:3–19. [Google Scholar]
- 70.Yalkowsky SH, Krzyzaniak JF, Ward GH. J. Pharm. Sci. 1998;87:787–796. doi: 10.1021/js980051i. [DOI] [PubMed] [Google Scholar]
- 71.Hu Y, Xie J, Tong YW, Wang CH. J. Controlled Release. 2007;118:7–17. doi: 10.1016/j.jconrel.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 72.Müller RH, Wallis KH. Int. J. Pharm. 1993;89:25–31. [Google Scholar]
- 73.Brigger I, Dubernet C, Couvreur P. Adv. Drug Delivery Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 74.Brigger I, Dubernet C, Couvreur P. Adv. Drug Delivery Rev. 2012;64:24–36. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 75.Grislain L, Couvreur P, Lenaerts V, Roland M, Deprezdecampeneere D, Speiser P. Int. J. Pharm. 1983;15:335–345. [Google Scholar]
- 76.Jain NK, Nahar M. Methods Mol. Biol. 2010;624:221–234. doi: 10.1007/978-1-60761-609-2_15. [DOI] [PubMed] [Google Scholar]
- 77.Jain A, Jain SK. Crit. Rev. Ther. Drug Carrier Syst. 2008;25:403–447. doi: 10.1615/critrevtherdrugcarriersyst.v25.i5.10. [DOI] [PubMed] [Google Scholar]
- 78.Chatikobo P, Choga T, Ncube C, Mutambara J. Prev. Vet. Med. 2013;109:327–333. doi: 10.1016/j.prevetmed.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Bourzac K. Nature. 2012;491:S58–S60. doi: 10.1038/491s58a. [DOI] [PubMed] [Google Scholar]
- 80.Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J. Nanomedicine. 2011;6:365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hussain N, Florence A. Pharm. Res. 1998;15:153–156. doi: 10.1023/a:1011981610840. [DOI] [PubMed] [Google Scholar]
- 82.Salman HH, Gamazo C, Campanero MA, Irache JM. J. Controlled Release. 2005;106:1–13. doi: 10.1016/j.jconrel.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 83.Dai S, Tam KC, Jenkins RD. Eur. Polym. J. 2000;36:2671–2677. [Google Scholar]
- 84.Ganta S, Devalapally H, Shahiwala A, Amiji M. J. Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 85.Carlstedt I, Sheehan JK. Ciba Foundation Symposium 109-Mucus and Mucosa. John Wiley & Sons, Ltd.; 2008. pp. 157–172. [DOI] [PubMed] [Google Scholar]
- 86.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Biophys. J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paerregaard A, Espersen F, Jensen OM, Skurnik M. Infect. Immun. 1991;59:253–260. doi: 10.1128/iai.59.1.253-260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fasinu P, Pillay V, Ndesendo VM, du Toit LC, Choonara YE. Biopharm. Drug Dispos. 2011;32:185–209. doi: 10.1002/bdd.750. [DOI] [PubMed] [Google Scholar]
- 89.Tanaka Y, Masaoka Y, Kataoka M, Sakuma S, Yamashita S. Eur. J. Pharm. Sci. 2006;29:240–250. doi: 10.1016/j.ejps.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 90.Schenk M, Mueller C. Best Pract. Res. Clin. Gastroenterol. 2008;22:391–409. doi: 10.1016/j.bpg.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Cone RA. Adv. Drug Delivery Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 92.Crater JS, Carrier RL. Macromol. Biosci. 2010;10:1473–1483. doi: 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 93.Lai SK, Wang YY, Hanes J. Adv. Drug Delivery Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohanraj V, Chen Y. Trop. J. Pharm. Res. 2006;5:561–573. [Google Scholar]
- 95.Prego C, Torres D, Fernandez-Megia E, Novoa-Carballal R, Quinoa E, Alonso MJ. J. Controlled Release. 2006;111:299–308. doi: 10.1016/j.jconrel.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 96.Wang XQ, Zhang Q. Eur. J. Pharm. Biopharm. 2012;82:219–229. doi: 10.1016/j.ejpb.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 97.Sahana DK, Mittal G, Bhardwaj V, Kumar MN. J. Pharm. Sci. 2008;97:1530–1542. doi: 10.1002/jps.21158. [DOI] [PubMed] [Google Scholar]
- 98.Cui F, He C, Yin L, Qian F, He M, Tang C, Yin C. Biomacromolecules. 2007;8:2845–2850. doi: 10.1021/bm070339e. [DOI] [PubMed] [Google Scholar]
- 99.Holpuch AS, Hummel GJ, Tong M, Seghi GA, Pei P, Ma P, Mumper RJ, Mallery SR. Pharm. Res. 2010;27:1224–1236. doi: 10.1007/s11095-010-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ensign LM, Cone R, Hanes J. Adv. Drug Delivery Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shoyele SA, Slowey A. Int. J. Pharm. 2006;314:1–8. doi: 10.1016/j.ijpharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Shoyele SA. Methods Mol. Biol. 2008;437:149–160. doi: 10.1007/978-1-59745-210-6_7. [DOI] [PubMed] [Google Scholar]
- 103.Fricker G, Kromp T, Wendel A, Blume A, Zirkel J, Rebmann H, Setzer C, Quinkert RO, Martin F, Müller-Goymann C. Pharm. Res. 2010;27:1469–1486. doi: 10.1007/s11095-010-0130-x. [DOI] [PubMed] [Google Scholar]
- 104.Bonner JC, Card JW, Zeldin DC. Hypertension. 2009;53:751–753. doi: 10.1161/HYPERTENSIONAHA.108.122846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Damgé C, Michel C, Aprahamian M, Couvreur P, Devissaguet JP. J. Controlled Release. 1990;13:233–239. [Google Scholar]
- 106.Russell-Jones GJ. Adv. Drug Delivery Rev. 2001;46:59–73. doi: 10.1016/s0169-409x(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 107.Haltner E, Easson JH, Lehr CM. Eur. J. Pharm. Biopharm. 1997;44:3–13. [Google Scholar]
- 108.Russell-Jones GJ, Arthur L, Walker H. Int. J. Pharm. 1999;179:247–255. doi: 10.1016/s0378-5173(98)00394-9. [DOI] [PubMed] [Google Scholar]
- 109.Schipper NGM, Varum KM, Artursson P. Pharm. Res. 1996;13:1686–1692. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- 110.Akiyama Y, Nagahara N, Kashihara T, Hirai S, Toguchi H. Pharm. Res. 1995;12:397–405. doi: 10.1023/a:1016208703380. [DOI] [PubMed] [Google Scholar]
- 111.Bjork E, Isaksson U, Edman P, Artursson P. J. Drug Targeting. 1995;2:501–507. doi: 10.3109/10611869509015920. [DOI] [PubMed] [Google Scholar]
- 112.Albanese A, Tang PS, Chan WC. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 113.Jepson M, Simmons N, Savidge T, James P, Hirst B. Cell Tissue Res. 1993;271:399–405. doi: 10.1007/BF02913722. [DOI] [PubMed] [Google Scholar]
- 114.Cevc G. Adv. Drug Delivery Rev. 2004;56:675–711. doi: 10.1016/j.addr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 115.Elias PM, Prausnitz MR, Franz TJ, Schmuth M, Tsai J-C, Menon GK, Holleran WM, Feingold KR. Medical Therapy. 2012:2065–2073. [Google Scholar]
- 116.Aqil M, Rizwan M, Talegaonkar S, Azeem A, Sultana Y, Ali A. Recent Pat. Drug Delivery Formulation. 2009;3:105–124. doi: 10.2174/187221109788452285. [DOI] [PubMed] [Google Scholar]
- 117.Tiwary AK, Sapra B, Jain S. Recent Pat. Drug Delivery Formulation. 2007;1:23–36. doi: 10.2174/187221107779814087. [DOI] [PubMed] [Google Scholar]
- 118.Kreilgaard M, Pedersen EJ, Jaroszewski JW. J. Controlled Release. 2000;69:421–433. doi: 10.1016/s0168-3659(00)00325-4. [DOI] [PubMed] [Google Scholar]
- 119.Cevc G, Vierl U. J. Controlled Release. 2010;141:277–299. doi: 10.1016/j.jconrel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 120.Smola M, Vandamme T, Sokolowski A. Nanomedicine. 2008;3:1–19. [PMC free article] [PubMed] [Google Scholar]
- 121.Groneberg DA, Witt C, Wagner U, Chung KF, Fischer A. Respir. Med. 2003;97:382–387. doi: 10.1053/rmed.2002.1457. [DOI] [PubMed] [Google Scholar]
- 122.Henning A, Hein S, Schneider M, Bur M, Lehr CM. Handb. Exp. Pharmacol. 2010;197:171–192. doi: 10.1007/978-3-642-00477-3_6. [DOI] [PubMed] [Google Scholar]
- 123.Bailey MM, Berkland CJ. Med. Res. Rev. 2009;29:196–212. doi: 10.1002/med.20140. [DOI] [PubMed] [Google Scholar]
- 124.Mansour HM, Rhee YS, Wu X. Int. J. Nanomed. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rytting E, Nguyen J, Wang X, Kissel T. Expert Opin. Drug Delivery. 2008;5:629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- 126.El-Sherbiny IM, Smyth HD. Int. J. Pharm. 2010;395:132–141. doi: 10.1016/j.ijpharm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takami T, Murakami Y. Colloids Surf., B. 2011;87:433–438. doi: 10.1016/j.colsurfb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 128.Odziomek M, Sosnowski TR, Gradon L. Int. J. Pharm. 2012;433:51–59. doi: 10.1016/j.ijpharm.2012.04.067. [DOI] [PubMed] [Google Scholar]
- 129.Nanjwade BK, Adichwal SA, Gaikwad KR, Parikh KA, Manvi FV. PDA J. Pharm. Sci. Technol. 2011;65:513–534. doi: 10.5731/pdajpst.2011.00704. [DOI] [PubMed] [Google Scholar]
- 130.Jacobs C, Muller RH. Pharm. Res. 2002;19:189–194. doi: 10.1023/a:1014276917363. [DOI] [PubMed] [Google Scholar]
- 131.Shekunov B. Idrugs. 2005;8:399–401. [PubMed] [Google Scholar]
- 132.Joshi M, Misra A. Clin. Exp. Pharmacol. Physiol. 2003;30:153–156. doi: 10.1046/j.1440-1681.2003.03813.x. [DOI] [PubMed] [Google Scholar]
- 133.Carpenter M, Epperly MW, Agarwal A, Nie S, Hricisak L, Niu Y, Greenberger JS. Gene Ther. 2005;12:685–693. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 134.Khanna C, Anderson PM, Hasz DE, Katsanis E, Neville M, Klausner JS. Cancer. 1997;79:1409–1421. doi: 10.1002/(sici)1097-0142(19970401)79:7<1409::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Q, Shen Z, Nagai T. Int. J. Pharm. 2001;218:75–80. doi: 10.1016/s0378-5173(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 136.Sharma BB, Jain SK, Vyas SP. J. Microencapsulation. 1994;11:279–286. doi: 10.3109/02652049409040457. [DOI] [PubMed] [Google Scholar]
- 137.Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. J. Controlled Release. 2007;123:148–154. doi: 10.1016/j.jconrel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Chauhan AS, Sridevi S, Chalasani KB, Jain AK, Jain SK, Jain NK, Diwan PV. J. Controlled Release. 2003;90:335–343. doi: 10.1016/s0168-3659(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 139.Venuganti VV, Perumal OP. Int. J. Pharm. 2008;361:230–238. doi: 10.1016/j.ijpharm.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 140.Huang X, Du YZ, Yuan H, Hu FQ. Carbohydr. Polym. 2009;76:368–373. [Google Scholar]
- 141.Joshi M, Patravale V. Int. J. Pharm. 2008;346:124–132. doi: 10.1016/j.ijpharm.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 142.Mei Z, Chen H, Weng T, Yang Y, Yang X. Eur. J. Pharm. Biopharm. 2003;56:189–196. doi: 10.1016/s0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 143.Irache JM, Merodio M, Arnedo A, Camapanero MA, Mirshahi M, Espuelas S. Mini-Rev. Med. Chem. 2005;5:293–305. doi: 10.2174/1389557053175335. [DOI] [PubMed] [Google Scholar]
- 144.Bochot A, Fattal E, Gulik A, Couarraze G, Couvreur P. Pharm. Res. 1998;15:1364–1369. doi: 10.1023/a:1011989202488. [DOI] [PubMed] [Google Scholar]
- 145.Sahoo SK, Diinawaz F, Krishnakumar S. Drug Discovery Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 146.Vyas SP, Mysore N, Jaitely V, Venkatesan N. Pharmazie. 1998;53:466–469. [PubMed] [Google Scholar]
- 147.Saettone MF, Perini G, Carafa M, Santucci E, Alhaique F. STP Pharma Sci. 1996;6:94–98. [PubMed] [Google Scholar]
- 148.El-Gazayerly ON, Hikal AH. Int. J. Pharm. 1997;158:121–127. [Google Scholar]
- 149.Card JW, Zeldin DC, Bonner JC, Nestmann ER. Am. J. Physiol.: Lung Cell. Mol. Phys. 2008;295:L400–L411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buxton D. Expert Opin. Drug Delivery. 2006;3:173–175. doi: 10.1517/17425247.3.2.173. [DOI] [PubMed] [Google Scholar]
- 151.Fernandez-Martinez A, Mula P, Cravo P, Charle P, Amor A, Ncogo P, Benito A, Berzosa P. Am. J. Trop. Med. Hyg. 2013;88:43–47. doi: 10.4269/ajtmh.2012.12-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lallemand F, Daull P, Benita S, Buggage R, Garrigue JS. J. Drug Delivery. 2012;2012:604204. doi: 10.1155/2012/604204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang S, Li D, Ito Y, Nabekura T, Wang S, Zhang J, Wu C. Curr. Eye Res. 2004;29:51–58. doi: 10.1080/02713680490513209. [DOI] [PubMed] [Google Scholar]
- 154.Sultana Y, Maurya DP, Iqbal Z, Aqil M. Drugs Today. 2011;47:441–455. doi: 10.1358/dot.2011.47.6.1549023. [DOI] [PubMed] [Google Scholar]
- 155.Gaudana R, Jwala J, Boddu SH, Mitra AK. Pharm. Res. 2009;26:1197–1216. doi: 10.1007/s11095-008-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mahmoud AA, El-Feky GS, Kamel R, Awad GE. Int. J. Pharm. 2011;413:229–236. doi: 10.1016/j.ijpharm.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 157.Chaiyasan W, Srinivas SP, Tiyaboonchai W. J. Ocul. Pharmacol. Ther. 2013;29:200–207. doi: 10.1089/jop.2012.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kambhampati SP, Kannan RM. J. Ocul. Pharmacol. Ther. 2013;29:151–165. doi: 10.1089/jop.2012.0232. [DOI] [PubMed] [Google Scholar]
- 159.Fadeel B, Garcia-Bennett AE. Adv. Drug Delivery Rev. 2010;62:362–374. doi: 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 160.Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Biochim. Biophys. Acta, Gen. Subj. 2011;1810:361–373. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 161.Song Y, Li X, Du X. Eur. Respir. J. 2009;34:559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- 162.Wiesner MR, Lowry GV, Jones KL, Hochella MF, Jr, Di Giulio RT, Casman E, Bernhardt ES. Environ. Sci. Technol. 2009;43:6458–6462. doi: 10.1021/es803621k. [DOI] [PubMed] [Google Scholar]
- 163.Palmateer NE, Hope VD, Roy K, Marongiu A, White JM, Grant KA, Ramsay CN, Goldberg DJ, Ncube F. Emerging Infect. Dis. 2013;19:29–34. doi: 10.3201/eid1901.120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sharma A, Madhunapantula SV, Robertson GP. Expert Opin. Drug Metab. Toxicol. 2012;8:47–69. doi: 10.1517/17425255.2012.637916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oberdorster G. J. Intern. Med. 2010;267:89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 166.Jong WHD, Borm PJ. Int. J. Nanomed. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chang J-S, Chang KLB, Hwang D-F, Kong Z-L. Environ. Sci. Technol. 2007;41:2064–2068. doi: 10.1021/es062347t. [DOI] [PubMed] [Google Scholar]
- 168.Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE. Nanomedicine. 2007;2:789–803. doi: 10.2217/17435889.2.6.789. [DOI] [PubMed] [Google Scholar]
- 169.Liang XJ, Kumar A, Shi DL, Cui DX. J. Nanomater. 2012;2012:127–129. [Google Scholar]
- 170.Landsiedel R, Kapp MD, Schulz M, Wiench K, Oeseh F. Mutat. Res., Rev. Mutat. Res. 2009;681:241–258. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 171.Lin Y-S, Haynes CL. J. Am. Chem. Soc. 2009;132:4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- 172.Yu T, Malugin A, Ghandehari H. ACS Nuno. 2011;5:5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dhawan A, Sharma V. Anal. Bioanal. Chem. 2010;398:589–605. doi: 10.1007/s00216-010-3996-x. [DOI] [PubMed] [Google Scholar]
- 174.Vega-Villa KR, Takemoto JK, Yanez JA, Remsberg CM, Forrest ML, Davies NM. Adv. Drug Delivery Rev. 2008;60:929–938. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 175.Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. Nat. Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 176.Champion JA, Mitragotri S. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnson S. Am. J. Bioeth. 2009;9:1–2. doi: 10.1080/15265160903178754. [DOI] [PubMed] [Google Scholar]
- 179.Allhoff F. Am. J. Bioeth. 2009;9:3–11. doi: 10.1080/15265160902985027. [DOI] [PubMed] [Google Scholar]
- 180.Bawa R, Johnson S. Med. Clin. North Am. 2007;91:881–887. doi: 10.1016/j.mcna.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 181.Gao J, Gu H, Xu B. Acc. Chem. Res. 2009;42:1097–1107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 182.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]