Abstract
The use of animal models to investigate experimental questions about impulsive behavior can provide valuable insight into problems that affect human health. The delay-discounting paradigm involves subjects choosing between smaller reinforcers delivered immediately and larger reinforcers that are delivered after a delay. This is an important experimental paradigm for examining impulsive choice in both laboratory species and humans. However, a shortcoming of previously published delay-discounting studies in animals is that typically only males were studied, reducing the applicability of these studies to human populations. In the present study, both female and male adult Long-Evans rats were trained to perform a delay-discounting task, with delays of 0, 5, 10, 20 and 40 s before delivery of the larger reinforcer. Because dopaminergic signaling is important in mediating this task, the effects of d-amphetamine and the dopamine receptor antagonist, cis-flupenthixol, on task performance were then examined. The main experimental measure was percent larger-reinforcer choice, which was defined as the percentage of experimental trials at each delay in which the delayed, larger reinforcer was chosen. There was not a sex difference in percent larger-reinforcer choice during baseline performance of the task. However, d-amphetamine administration disrupted choice in females, as evidenced by < 80% larger-reinforcer choice in half of the females, but none of the males, at 0.5 mg/kg. d-Amphetamine also differentially altered the latency to choose between immediate versus delayed reinforcers in females compared to males. In contrast, cis-flupenthixol did not have a sex-related effect on percent larger-reinforcer choice. These findings parallel the sex differences in response to amphetamine seen in human delay-discounting studies and underscore the importance of evaluating sex-based differences in baseline performance and in response to pharmacologic agents when utilizing animal models.
Keywords: Delay-discounting, Amphetamine, Flupenthixol, Impulsivity, Sex differences
1. Introduction
Excessive impulsive behavior, defined as a pattern of behavior that involves unplanned actions performed without regard to the consequences (Moeller et al., 2001), is a behavioral pathology that is common to a variety of neuropsychiatric diseases. Impulsive behavior in children reflects a vulnerability to future psychopathology (Beauchaine and Neuhaus, 2008). Thus, efforts to understand the biological basis of impulsive behavior are likely to bring new insights to the diagnosis and treatment of neuropsychiatric disorders in which it is an important component (Robbins et al., 2012).
One challenge to studying impulsive behavior is that impulsivity is not a unitary construct (Evenden, 1999). Impulsive choice, as assessed through delay-discounting (DD) tasks, is one of the better-studied types of impulsivity in laboratory animal and human studies (Winstanley, 2011). In DD paradigms, subjects must choose between a smaller reinforcer that is delivered immediately and a larger reinforcer that is delivered after a delay; the choice of the smaller, immediate reinforcer is considered impulsive. Rodent models have been valuable in understanding the neural pathways and neurochemical signaling underlying impulsive behavior in the DD paradigm (Dalley et al., 2008; Pattij and Vanderschuren, 2008; Winstanley, 2011). Dopaminergic systems, in particular, have been shown to have an important role in mediating DD performance (van Gaalen et al., 2006; Winstanley, 2011).
Developing predictive animal models of behavior involves making the models as applicable to human populations as possible. A strength of the DD paradigm is that the relationship between delay and the subjective value of the reinforcer is best described by a hyperbolic function in both humans and animals (Paule et al., 2012), although the reinforcers are typically hypothetical in human studies and real (i.e. food) in animal studies. A recent meta-analysis of different forms of impulsivity in humans found no sex differences in performance of DD tasks (Cross et al., 2011); yet, administration of the dopaminergic drug, d-amphetamine (AMPH), resulted in sex differences on both cognitive-task performance and dopamine release in humans (Riccardi et al., 2006; Riccardi et al., 2011). Because sex differences in either baseline performance or response to drug challenge could confound interpretation of data, it is important to know whether such differences exist when utilizing animal models.
One shortcoming of animal DD models is that sex differences in performance have rarely been reported, with most published studies only reporting findings from males. Studies have been conducted on selectively bred strains of rodents, but the only commonly utilized outbred strains that have been evaluated for sex differences have been Sprague-Dawley rats and CD-1 mice. Female CD-1 mice were found to be more impulsive than males (Koot et al., 2009), whereas sex differences were not seen in Sprague-Dawley rats (Adriani et al., 2003). Additionally, there are strain differences in DD performance, with Lewis rats exhibiting a decreased choice for the large reinforcer as compared to Wistar and Fischer 344 strains (Garcia and Kirkpatrick, 2012; Huskinson et al., 2012). Also, sex and strain differences in both the response to dopaminergic drugs and dopaminergic signaling have been reported. Sex differences in response to dopaminergic drugs include that the peak locomotor effects of AMPH were seen at a lower dose, and greater locomotor and stereotypic effects of AMPH were seen at the same dose, in female versus male Sprague-Dawley rats (Milesi-Halle et al., 2007; Simpson et al., 2012). Also, female CD-1 mice have been shown to have increased dopamine transporter (DAT) and vesicular monoamine transporter functioning as compared to males (Dluzen and McDermott, 2008), which suggests that sex differences in dopaminergic signaling exist. One reported strain difference in response to dopaminergic drugs is that AMPH increased choice for the large reinforcer in DD in Lewis but not Fischer 344 rats (Huskinson et al., 2012), while strain differences in dopaminergic signaling include that Fischer 344 and Wistar rats have increased DAT and dopamine receptor binding in the striatum and nucleus accumbens as compared to Lewis and Sprague-Dawley rats, respectively (Flores et al., 1998; Zamudio et al., 2005). Because of differences such as these, it is important to investigate whether sex differences exist in other rat strains commonly used for behavioral toxicology and addiction research.
This study explored potential sex differences in adult Long-Evans rats in baseline DD, in response to the dopaminergic drug AMPH, which increases synaptic dopamine concentrations via its effects on dopamine transporter and vesicles (Zhu and Reith, 2008), and in response to the D1/D2 dopamine receptor antagonist cis-flupenthixol (FLU) (Hyttel et al., 1989). Based on the aforementioned, small number of studies to date (Adriani et al., 2003; Koot et al., 2009; Perry and Carroll, 2008), it was hypothesized that there would not be sex differences in baseline DD performance or in response to AMPH or FLU. Increasing synaptic dopamine concentrations has been shown to have a biphasic effect on many behaviors modulated by dopaminergic signaling (Cools and D'Esposito, 2011). Prior DD studies suggest a similar dose-response of impulsive choice to AMPH administration. Thus, for this study, AMPH was hypothesized to have a biphasic effect, regardless of sex: increasing choice of the large reinforcer at doses in the range of 0.25 to 0.5 mg/kg (Floresco et al., 2008; van den Bergh et al., 2006; van Gaalen et al., 2006) and decreasing choice of the large reinforcer (increased impulsivity) at 1.0 mg/kg (Cardinal et al., 2000; Evenden and Ryan, 1996; Slezak and Anderson, 2009). FLU was hypothesized to decrease choice of the large reinforcer at doses in the range of 0.05 to 0.25 mg/kg (Cardinal et al., 2000; Floresco et al., 2008; Wade et al., 2000), regardless of sex, due to its competitive antagonism of AMPH binding to dopamine receptors.
2. Methods
2.1. Animals
Six male and six female Long-Evans rats, approximately 75 days of age, were purchased from Harlan (Indianapolis, IN). Rats were pair-housed in standard polycarbonate shoebox cages with pine bedding, in a temperature- and humidity-controlled room (22 °C, 40–55% humidity) on a 12-hour reverse light–dark cycle (lights off at 0830 h). Beginning 6 days after arrival, food access was restricted to reduce body weights to target weights of 85% of the free-feed body weights on that 6th day. Thereafter, the target weights were incrementally increased by 5–10 g every 2 weeks, with a maximum weight of 250±10 g for female rats and 350±10 g for male rats, to allow for continued growth. Food was restricted so that food reinforcement could be used in the DD task. Tap water was available ad libitum. Rats used in these procedures were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana–Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Research Council, Institute for Laboratory Animal Research, 2011).
2.2. Apparatus
Behavioral testing was conducted in 8 automated operant-conditioning chambers (Med Associates; St. Albans, VT) housed in sound attenuated cubicles, each ventilated by a fan. All operant-conditioning chambers contained 3 stimulus cue lights, each of which was positioned above one of 2 retractable response levers or the centrally located pellet trough. The levers, which were 6 cm above the floor, were located symmetrically on both sides of the pellet trough. An external pellet dispenser delivered 45 mg AIN-76A purified rodent food pellets (TestDiet; Richmond, IN). An 80 decibel white-noise generator masked extraneous sounds. A house light positioned at the rear of the chamber provided general illumination. The experimental contingencies were programmed using Medstate Notation behavioral programming language (Med Associates; St. Albans, VT).
2.3. Procedures
2.3.1. Initial lever press training
One daily session of operant testing was conducted 6 days per week. Beginning at approximately 19 weeks of age, an autoshaping program was used to familiarize the rats with the operant-conditioning chambers and to train them to begin pressing the response levers. Both levers were extended at the onset of the session. The illumination of the right cue light (above the right response lever) was programmed according to a fixed-time 3 min (FT3) schedule in which the light was illuminated for 15 s every 3 min, after which a food pellet was dispensed regardless of whether the lever was pressed during the allotted time period. If a rat pressed either lever while the cue light was illuminated, a pellet was delivered and the cue light was extinguished. After a total of 10 lever presses on either response lever, delivery of reinforcers became contingent on lever presses, with every lever press resulting in the delivery of a food pellet (i.e., a fixed-ratio 1 (FR1) contingency). The house light remained on for the entire session. Autoshaping sessions terminated after either 60 min had elapsed or 100 reinforcers were delivered. Criterion for advancement to the next phase of training was 95 to 100 lever presses within a single session. Autoshaping was completed in 1 session on average (range 1 to 2 sessions).
2.3.2. Fixed ratio training
The purpose of this phase of training was to elicit lever pressing in response to cue light illumination. At the onset of the session, one of the levers was extended at random, and the corresponding cue light was illuminated. Every response resulted in the delivery of a food pellet. After 5 lever presses, the lever retracted and the opposite lever was extended. The reinforcement lever alternated after every 5th lever press so the rat would not develop a side bias. The house light remained on for the entire session. Rats were required to earn 100 reinforcers across three consecutive sessions in order to move to the next training phase. All rats completed this phase in 3 sessions.
2.3.3. Delay-discounting training and testing
DD testing occurred in three phases: two training phases and a testing phase. The purpose of the first phase was to introduce alternating levers and time-controlled trials. This phase consisted of 60 trials/session with each trial lasting 40 s. Rats were required to initiate each trial with a nosepoke into the food trough when the center cue light was illuminated. Also, 10 s limited holds on nosepokes and lever presses were introduced, wherein a rat had 10 s to nosepoke and then 10 s to lever press. Failure to nosepoke or lever press resulted in the trial being counted as an omission. If the rat initiated a trial with a nosepoke, the center cue light was extinguished and a single response lever was extended at random. If the rat pressed the lever, a food pellet was immediately delivered, and the lever retracted. After the next nose poke, the opposite lever was extended. An extended lever was always accompanied by an illuminated cue light. For this and all subsequent phases of DD testing, the house light was on during the times the rat could nosepoke or lever press, but off for the remainder of the time. Progression to the next phase of testing required ≥40 successful responses, with at least 20 responses on the left lever and 20 responses on the right lever. All rats completed this phase in 1 to 2 sessions.
The second training phase consisted of two sessions where rats were introduced to large and small reinforcers. Half of the rats were assigned to the left lever as the large reinforcer lever, and the other half to the right lever. This phase also consisted of 60 trials/session (5 blocks of 12 trials), but the length of each trial was increased to 80 s. The first 2 trials of each block were forced-choice trials in which the large reinforcer lever (resulting in 4 pellets if pressed) and the small reinforcer lever (resulting in 1 pellet if pressed) were alternately introduced. Both levers were presented in the remaining 10 trials of each block, termed free-choice trials. There was no delay in delivery of either the small or large reinforcers in this phase.
Delays to the large reinforcer were introduced in the DD testing phase. Parameters of this phase remained the same as in the second training phase, but delays to the larger reinforcer increased across the 5 trial blocks, progressing from 0 s in the first block of 12 trials to 5, 10, 20, and then 40 s over the course of the 5 blocks of trials in each session. Importantly, if the rat chose the delayed reinforcer, the cue light above that lever remained illuminated until the delay was complete and delivery of the food pellets began. Percent larger-reinforcer choice was evaluated for the development of a sensitivity to delay, according to the criteria discussed below. Rats were tested for a total of 105 to 106 sessions before drug trials were initiated due to an unanticipated delay in the commencement of drug trials. Rats were tested 6 days/week for the first 50 sessions and then 5 days/week for the remainder of this phase when it became evident that the drug trials would be delayed.
2.3.4. Flupenthixol and amphetamine drug challenges
Drug trials began when rats were approximately 48 weeks old. Testing 6 days/week resumed at this point. The experimental parameters were identical to those in the DD testing phase. Drug doses were selected based on their previous use in similar behavioral experiments (Cardinal et al., 2000; Floresco et al., 2008; Sable et al., 2009). Both drugs were prepared on the day of administration and then protected from light to prevent photodecomposition. There were 4 doses of each drug, including a saline-only vehicle control that ensured that each rat served as its own control. cis-Flupenthixol (FLU; Sigma-Aldrich; St. Louis, MO) was prepared on injection days by dissolving the drug in 0.9% sterile saline to concentrations of 0 (vehicle), 0.05, 0.125, and 0.25 mg/mL for respective doses of 0, 0.05, 0.125, and 0.25 mg/kg. d-Amphetamine sulfate (AMPH; Sigma-Aldrich; St. Louis, MO) was similarly prepared on injection days by dissolving the drug in 0.9% sterile saline to concentrations of 0 (vehicle), 0.25, 0.5, and 1.0 mg/mL for respective doses of 0, 0.25, 0.5, and 1.0 mg/kg. Based on the pharmacokinetics of the drugs (Jorgensen et al., 1969; Kuhn and Schanberg, 1978), FLU and AMPH injections were given 30 and 10 min, respectively, before testing.
Two successive sets of drug injections were given: FLU then AMPH. Each dose within a set was administered a single time, with the order of dosing for each rat randomized within each set using a balanced Latin Square design. Drug injections were given on Tuesdays and Fridays, with 7 calendar days between the FLU injections and the beginning of the AMPH injections. After the drug trials, testing was continued for 5 additional days. Additional saline injections were given the day before the FLU injections began, the day before the AMPH injections began, and 5 days after the AMPH injections ended. Results from these additional saline-testing days were examined to assess whether baseline choice remained stable across the period of the drug challenges.
2.4. Data analysis
All statistical analyses were conducted using SPSS for Windows (version 20.0, SPSS Inc.; Chicago, IL) with statistical significance set at p<0.05. Repeated-measures ANOVAs were performed to test for differences in experimental measures, with the experimental factors of drug dose, delay to reinforcement, and session block as within-subjects factors and sex as a between-subjects factor. If sphericity assumptions were violated, a Greenhouse–Geisser correction was used to reduce the risk of a Type I error (Rogan et al., 1979). Analyses requiring such corrections are reported using the appropriately adjusted degrees of freedom rounded to the nearest integer. There were not enough residual degrees of freedom to use multivariate analysis to examine interactions between dose and delay, or between dose and block. In these instances, univariate results using a Greenhouse-Geisser correction to protect against Type I error were reported. When appropriate, one-way ANOVA followed by Tukey’s least significant difference (LSD) analyses were used to further analyze significant main effects and interactions. Data are reported as mean±SEM.
2.4.1. Sensitivity to delay during testing phase
Percent larger-reinforcer choice was defined as the number of lever presses for the larger, delayed reinforcer during free-choice trials divided by the total number of lever presses for both delayed and immediate reinforcers during free-choice trials. Discounting curves were obtained by plotting percent larger-reinforcer choice on the ordinate versus delay length in s on the abscissa of graphs. To examine for the development of sensitivity to delay in rats, percent larger-reinforcer choice for each delay was averaged across 5 consecutive daily sessions for each rat to form 5-session blocks. Percent larger-reinforcer choice was then analyzed via a 21 (block) x 5 (delay) x 2 (sex) repeated-measures ANOVA. Criteria for sensitivity to delay were met when there was either a significant main effect of delay or significant block x delay interaction, and when post-hoc analysis confirmed significantly smaller percent larger-reinforcer choice for one or more of the longer delays, indicating that a sensitivity to delay had developed (Mar and Robbins, 2007).
Percent larger-reinforcer choice from the subsequent drug challenge data was analyzed via a 3 (dose) x 5 (delay) x 2 (sex) repeated-measures ANOVA. Data from only 3 doses were analyzed because the highest FLU and AMPH doses were excluded (see Section 2.4.3).
2.4.2. Area under the curve
Area under the curve (AUC) was defined as the area under the discounting curve divided by the total area of the discounting graph (Slezak and Anderson, 2009), and thus was in the range of 0 to 100%. AUC calculations allow comparison between individuals or groups of percent larger-reinforcer choice across the delays in a testing session, with increases in AUC indicating increases in larger-reinforcer choice (Myerson et al., 2001). GraphPad Prism (version 5, GraphPad Software, Inc.; La Jolla, CA) was used to calculate AUCs from discounting curves. AUC from the drug challenges was analyzed via a 3 (dose) x 2 (sex) repeated-measures ANOVA.
2.4.3. Omissions and latencies
Omissions occurred when a rat either did not make a nose poke to initiate a trial (trial-initiation omission) or did not make a choice when the levers were extended (choice omission). Total omissions, comprised of both omission types across all trials (free- and forced-choice), were analyzed via a 2 (omission type) x 4 (dose) x 2 (sex) repeated-measures ANOVA. In these studies, the 0.25 mg/kg dose of FLU and the 1.0 mg/kg dose of AMPH caused ≥ 6 omissions in at least one block of free-choice trials in 7 and 3 rats, respectively. Furthermore, an entire block of free-choice trials was omitted by 3 rats at FLU 0.25 mg/kg and by 1 rat at AMPH 1.0 mg/kg. When a rat omitted several trials in a free-choice block, it was not clear whether the rat’s remaining choices accurately represented the rat’s choice or if other factors, such as diminished motivation to perform, had confounded the results. Because of this concern, data of the other dependent measures (percent larger-reinforcer choice, AUC, and latencies) at the FLU 0.25 mg/kg and AMPH 1.0 mg/kg doses were excluded from further analysis.
Trial-initiation latency was defined as the time at the beginning of a trial from when the cue light above the pellet trough was illuminated to when the rat poked its nose into the food trough to initiate the trial. Choice latency was defined as the time from when the levers extend to when the rat presses one of the levers. Latencies were analyzed via a 3 (dose) x 2 (sex) repeated-measures ANOVA.
3. Results
3.1. Delay-Discounting
ANOVA analysis of the 5-session blocks during the DD testing phase revealed a significant main effect of delay [F(2,16)=36.57, p<0.001] and a significant block x delay interaction [F(5,55)=3.56, p=0.006], but there was no main effect of sex and no sex by block or delay interactions [all F≤0.96, ns]. Post-hoc analysis comparing delays within each block confirmed that during the 2nd block of sessions (testing days 6–10) a sensitivity to delay developed as evidenced by a decrease in percent larger-reinforcer choice as delay to reinforcement increased. Once the sensitivity to delay developed, it was maintained throughout the remainder of the experiment, as all subsequent repeated-measures ANOVAs on percent larger-reinforcer choice were significant for the main effect of delay [all F≤20.96, all p<0.001].
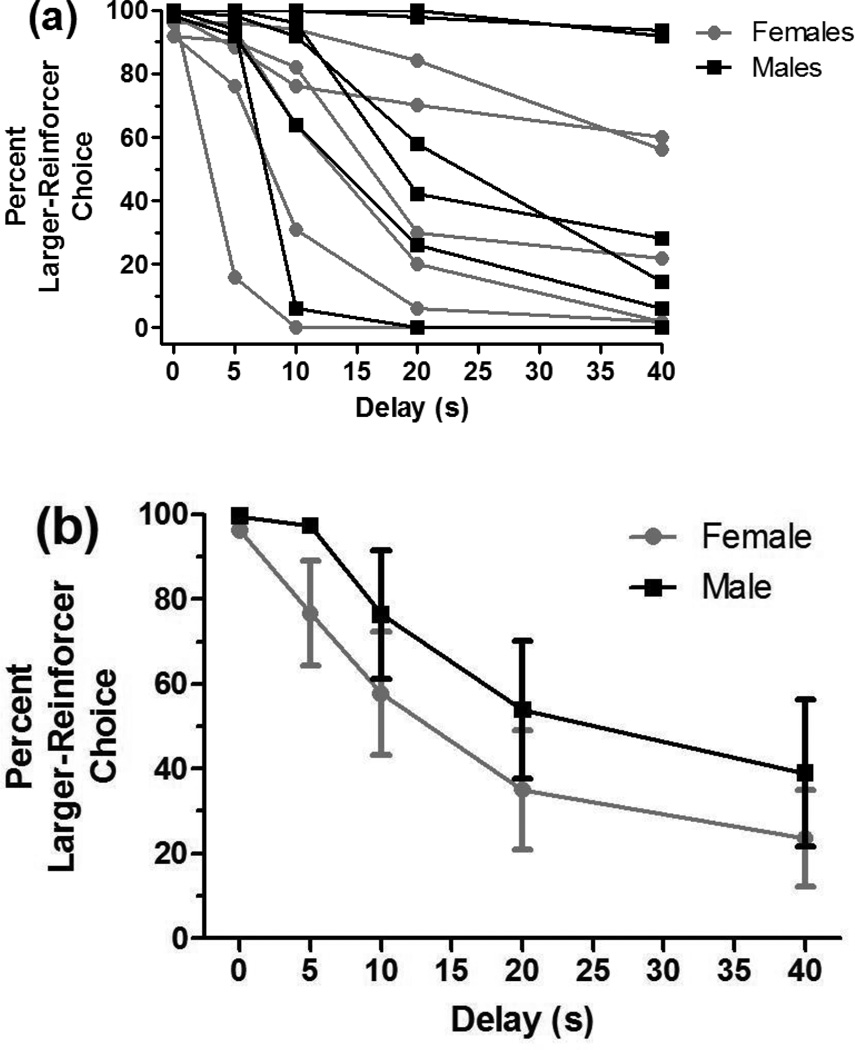
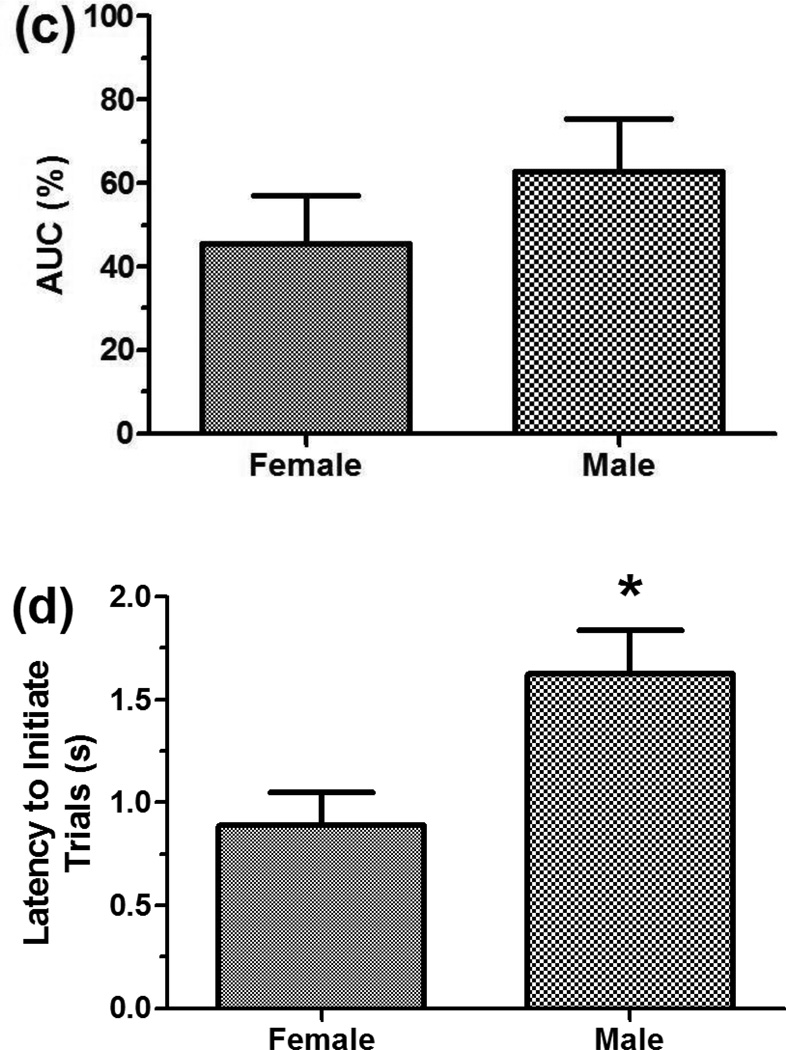
Examination of the final 5-session block of testing was undertaken to document baseline performance just prior to commencing the drug challenges (Figure 1a–e). With respect to percent larger-reinforcer choice, inspection of the individual discounting curves suggested that female rats showed a reduced choice for larger, delayed reinforcers (see Figure 1a); yet, analysis of the data did not reveal a significant main effect of sex [F(1,10)=1.66, ns] or a sex x delay interaction [F(4,40)=0.52, ns] (see Figure 1b). Analysis of AUC also did not reveal a significant main effect of sex on choice (45.50±11.45% for females vs. 62.69±12.71% in males, see Figure 1c; F(1,10)=1.01, ns). Analysis of the trial-initiation latency data revealed a main effect of sex [F(1,11)=7.65, p=0.02]. Females initiated trials faster than males (0.89±0.16 s vs. 1.63±0.21 s, see Figure 1d). No baseline sex differences were seen in choice latency (1.29±0.15 s for females vs. 0.98±0.11 s for males, see Figure 1e) or omissions (0.62±0.22 for all subjects). Because sex differences were seen in all measures except omissions during the drug trials, Figure 1 displays female and male performance separately for percent larger-reinforcer choice, AUC, initiation latency, and choice latency.
Fig. 1.
Performance during the last 5-session block of training. Although there was only a sex difference in trial-initiation latency, each dependent measure is shown separately for females and males to allow comparison to results during the drug trials, when sex differences were seen for all measures except omissions. (a) Discounting curves for individual rats. The larger, delayed reinforcer was chosen less frequently as the delay to the reinforcer increased. Choice did not differ between sexes. (b) Discounting curves of mean-female choice vs. mean-male choice across delays. (c) AUC for females vs. males. AUC did not differ between sexes. (d) Females initiated trials significantly faster than males. (e) There was no sex difference in the latency to choose between the small, immediate versus the large, delayed reinforcer. *p<0.05.
3.2. Flupenthixol challenge
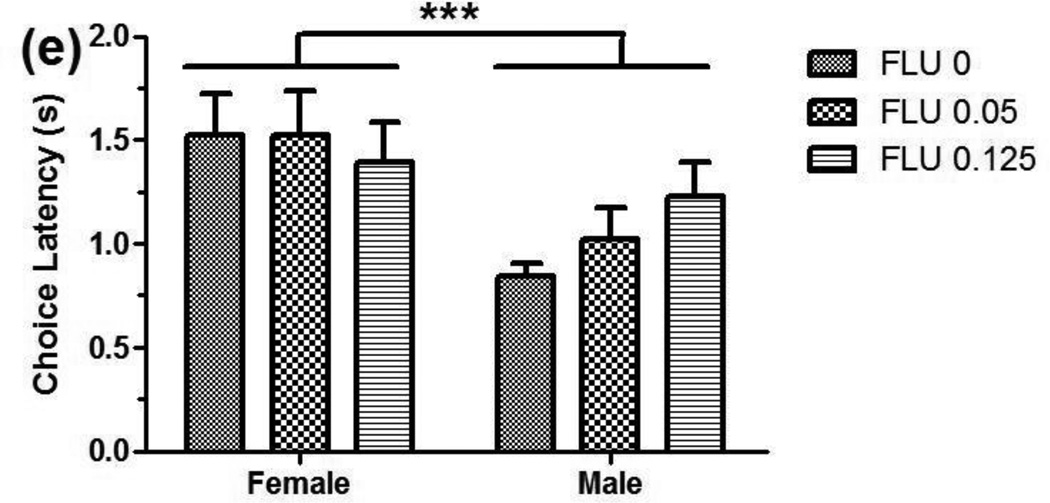
Analysis of the choice data obtained after administration of FLU did not yield significant main effects of dose [F(2,20)=2.06, ns] or sex [F(1,10)=1.42, ns] (see Figure 2a, individual data not shown), nor were the interactions of sex by dose [F(2,20)=0.79, ns] or dose by delay [F(3,33)=1.20, ns] significant. For AUC, the main effects of dose [F(2,20)=2.30, ns] and sex [F(1,10)=1.04, ns] (see Figure 2b), and the sex x dose interaction [F(2,20)=1.02, ns], also were not significant.
Fig. 2.
Flupenthixol drug trials. There was not a significant effect of FLU dose or sex on percent larger-reinforcer choice (a) or AUC (b). (c) Total omissions were increased at 0.25 mg/kg in both sexes. The effect was primarily due to an increase in trial-initiation omissions (see inset). (d) Females initiated trials significantly faster than males across doses. (e) Choice latency in females was longer across doses. ***p≤0.001.
Flupenthixol caused a dose-dependent increase in omissions (see Figure 2c), with omission type [F(1,10)=19.33, p=0.001], dose [F(1,11)=14.20, p=0.003], and the omission type x dose interaction [F(1,11)=11.23, p=0.005] all being significant. The main effect of sex was not significant [F(1,10)=0.80, ns]. Post-hoc analysis revealed that the effect was primarily due to an increase in trial-initiation omissions (p<0.001). Rats failed to initiate 10.7±2.7 trials (out of 60) at 0.25 mg/kg FLU compared to <1 trial at control and smaller FLU doses (see Figure 2c inset). Although there was also a significant dose-dependent increase in choice omissions (p=0.009), this is of questionable importance since choice omissions at all FLU doses were <1 per session.
For trial-initiation latency, the main effect of sex [F(1,10)=20.27, p=0.001] was significant, with females taking less time to initiate trials than males (see Figure 2d). The main effect of dose [F(2,20)=0.81, ns] and the dose x sex interaction [F(2,20)=0.54, ns] were not significant. For choice latency, a sex difference was detected in which females were slower to press levers [F(1,10)=6.57, p=0.028] (see Figure 2e). There was not a significant effect of dose on choice latency [F(2,20)=0.39, ns], nor was the sex x dose interaction significant [F(2,20)=1.56, ns].
3.3. Amphetamine challenge
Analysis of percent larger-reinforcer choice for AMPH doses revealed a significant main effect of sex [F(1,10)=8.48, p=0.016] and a significant sex by dose interaction [F(2,20)=4.98, p=0.018]. However, the main effect of dose [F(2,20)=2.03, ns] and the dose by delay interaction [F(4,44)=1.40, ns] were not significant. Inspection of the data of individual rats suggests that 0.5 mg/kg AMPH disrupted choice in females as compared to males, which was evidenced by < 80% percent larger-reinforcer choice at 0 s delay in 3 females (see Figure 3a, 3b for 0 vs. 0.5 mg/kg). Because a decrease in percent larger-reinforcer choice below 80% at 0 s delay can be for reasons other than a change in the ability to discriminate the difference in reinforcer magnitude, the analysis was repeated with the 3 female’s data excluded. The main effect of sex remained significant [F(1,7)=5.86, p=0.046], but the sex x dose interaction was not significant [F(2,14)=1.60, ns] (see Figure 3c). The main effect of dose and the dose by delay interaction remained non-significant. Visual inspection of Figures 3 a–c and comparison to Figure 1b suggests that AMPH decreased percent larger-reinforcer choice in the remaining females and increased percent larger-reinforcer choice at the longer delays in males.
Fig. 3.
Amphetamine drug trials. Discounting curves for individual rats at 0 (a) and 0.5 (b) mg/kg AMPH. Curves for the female rats for which the data was excluded from analysis are shown as dotted lines. (c) Discounting curves of mean-female choice vs. mean-male choice across delays. Although data from 3 females was excluded, percent larger-reinforcer choice for females was significantly less than that for males across delays (p=0.046), but there was not a main effect of dose. (d) AUC for all females (Female (6)) vs. males significantly differed at 0.5 mg/kg AMPH, but the sex difference was lost when 3 female’s data was excluded (Female (3)). (e) Rats had significantly more total trial omissions at 1.0 mg/kg AMPH than at other doses. (f) Trial-initiation latency did not differ with dose or between sexes, regardless of whether 3 female’s data was included (Female (6)) or excluded (Female (3)). (g) Choice latency was significantly longer in females across doses, even when the data from 3 females was excluded. *p<0.05, **p<0.01.
Analysis of AUC with all females included revealed a significant main effect of sex [F(1,10)=5.55, p=0.04] and a significant sex by dose interaction [F(2,20)=3.57, p=0.047], with post-hoc analysis revealing that AUC for females significantly differed from that of males at 0.5 mg/kg AMPH (see Figure 3d, Female (6) vs. Male). Exclusion of the 3 females resulted in a main effect of sex that approached significance [F(1,7)=5.04, p=0.06] (see Figure 3d, Female (3) vs. Male) and a non-significant sex by dose interaction [F(2,14)=2.39, ns].
Because a main effect of sex on percent larger-reinforcer choice was not seen during baseline testing or during the FLU trials, it was important to determine if the effect was due to AMPH or due to a change in baseline choice over the course of the drug trials. To assess this, percent larger-reinforcer choice was compared between sexes for the last 5-session block of testing prior to the drug trials and the saline-injection days before the FLU and AMPH trials and after all drug trials were completed. We did not observe any sex differences in percent larger-reinforcer choice on non-drug days before, during, or after the drug challenges [all F≤2.11, ns]. This indicates that the baseline choice of females relative to males did not change during the course of the drug trials.
Analysis of omissions of all rats revealed a significant main effect of dose [F(3,30)=4.64, p=0.009], with post-hoc analysis revealing that total omissions at 1.0 mg/kg were significantly greater than those at 0, 0.25, and 0.5 mg/kg (all p<0.029, see Figure 3e). Rats failed to initiate 7.3±3.3 trials (out of 60) at 1 mg/kg AMPH compared to <1 trial at control and smaller AMPH doses. The main effects of omission type [F(1,10)=0.68, ns] and sex F(1,10)=0.48, ns], and the type x dose interaction [F3,30)=0.90, ns], were not significant.
The difference in trial-initiation latency between sexes at baseline was not present during AMPH trials, regardless of whether the 3 females were excluded (see Figure 3f) ([F1,10)=1.17, ns] for all females, [F(1,7)=0.09, ns] with 3 females excluded). Yet, the sex difference for choice latency seen with FLU was present during AMPH challenges ([F(1,10)=10.64, p=0.009] for all females, [F(1,7)=10.71, p=0.014] with 3 females excluded). Females took longer to press levers (see Figure 3g). The main effect of AMPH dose and the sex x dose interaction were not significant, regardless of whether the 3 females were excluded.
4. Discussion
A sex difference in baseline percent larger, delayed-reinforcer choice of Long-Evans rats was not demonstrated under the experimental conditions of this study. AMPH administration resulted in a sex difference in choice by disrupting choice. This was evidenced by percent larger-reinforcer choice of half of the females being < 80% at 0.5 mg/kg AMPH. In contrast, AMPH tended to increase choice at the longer delays in males in the dose range of 0.25 to 0.5 mg/kg. However, FLU administration in the range of 0.05 to 0.125 mg/kg did not affect choice. Amphetamine administration also resulted in the loss of the difference between sexes for trial-initiation latency that was present both at baseline and with FLU administration.
4.1. Sex effect on percent larger-reinforcer choice with amphetamine administration
The findings suggest that in adult Long-Evans rats, females were more sensitive than males to the effects of AMPH on choice between immediate and delayed reinforcers. In other studies, greater behavioral response to AMPH in females was demonstrated using locomotor-activity assessment (Milesi-Halle et al., 2007; Simpson et al., 2012). In addition, estrogen, but not testosterone, increased dopamine release in the striatum and nucleus accumbens and enhanced performance on dopamine-mediated tasks in female rats relative to males (Becker, 1999). Imaging studies in humans also have demonstrated sex differences in dopamine release in response to AMPH (Munro et al., 2006) and in concurrent cognitive-task performance (Riccardi et al., 2006; Riccardi et al., 2011).
One reason for the sex difference in response to AMPH may have been that the biotransformation of AMPH to its metabolites was slower in female rats than in males, resulting in higher systemic and brain concentrations of AMPH in females, as has been demonstrated by other researchers (Becker et al., 1982; Meyer and Lytle, 1978). If the sex difference in AMPH biotransformation was true for the current study then the average response of females to AMPH would have been at a different point on the dose-response curve than that of males due to higher brain concentrations of AMPH in females. However, the effects of estrous cycle, which was not monitored in the females, may confound this interpretation.
Sex differences in basal extracellular dopamine concentrations in the striatum have been reported, with the dopamine concentrations in females varying with the stage of the estrous cycle from being the same as those of males to being greater (Xiao and Becker, 1994). Also, estrous cycle-dependent variations in striatal dopamine release in female rats in response to AMPH have also been reported (Becker and Cha, 1989). In addition, Fentie et al. (2004) showed that approximately 40% of female Long-Evans rats had either irregular estrous cycles or extended estrous phase at 12 months of age, which was the age of the rats during the AMPH trials in the current study. Although food deprivation is another factor that can impact estrous cycling, it has been shown that mild food restriction to 85% of free-fed body weight, such as in the current study, did not affect estrous cycling of Long-Evans rats (Tropp and Markus, 2001). The implications for the current study are that the effects of the estrous cycle on the response to AMPH could have accentuated the difference in response to AMPH between females and males.
Despite the sex difference in choice, we did not observe a significant main effect of AMPH dose in either sex. We had hypothesized that AMPH would induce a biphasic effect with an increase in percent larger-reinforcer choice at doses through 0.5 mg/kg and then a decrease in percent larger-reinforcer choice at 1.0 mg/kg. The hypothesis was based on reports of 0.25 mg/kg (Floresco et al., 2008; van den Bergh et al., 2006) and 0.5 mg/kg (van den Bergh et al., 2006; van Gaalen et al., 2006) AMPH increasing percent larger-reinforcer choice, and 1.0 mg/kg decreasing percent larger-reinforcer choice at one or more delays (Cardinal et al., 2000; Evenden and Ryan, 1996; Koffarnus et al., 2011; Slezak and Anderson, 2009), although the effects of 1.0 mg/kg AMPH are not consistent across studies. These prior studies suggest that the effects of AMPH on percent-larger reinforcer choice follows a non-monotonic dose-response curve, similar to the findings from other studies that have examined the effect of dopaminergic drugs on different behavioral measures (Cools and D'Esposito, 2011). The differences in percent larger-reinforcer choice between female and male rats in the current study suggest that a non-monotonic dose-response to AMPH was also seen in the current study, with females and males being at different points on the curve. This can be seen by inspecting the individual and group data of the dose-responses to 0.5 mg/kg as compared to 0 mg/kg AMPH: males tended to increase their percent larger-reinforcer choice at the longer delays while females began to be disrupted in their choice. This interpretation, that the same AMPH doses affect DD performance in females differently than in males, corresponds with what is known about sex differences in AMPH biotransformation, as discussed above.
Another factor to consider is environmental enrichment (EE), one form of which is housing with conspecifics. EE has been shown to influence the effect of AMPH on impulsive choice in rats performing an adjusting-delay procedure (Perry et al., 2008). However, it is difficult to extrapolate the findings from that study, in which EE rats housed in groups of 10 were compared to single-housed rats, to the current study in which rats were pair-housed and performed a DD procedure. Hellemans et al. (2005) showed that, after DD training was complete, the choice of pair-housed rats did not statistically differ from either single-housed rats or rats housed in groups of 12. Yet, the effect of EE on the response to AMPH was not examined in the study by Hellemans et al. (2005), so it is possible that pair-housing influenced the findings of the current study. Another interesting question to pose is whether sex interacts with EE, which has implications for the current study. However, no studies that address this question were located.
A limitation of this study is the small sample size (N=6 for each sex). This likely contributed to the inability to detect a significant difference between AMPH doses. Another factor that may have influenced the ability to detect a significant main effect of AMPH dose is the cue that was present during the delay until the larger reinforcer was delivered in trials with > 0 s delays. Cardinal et al. (2000) found that 1.0 mg/kg AMPH decreased percent larger-reinforcer choice when the delay was uncued, but that percent larger-reinforcer choice was unaffected if the delay was cued. In contrast, van Gaalen et al. (2006) determined that 1.0 mg/kg AMPH increased percent larger-reinforcer choice when the delay was cued, although that dose did not increase percent larger-reinforcer choice any more than 0.5 mg/kg AMPH did. Cueing the delay has been suggested to serve as a conditioned reinforcer that bridges the interval between choice of the delayed-reinforcer lever and the delivery of the reinforcer, with AMPH increasing the conditioned reinforcing properties of the cue (Cardinal et al., 2000). However, as the dose of AMPH continues to increase, sensitivity to reinforcement decreases (Evenden and Robbins, 1983), regardless of cueing, which results in diminished percent larger-reinforcer choice across all delays (Cardinal et al., 2000). In the current study, cueing the delay to reinforcement may have reduced the differences in percent larger-reinforcer choice between AMPH doses so that it was difficult to distinguish a dose effect. If so, repeating the study with uncued delays may reveal a dose effect of AMPH.
Another potential drawback of this study design is that the effects of each dose of AMPH and FLU on choice were determined only once, and FLU was tested before AMPH. Because of the evidence that single or repeated doses of AMPH can induce long-lasting alterations in dopaminergic signaling (Vanderschuren et al., 1999; Vanderschuren and Kalivas, 2000), the concern when planning this study was that AMPH might influence the outcome of subsequent AMPH or FLU drug trials if repeated doses of each drug were administered in a completely randomized manner. Although a study by Stanis et al. (2008) suggests that repeated AMPH pretreatment did not alter the effect of subsequent AMPH administration on percent larger-reinforcer choice, that study greatly differs from the current one in that much higher AMPH doses were used (3.0 mg/kg), AMPH pretreatment occurred prior to training on DD, and AMPH was reintroduced over 50 days after the last pretreatment injection. Regarding FLU, experimental evidence suggests that effects such as dopamine receptor upregulation do not develop until at least 3 weeks of daily FLU administration in rodents (De La Concha et al., 1991; Hess et al., 1988). Hence, it was decided to administer the set of FLU doses before the set of AMPH doses. Still, the possibilities that the drug effects may have been less reliable due to only one injection of each dose being given, and that the order of FLU then AMPH injections may have influenced the findings, can not be completely discounted.
4.2. Flupenthixol did not affect percent larger-reinforcer choice
Neither an effect of dose nor sex on percent larger-reinforcer choice was seen with FLU once the data from 0.25 mg/kg was excluded. The findings for dose of FLU were similar to those seen in other DD studies, which only tested males. Cardinal et al. (2000) reported that FLU decreased percent larger-reinforcer choice at 0.25 mg/kg when the delay to reinforcement was cued and at 0.125 mg/kg when the delay was uncued. Floresco et al. (2008) reported that 0.25 mg/kg FLU decreased percent larger-reinforcer choice, but lower doses were not tested. Wade et al. (2000) found a similar effect for FLU at 0.05 and 0.1 mg/kg, but this with an adjusting-amount version of the DD task, which differs from the DD methodology in the current study. It has been argued that D1/D2 receptor antagonism produced by FLU diminishes the value of conditioned reinforcers (Wade et al., 2000), which is opposite the effect of AMPH. However, in the current study, the number of trial omissions was problematic at 0.25 mg/kg FLU, which is the dose where an effect of FLU on percent larger-reinforcer choice would have been most likely to be evident. It appears that, under the conditions of this experiment, the FLU doses we chose were outside of the relatively small margin between doses at which a significant effect on choice without a significant increase in trial omissions might have been seen. Based on the findings of Cardinal et al. (2000), it is possible that a dose effect may be more readily detected with the doses used in the current study if an uncued version of DD were used.
4.3. Differences in trial-initiation and choice latencies
There was a baseline difference in the latency to initiate trials, with females initiating faster than males. This difference remained with FLU administration. However, a difference in trial initiation was not observed following AMPH administration. In regards to the baseline difference, there have been relatively few studies using signal-detection tasks to assess potential for sex differences in latency to respond to the signal. In the current DD paradigm, initiating a trial involved nosepoking in the food trough when both a cue light and the houselight concurrently illuminated. Differences between sexes on this measure may have been due to differential sensitivity to detecting this cue. In one study that employed the 5-choice serial reaction time task in Long-Evans rats it was reported that there was not a sex difference in the latency to respond after a cue light illuminated (Bayless et al., 2012); no other studies were identified that reported latencies for both sexes. It is possible that the baseline sex difference seen in the current study was more evident due to an aspect of the DD experimental paradigm that was not present in the 5-choice task.
There was no significant baseline difference between the sexes on choice latency, although the graph suggests a trend toward females having a longer latency to lever press. Sex differences became significant with both FLU and AMPH administration. Under the effect of either drug, females took longer to lever press than males. This may suggest a sex difference in sensitivity to dopaminergic drugs, particularly AMPH, but also FLU, with this measure.
5. Conclusions
This study found no sex differences in baseline performance of a DD task in Long-Evans rats, similar to the findings of a recent meta-analysis of DD in humans (Cross et al., 2011). Yet AMPH resulted in sex differences on percent larger-reinforcer choice and choice latency, which parallels sex-based differences in performance in response to AMPH in humans (Riccardi et al., 2006; Riccardi et al., 2011).
These findings further reinforce the argument of Winstanley et al. (2011) that DD is a valuable experimental paradigm for exploring the effects of dopaminergic drugs on impulsive choice, with experimental findings being relatively consistent between rats and humans. Yet, sex differences in DD performance and in response to dopaminergic drugs in experimental species have rarely been reported. If findings from preclinical studies are to be successfully extrapolated to human populations, then investigating and reporting sex differences that occur in the DD model is important. Furthermore, evaluating sex differences, both in tasks that examine aspects of response inhibition other than impulsive choice, and in response to more selective dopaminergic agonists and antagonists, are also important future steps to take.
Highlights.
There was no sex difference in baseline impulsive choice in adult Long-Evans rats.
d-Amphetamine disrupted choice in female rats.
Assessing sex differences when utilizing animal models is important.
Acknowledgements
This work was funded by National Institute of Environmental Health Sciences K08 ES017045 to PAE and R01 ES015687 to SLS, and an operating grant from the Canadian Institutes of Health Research to SBF. The authors thank Mindy Howe and the undergraduate research assistants in our laboratory for the daily care and behavioral testing of the rats. We thank Leonard Green of Washington University in St. Louis and Joshua Gulley of the University of Illinois for assistance in designing this version of the DD task and the pharmacologic trials portion of the study, respectively. We also thank Mikhal Koffarnus of the Virginia Tech Carilion Research Institute for assistance with analysis of the experimental findings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Seta DD, Dessi-Fulgheri F, Farabollini F, Laviola G. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environ Health Perspect. 2003;111:395–401. doi: 10.1289/ehp.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Darling JS, Stout WJ, Daniel JM. Sex differences in attentional processes in adult rats as measured by performance on the 5-choice serial reaction time task. Behav Brain Res. 2012;235:48–54. doi: 10.1016/j.bbr.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E. Impulsivity and vulnerability to psychopathology. In: Beauchaine TP, Hinshaw SP, editors. Child and Adolescent Psychopathology. Hoboken, NJ: John Wiley & Sons, Inc; 2008. pp. 129–156. [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137:97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- De La Concha A, McKie J, Hodgkinson S, Mankoo BS, Gurling HM. Stereospecific effect of flupenthixol on neuroreceptor gene expression. Brain Res Mol Brain Res. 1991;10:123–127. doi: 10.1016/0169-328x(91)90102-4. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL. Sex differences in dopamine- and vesicular monoamine-transporter functions. Ann N Y Acad Sci. 2008;1139:140–150. doi: 10.1196/annals.1432.010. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Robbins TW. Dissociable effects of d-amphetamine, chlordiazepoxide and alpha-flupenthixol on choice and rate measures of reinforcement in the rat. Psychopharmacology (Berl) 1983;79:180–186. doi: 10.1007/BF00427808. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fentie IH, Greenwood MM, Wyss JM, Clark JT. Age-related decreases in gonadal hormones in Long-Evans rats: relationship to rise in arterial pressure. Endocrine. 2004;25:15–22. doi: 10.1385/ENDO:25:1:15. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Garcia A, Kirkpatrick K. Impulsive choice behavior in four strains of rats: Evaluation of possible models of Attention-Deficit/Hyperactivity Disorder. Behav Brain Res. 2012;238C:10–22. doi: 10.1016/j.bbr.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res. 2005;159:207–220. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Hess EJ, Norman AB, Creese I. Chronic treatment with dopamine receptor antagonists: behavioral and pharmacologic effects on D1 and D2 dopamine receptors. J Neurosci. 1988;8:2361–2370. doi: 10.1523/JNEUROSCI.08-07-02361.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Krebs CA, Anderson KG. Strain differences in delay discounting between Lewis and Fischer 344 rats at baseline and following acute and chronic administration of d-amphetamine. Pharmacol Biochem Behav. 2012;101:403–416. doi: 10.1016/j.pbb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttel J, Arnt J, van den Berghe M. Selective dopamine D1 and D2 receptor antagonists. Psychopharmacol Ser. 1989;7:109–122. doi: 10.1007/978-3-642-74430-3_12. [DOI] [PubMed] [Google Scholar]
- Jorgensen A, Hansen V, Larsen UD, Khan AR. Metabolism, distribution and excretion of flupenthixol. Acta Pharmacol Toxicol (Copenh) 1969;27:301–313. doi: 10.1111/j.1600-0773.1969.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot S, van den Bos R, Adriani W, Laviola G. Gender differences in delay-discounting under mild food restriction. Behav Brain Res. 2009;200:134–143. doi: 10.1016/j.bbr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM. Metabolism of amphetamine after acute and chronic administration to the rat. J Pharmacol Exp Ther. 1978;207:544–554. [PubMed] [Google Scholar]
- Mar AC, Robbins TW. Delay discounting and impulsive choice in the rat. Curr Protoc Neurosci. 2007 doi: 10.1002/0471142301.ns0822s39. Chapter 8:Unit 8.22. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Jr, Lytle LD. Sex related differences in the physiological disposition of amphetamine and its metabolites in the rat. Proc West Pharmacol Soc. 1978;21:313–316. [PubMed] [Google Scholar]
- Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, D.C: National Academies Press; 2011. [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Paule MG, Green L, Myerson J, Alvarado M, Bachevalier J, Schneider JS, et al. Behavioral toxicology of cognition: extrapolation from experimental animal models to humans: behavioral toxicology symposium overview. Neurotoxicol Teratol. 2012;34:263–273. doi: 10.1016/j.ntt.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari MS, Schmidt D, et al. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [(18)F]fallypride. Synapse. 2011;65:99–102. doi: 10.1002/syn.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, et al. Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006;163:1639–1641. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Rogan JC, Keselman HJ, Mendoza JL. Analysis of repeated measurements. Br J Math Stat Psych. 1979;32:269–286. [Google Scholar]
- Sable HJ, Eubig PA, Powers BE, Wang VC, Schantz SL. Developmental exposure to PCBs and/or MeHg: effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotoxicol Teratol. 2009;31:149–158. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Ryan C, Curley A, Mulcaire J, Kelly JP. Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:227–236. doi: 10.1016/j.pnpbp.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behav Pharmacol. 2009;20:424–436. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Marquez Avila H, White MD, Gulley JM. Dissociation between long-lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay-discounting task. Psychopharmacology (Berl) 2008;199:539–548. doi: 10.1007/s00213-008-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropp J, Markus EJ. Effects of mild food deprivation on the estrous cycle of rats. Physiol Behav. 2001;73:553–559. doi: 10.1016/s0031-9384(01)00487-5. [DOI] [PubMed] [Google Scholar]
- van den Bergh FS, Bloemarts E, Groenink L, Olivier B, Oosting RS. Delay aversion: effects of 7-OH-DPAT, 5-HT1A/1B-receptor stimulation and D-cycloserine. Pharmacol Biochem Behav. 2006;85:736–743. doi: 10.1016/j.pbb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ, Schoffelmeer AN. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Fregoso T, Miranda A, De La Cruz F, Flores G. Strain differences of dopamine receptor levels and dopamine related behaviors in rats. Brain Res Bull. 2005;65:339–347. doi: 10.1016/j.brainresbull.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]