Abstract
The key signaling networks regulating mammary stem cells are poorly defined. The leucine-rich repeat containing G-protein coupled receptor (Lgr) family has been implicated in intestinal, gastric, and epidermal stem cell functions. We investigated whether Lgr4 functions in mammary gland development and mammary stem cells. We found that Lgr4−/− mice had delayed ductal development, fewer terminal end buds (TEBs), and decreased side-branching. Crucially, the mammary stem cell repopulation capacity was severely impaired. Mammospheres from Lgr4−/− mice showed decreased Wnt signaling. Wnt3a treatment prevented the adverse effects of Lgr4 loss on organoid formation. ChIP analysis indicated that Sox2 expression was controlled by the Lgr4/Wnt/β-catenin/Lef1 pathway. Importantly, Sox2 overexpression restored the in vivo mammary regeneration potential of Lgr4−/− mammary stem cells. Therefore, Lgr4 activates Sox2 to regulate mammary development and stem cell functions via Wnt/β-catenin/Lef1.
Keywords: Lgr4, Gpr48, Mammary stem cell, Sox2, Wnt signaling
Introduction
Deciphering the complex signal transduction pathways regulating stem cell activity remains an ongoing challenge. Key pathways controlling mammary gland stem cells include the Wnt, Notch, and Hedgehog pathways 1-3. However, crucial components of these pathways and how they determine mammary stem cell behavior remain unexplored.
The leucine-rich repeat containing G protein-coupled receptors (LGRs) 4, 5, and 6 are vital for stem cell maintenance in several systems. Lgr5 marks stem cells in the intestine 4, 5, the stomach 6, and the hair follicle 7. Likewise, Lgr6 is a stem cell marker in the skin 8. Lgr4 (also called GPR48) is required for intestinal stem cell maintenance ex vivo 9, 10. Lgr4 loss causes developmental defects in multiple organs, including gall bladder 11, male reproductive tracts 12-14, and hair follicle development 15, as well as reduced embryonic growth 16. Recently, three separate groups have identified members of the R-spondin family of Wnt potentiators as ligands for LGR4-6, directly linking these receptors to stem cell signaling 10, 17, 18. Since disruption of Lgr4 causes developmental defects in multiple organs, a role in mammary stem cell regulation is likely; however, the function of Lgr4 in mammary stem cells has not been defined.
We have previously described the roles of Lgr4 in early eye development 19, 20, midgestational erythropoiesis 21, osteoblast differentiation and postnatal bone modeling 22, spermatogenesis 23 and Dextran Sodium Sulfate-induced Inflammatory Bowel Disease 24. Here, we report that the loss of Lgr4 compromised mammary development and stem cell self-renewal and function. Lgr4−/− mouse mammary glands have impaired terminal end buds (TEBs) and delayed ductal tree development. Defects in the basal epithelium were observed in both ducts and TEBs in Lgr4−/− mice. Crucially, the mammary stem cell pool was reduced in Lgr4−/− mice, and Lgr4−/− mammary stem cells had diminished regenerative potential. Finally, we discover Sox2 as a key transcription factor regulated by Lgr4 signaling in mammary stem cells.
Materials and Methods
Mouse Strains
Lgr4 homozygous mutant mice (Lgr4−/−) were generated by microinjecting gene trap-mutated Lgr4 embryonic stem cells into blastocyts of C57BL/6 mice 20. Mice were backcrossed to C57BL/6 for 5 to 10 generations or to FVB/NJ for 6 to 12 generations. Experimental procedures were approved by the animal care and use committee of Texas A&M University.
BrdU Incorporation, Tissue Havest, Histology, and Whole-mount
Two hours before euthanasia, BrdU (100 μg/g body weight, Sigma) was injected intraperitoneally into some mice to assay cell proliferation. Mammary glands were excised and processed as described in 25, with the following changes: Formalin fixation for histology was for 4 hours to overnight at 4°C. Fixation for whole mount was done in acetic acid/ethanol for 2-4 hours at room temperature, and staining was with either carmine alum or Neutral Red. After taking whole-mount pictures, tissues were embedded in paraffin for sectioning and analysis. Whole-gland β-galactosidase staining was performed as described 26. A minimum of three animals per genotype and time point were analyzed.
Immunohistochemistry, Immunofluorescence, and TUNEL assay
Immunohistochemistry and immunofluorescence were performed as described previously 25. MOM, Vectastain Elite ABC Rabbit, or Rat Kits (Vector Laboratories, cat no. PK-2000, PK-6101, PK-6104) were used according to manufacturer instructions. Apoptotic cells were determined by the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI). DAPI counterstain was used to visualize nuclei. TUNEL-positive cells were scored in at least 5 fields per section, and at least 1000 cells were counted for each section.
Isolation of Primary Mammary Epithelial Cells and Primary Mammary Organoids
Primary mammary epithelial cells were isolated from 12- to 16- week-old virgin female FVB, C57BL/6 mice as described 27, with the following modifications: initial digestion in DMEM/F-12 medium containing 0.2% FBS, 30,000 U/ml collagenase A (Worthington, Lakewood, NJ) and 1,000 U/ml hyaluronidase (Sigma, St. Louis, MO) for 1-2 hours followed by 5-10 minute digestion in 0.25% Trypsin-EDTA. Primary mammary organoid preparation was performed as previously described 28.
Mammosphere Culture
Mammosphere culture was performed as described in Dontu et al. 29, except that the culture medium (Serum-free DMEM/F12 supplemented with B27 (Invitrogen), 20ng/ml EGF (Invitrogen) and 20ng/ml bFGF (R&D systems) contained 1% methyl cellulose to prevent cell aggregation. Briefly, primary Mammary Epithelial Cells (MECs) were isolated as described above, and single cells were plated at 10,000 cells per well in 6-well, ultra-low attachment dishes. After culturing for 7-10 days mammospheres with diameter ≥50μm were counted. Mammospheres were then harvested for serial passage using 70 μm cell strainers, dissociated to single cells with trypsin, and 5000 cells/well were plated in a 6-well or 24-well ultra-low attachment plate and cultured for 10 days before counting and passage.
In vitro Branching Morphogenesis Assays (mammary organoid culture)
Organoid assays were performed in 24-well plates. Three-dimensional primary cultures were generated as described 30. Briefly, we embedded 5,000 MECs in 50 μl of growth factor-reduced Matrigel. Fragment organoids were obtained by embedding purified epithelial fragments into Matrigel, and stimulated with 2.5nM bFGF (R&D systems) according to 28.
Mammary Transplantation and Analysis
100-10,000 primary mammary epithelial cells from 13-to 15-week-old Lgr4−/− and wild-type glands were suspended in a 1:1 mix of Matrigel and DMEM/F12 medium and injected into cleared mammary fat pads of 3-week-old females. After 2 months, the fat pads were dissected, processed, and stained with Neutral Red as described above, then analyzed to evaluate the extent of mammary outgrowth. A minimum cutoff of 5% fat pad filling was used to indicate positive ductal outgrowths.
Cell Labeling, Flow Cytometry
Mammary epithelial cells were isolated as described above. We stained Lgr4−/− and wild-type MECs with lineage antibodies (biotinylated CD45/CD31/TER119 and Streptavidin-APC), CD24-PE, and CD49f-FITC on ice for 20 min in HBSS with 5% FBS, and established the FACS gating and positioning of the MRU, CFC and MYO populations 27; gates were set according to the isotype control antibody labeled with the corresponding fluorochromes. All cell sorts were performed using FACSAria (Beckton Dickinson).
Quantitative real-time PCR analysis
Mammosphere culture was performed as described above, and total RNA was extracted using the Rneasy Mini Kit (Qiagen, Valencia, CA). Following reverse transcription to generate single-stranded cDNA, quantitative PCR was performed using the SYBR Green-based system on the ABI 7900HT according to the manufacturer's instructions (Applied Biosystems, Carlsbad, CA). ΔΔCt values were calculated. 18s as a reference was used for normalization. Primers are described in detail in Supplementary Table S1.
Human SOX2 Reporter Constructs
The Human SOX2 proximal promoter region was obtained by PCR using human genomic DNA, then subcloned to pGL3-Basic Vector (Promega). For generation of the promoter region, forward primer: 5′-TTGGAGCTCGTGAGTTTGACAGTAACAGGCTAGG-3′ and reverse primer: 5′-TGCACATCTCAGCCACGTAGAAACCTTTGTATC-3′ were used. The constructs were verified by sequencing.
Cell Transfection and Luciferase Assays
Human MCF10A cells were maintained in DMEM/F12 supplemented with 15 mM hepes buffer, 5% horse serum, 10 ug/ml insulin, 20 ng/ml EGF, 100 ng/ml cholera toxin, 0.5 ug/ml hydrocortisone, 100 μg/ml penicillin and 100 μg/ml streptomycin. For the luciferase reporter assay, 48 hours after plasmid transfection, cells were lysed and harvested in Reporter Lysis Buffer (Promega). Extracts were normalized by β-galactosidase activity using the Galacto-Light plus β-Galactosidase Reporter Gene Assay System (Promega).
ChIP Assays
The chromatin immunoprecipitation (ChIP) assay was performed on lysates of MECs derived from Lgr4+/+ or Lgr4−/− mice grown in organoid culture using the Chip Assay kit (Cell Signaling), according to the manufacturer's instructions. The regions of LEF1 sites of Sox2 in both human and mouse species were amplified from the immunoprecipitated chromatin by using the primers in Table S1.
Lentivirus Infection
The mouse Sox2 lentivirus plasmid was constructed in pLVX-IRES-ZsGReen vector (Clonetech). Lentivirus was produced by transient transfection in 293T cells. Mammary cells were isolated from 12- to 16-week-old virgin female glands as described above, followed by plating for 30 min to deplete stromal cells. The suspended MECs were collected and plated in 5% FBS Epicult medium (Stem Cell Technologies) with virus. 24hr after infection, cells were washed twice with DMEM/F12 medium. Rescue by overexpression of Sox2 was tested by in vitro colony assay and in vivo transplantation assay.
In vitro colony assay
Primary MECs were mixed with 100 μl of Matrigel mix (a 1:1 mixture of 5% FBS Epicult medium (Stem Cell Technologies) and Matrigel) and plated into Matrigel-coated 8-well chambers. After 8-10 days, Matrigel culture was fixed in 4% paraformaldehyde and photographed.
Antibodies
Primary antibodies used in these experiments were against: Lgr4 (Abcam), keratin 8 (TROMA1, DSHB), keratin 5 (Covance), keratin 14 (Covance), Ki67 (Novacastra), Lgr6 (Epitomics), BrdU (Beckon Dickinson), LEF1 (Cell Signaling Technologies); CD45/CD31/TER119 (Ebioscience), Streptavidin-APC, CD24-PE, and CD49-FITC (BD Biosciences). Secondary antibodies were from Cell Signaling Technologies except for secondary antibodies for FACS (BD Biosciences).
Statistical analysis
Apart from mammary transplantation experiments, all comparisons were analyzed using two-tailed student T tests. p-values of 0.05 or less were defined as statistically significant. Error bars represent standard deviation. Limiting dilution analysis was performed according to 31 to estimate mammary stem cell frequency, and the Sox2 rescue of mammary outgrowth potential was analyzed using Fisher's exact test.
Results
Lgr4 loss impairs mammary development
To explore the role of Lgr4 in the mammary gland, we used Lgr4 mutant mice in which a gene-trapping cassette encoding the β-galactosidase (β-gal) protein was inserted into the first intron of the Lgr4 gene 20. We assessed β-gal activity in Lgr4+/− mammary glands to track Lgr4 expression. In 6-week-old virgin mice, β-gal activity was detected in most terminal end bud (TEB) cap cells and in some body cells (Fig 1A), as well as in mammary duct basal cells (Fig 1B). Using immunohistochemistry staining to detect Lgr4 in age-matched wild-type mice, we confirmed the Lgr4 expression pattern reported by β-gal (Fig 1C-D). In addition, we detected occasional staining of luminal epithelial cells (Fig 1D). In mature ducts of 13-week-old virgin mice, β-gal activity and anti-Lgr4 immunohistochemical staining was limited to the basal layer (Supplemental Fig S1), where mammary stem cells have been suggested to reside 27, 32. The Lgr4 family members Lgr5 and Lgr6 are also receptors for R-spondins. Lgr5 has been reported in rare basal mammary epithelial cells33, 34, a pattern we also observed using lacZ staining of Lgr5+/− mice (data not shown). Lgr6 is expressed in occasional clusters of luminal epithelial cells; we did not observe Lgr6 expression in basal cells, and the Lgr6 expression pattern was unaffected by Lgr4 genotype (Supplemental Fig S2).
Figure 1. Lgr4−/− mice have defects in ductal development and terminal end buds, and reduced cell proliferation.
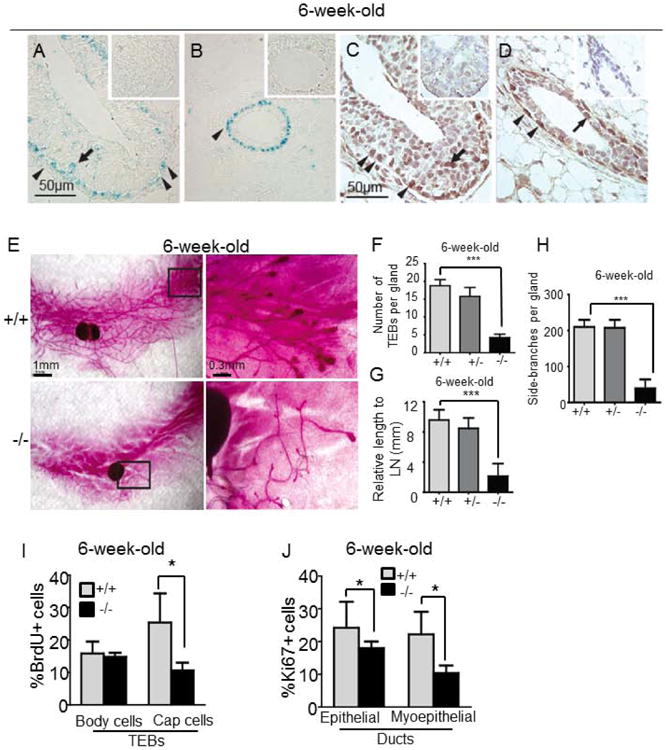
(A-B) β-galactosidase staining in wild-type (insets) and Lgr4+/− mammary ducts (A) and TEBs (B). (C-D) Immunohistochemical staining for Lgr4 in TEBs (C) or ducts (D) of 6-week-old Lgr4+/+ or Lgr4−/− (insets) mammary glands. Body cells (arrow, TEB), cap cells (arrowhead, TEB) and myoepithelial cells (arrowhead, ducts) are indicated.
(E) Mammary gland whole mounts. Right: magnification of boxed areas. (F-H) Average number of TEBs (F, ***p=0.0016), duct length relative to lymph node (G, *** p<0.001), and side branches per gland (H, ***p=0.006) in indicated whole mounts (n=5).
(I) BrdU incorporation in TEBs (*p=0.028, n=5).
(J) Percentage of Ki67+ cells in mammary gland ducts (*p=0.04, n=5).
The mammary glands of 6-week old Lgr4−/− mice exhibited marked developmental defects. The number of TEBs was reduced by 41% in Lgr4−/− mice compared to wild-type littermates (p=0.0016, n=5; Fig 1E-F). Ductal elongation and branching morphogenesis were also impaired: 6-week-old wild-type ducts extended past the inguinal lymph node into the distal fat pad, while Lgr4−/− ducts ended approximately at the lymph node (p<0.001, n=5; Fig 1E, G). Furthermore, the number of side branches per gland was significantly reduced (p=0.0006, n=5; Fig 1E, H). The decrease in side-branching persisted into adulthood (Supplemental Fig S1C-F). Heterozygous mice displayed normal ductal outgrowth similar to wild-type (Fig 1F-H), suggesting that one copy of Lgr4 is sufficient for normal mammary development.
To investigate potential causes of the reduced TEBs and delayed ductal elongation, we examined cell proliferation and apoptosis in 6-week old wild type or Lgr4−/− mammary glands. While body cell proliferation was similar to wild-type, cap cell proliferation in Lgr4−/− TEBs was markedly decreased (Fig 1I and Supplemental Fig S3A). In addition, Lgr4−/− TEBs displayed increased apoptosis (Supplemental Fig S4). Finally, while luminal cell proliferation in mature Lgr4−/− ducts was similar to wild-type, basal Lgr4−/− cells had a sharp decrease in proliferation (25±10% versus 10±5%, p=0.028, n=5; Fig 1J and Supplemental Fig S3B), suggesting a potential effect of Lgr4 loss on mammary stem cell and basal progenitor cell proliferation.
Lgr4 loss impairs lineage specification, branching morphogenesis, and basal cell colony formation
To test whether these developmental defects resulted from impaired stem cell functions and altered cellular hierarchy and lineage specification, we first co-stained cell lineage markers cytokeratin 14 (K14) and cytokeratin 8 (K8) in 4-6-week-old Lgr4−/− mammary epithelia. Lgr4+/+ TEBs showed a distinct, organized K5+ cap cell layer (proposed to contain the mammary stem cells) surrounding well-ordered body cells; however approximately 37% of Lgr4−/− TEBs exhibited a more disorganized body cell layer occasionally breaking through a discontinuous cap cell layer to contact the stroma (n=8, Fig 2A, arrowheads indicate gaps). Interestingly, a large number of Lgr4−/− ducts contained cells that were double positive for K14 and K8, whereas no double-positive cells were detected in wild-type ducts of the same age (p=0.026, n=6; Fig 2B). These data suggest a role for Lgr4 in lineage specification and ductal morphogenesis. In accord with this suggestion, Lgr4−/− mammary ducts occasionally exhibited multiple layers of luminal cells as well as mis-localization of basal cells in the lumen (Supplemental Fig S5A). These defects in ductal morphogenesis and lineage specification were also observed in outgrowths of Lgr4−/− mammary epithelial cells (MECs) transplanted into cleared mammary fat pads (Supplemental Fig S5B), indicating that these Lgr4 functions are intrinsic to the mammary epithelium per se. Of note, some of the Lgr4−/− ductal growths lacked a basal cell layer (Supplemental Fig S5B right panel), further confirming a crucial role of Lgr4 in the mammary basal compartment.
Figure 2. Lgr4 loss disrupts multipotent mammary epithelial progenitor differentiation and branching morphogenesis.

(A) Mammary TEB co-immunofluorescence (K5/K8, n=8). Note cap cell layer gaps (arrowhead).
(B) Mammary duct K14/K8 co-immunofluorescence. Arrowheads indicate double positive cells. Double positive cells graphed (*p=0.026, n=6).
(C) Representative organoid images. (D) Proportion of branched organoids. (**p=0.012, n=4) (E) Branches per organoid. (***p<0.0001, n=3, > 100 organoids/genotype)
We then examined the colony forming abilities of cells expressing Lgr4 compared to cells not expressing Lgr4 from the same mammary gland. According to Stingl et al.27, the mammary stem cell-enriched cell population generates small, solid colonies when grown in Matrigel, whereas the luminal-enriched cell population forms hollow, acinar-like colonies. When we FACS sorted mammary cells from Lgr4+/− mice on the basis of lacZ staining, we found an increase in small, solid colony formation and decreased acinar-like colony formation in the lacZ+ (i.e. Lgr4+) population (Supplemental Figure S6). This further supports a role for Lgr4 in basal mammary cells and mammary stem cells.
Next we used a mammary organotypic culture model to study the role of Lgr4 in regulating mammary stem cell functional differentiation 28, 35, 36. Lgr4−/− MECs generated much fewer branching organoids than wild-type controls (p=0.0142, n=4, Fig 2C-D), and the average number of branches was one-third of that of wild-type controls (p<0.0001; Fig 2E). These in vivo branching morphogenesis defects further suggest a role for Lgr4 in regulating mammary stem cells. In addition, wild-type organoids showed an outer layer of K14+ cells surrounding an inner population of K8+ luminal cells, with the appearance of a hollow lumen; in contrast, Lgr4−/− organoids had only sparse K14+ basal cells and a solid mass of K8+ cells filling the entire interior (Fig 2C, right). This organoid defect resembles the mammary ductal phenotype in the Lgr4−/− mice (Supplementary Fig S4A). Together, these observations suggest that loss of Lgr4 impairs mammary morphogenesis, lineage specification, and stem cell function.
The above experiments demonstrated an impaired basal compartment in Lgr4−/− mice. In agreement with this, by staining single cell preparations of mammary glands for CD24 and CD49f, FACS analysis showed a decreased ratio of basal (CD24lo/CD49f+) vs. epithelial cells (CD24+/CD49flo Fig 3A-C). Importantly, these Lgr4−/− CD24lo/CD49f+ basal cells resulted in fewer and smaller colonies in Matrigel than their wild-type counterparts (Fig 3D-F), again suggesting that Lgr4 is important for stem cell proliferation and differentiation.
Figure 3. Lgr4 loss decreased mammary basal population.

(A) Lin− mammary cells from indicated mice were FACS sorted to separate luminal (CD24+CD49f−, green) and basal (CD24+CD49f+, blue) populations. (B) Decrease in basal cell number in Lgr4−/− mammary glands (n=6, * p=0.0188). (C) Luminal:basal epithelial cell ratio (* p=0.04).
(D-F) Representative photomicrographs (D), quantification of colony number (E), and size (F) of primary FACS-sorted basal MECs grown in Matrigel culture (*p<0.05; ***p<0.0005).
Lgr4 ablation impairs stem cell self-renewal and regenerative capacity
To further investigate the role of Lgr4 in mammary stem cells, we isolated MECs from 12-16-week-old Lgr4+/+ and Lgr4−/− mice, stained for CD24 and CD49f as described by Stingl et al 27, and observed a similar distribution of wild type mammary cells into the Mammary Repopulating Unit (MRU), Colony Forming Cell (CFC) and Myoepithelial (MYO) populations based on relative CD24 and CD49f expression. When we used FACS to quantify the proportion of cells in each population, the CFC and MYO populations were not significantly affected by Lgr4 loss, but the MRU population in Lgr4−/− mice was less than half of that in wild-type (p=0.006, n=10; Fig 4A-B), suggesting that the stem cell pool is sharply decreased in Lgr4−/− mammary glands.
Figure 4. Lgr4 loss decreases MRU population and impairs mammosphere formation and self-renewal.

(A) Distribution of Lgr4+/+ and Lgr4−/− mammary epithelial CD45−Ter119−CD31−CD140a− cells by CD24 and CD49f expression. CFC = Colony Forming Cells MYO = myoepithelial cells MRU = Mammary Repopulating Unit. Population gates were set on the basis of comparison with the published populations in Stingl et al. 27.
(B) Subpopulation quantitation (*p = 0.006, n=10).
(C) Mammospheres from Lgr4+/+ and Lgr4−/− MECs over 5 serial passages (**p<0.005; ***p<0.0005; n=5).
(D, E) Photomicrographs of 2nd passage mammospheres (D) and mammosphere size quantification (≥50μm) (E) (***p=0.0002, n=3).
Next, we performed a mammosphere formation assay to quantify self-renewal and differentiation. Loss of Lgr4 had no apparent effect on either the number (n=5; Fig 4C) or size (data not shown) of primary mammospheres relative to wild-type, suggesting that short-term proliferation (i.e. the population of cells with limited replicative potential) is not significantly affected by Lgr4 loss. Lgr4−/− epithelial cells gave rise to significantly smaller secondary mammospheres than wild-type cells (61 μm vs 72 μm p=0.0002, Fig 4D-E), without significant change in the number of secondary mammospheres (n=5; Fig 4C). Lgr4−/− cells initiated tertiary mammospheres at a much lower rate than wild-type cells (p=0.0034, n=5; Fig 4C). This decline was accelerated in successive generations, and by the 5th passage there were almost no mammospheres observed in the Lgr4−/− group (p=0.0011, n=5; Fig 4C). These data suggest a defect in long-term mammary stem cell self-renewal in Lgr4−/− mice.
To functionally assess Lgr4−/− mammary stem cell defects in vivo, we transplanted MECs from adult Lgr4−/− and wild-type mice into cleared fat pads of 3-week-old wild-type recipients in a limiting dilution assay. After 2 months, transplanted mammary tissue was harvested to evaluate the extent of mammary outgrowth. In three independent transplantation experiments, cells from Lgr4−/− mammary glands exhibited an 80% decrease in repopulating frequency compared to wild-type cells (Table 1, p<0.001). Furthermore, the extent of Lgr4−/− ductal outgrowth was decreased (Table 1), strongly suggesting that Lgr4 loss leads to compromised stem cell activity.
Table 1.
Mammary outgrowths derived from Lgr4+/+ and Lgr4−/− mammary cells.
| Number of injected cells | Lgr4+/+ | Lgr4−/− |
|---|---|---|
| 10,000 |
|
|
| 1000 |
|
|
| 100 |
|
|
| Estimated repopulating frequency | 1/1255 (1/630-1/2499) | 1/8082 (1/3924-1/16645) |
P<0.001 ● 100% ◕ 80%∼100% ◑ 50%∼80% ◔ 30%∼50%
 5%∼3% ○ 0%
5%∼3% ○ 0%
Canonical Wnt signaling rescues the Lgr4-null mammary organoid phenotype
Lgr4 has recently been reported to potentiate Wnt signaling after binding to its ligand R-spondin 10, 17, 18. To test whether the Wnt pathway mediates Lgr4 in mammary stem cell maintenance, we generated mammary organoids from wild-type or Lgr4−/− MECs and treated them with Wnt3a or R-spondin1 alone or in combination. In the wild-type culture, treatment of R-spondin1 or Wnt3a alone increased the fraction of branching organoids (Fig 5A-B), suggesting that R-spondin1 or Wnt3a can promote mammary stem cell function as previously reported 1. Combinatorial treatment with both Wnt3a and R-spondin1 resulted in a significant increase in the percentage of branching organoids arising from wild-type MECs (Fig 5A-B), suggesting that R-spondin1 and Wnt3a can synergize in stimulating mammary gland stem cell functions. However, in the Lgr4−/− culture, R-spondin1 treatment alone did not affect the fraction of branching organoids (Fig 5A-B), confirming the role of Lgr4 as a potential receptor for R-spondin1-mediated response. Wnt3a treatment significantly increased the frequency of branching organoids from Lgr4−/− MECs, but this new level did not reach that of wild-type (WT) cells treated with Wnt3a, suggesting that Wnt can partially activate its downstream components in the absence of Lgr4 and that Lgr4 potentiates Wnt signaling in the mammary gland. Adding R-spondin1 to Wnt3a treatment did not increase the fraction of branching organoids from the Lgr4−/− culture, confirming that Lgr4 is obligatory for the R-spondin1 effect on Wnt signaling and mammary stem cells.
Figure 5. Wnt restores the effects of Lgr4 loss.

(A) Representative images of Lgr4+/+ and Lgr4−/− organoids from 12-16 week old mice treated as indicated.
(B) Quantification of branched Lgr4+/+ and Lgr4−/− organoids. *p<0.05, n = 4.
(C) q-RT-PCR analysis of genes from mammosphere culture. Gene expression in Lgr4−/− samples was graphed relative to wild-type (*p<0.05, **p <0.01 n=3-5).
To identify key transcriptional targets by which Lgr4 and Wnt signaling regulate mammary stem cell functions, we used qPCR to compare Lgr4−/− and wild-type primary mammospheres for expression differences in genes that are known to be critical for stem cell maintenance in breast and other organs. These include known and potential Wnt signaling targets 1, and members of the Notch 2 and Hedgehog 3 signaling pathways, which have been reported to be critical for the maintenance of stem cells in the mammary gland and in other organs. Hedgehog pathway component expression was unchanged, along with most Notch pathway members, though two Notch target genes (Hey1 and Gata3) were altered in Lgr4−/− mammospheres (Supplemental Fig 7A-B). However, many Wnt target genes including Axin2, Sox9, Nanog, Cyclin D1 and Cyclin E were downregulated in Lgr4−/− mammospheres (p<0.05; Fig 5C). These data imply that the Wnt/β-catenin signaling pathway is reduced in Lgr4−/− mammospheres, and suggest a role for the Wnt pathway in mediating Lgr4 signaling in mammary stem cells.
Lgr4 regulates mammary stem cells through Sox2
Sox2 was reported to be up-regulated by Wnt in the Xenopus retina 37, but whether Sox2 is direct target of Wnt signaling is still unknown. Our gene expression screening indicated that Sox2 was downregulated by more than 50% in Lgr4−/− mammospheres (Fig 5C), suggesting that Sox2 is a target gene of Lgr4-mediated signaling. Since Lgr4 is involved in R-spondin-mediated Wnt signaling, we examined whether Sox2 is a direct target of Wnt signaling by analyzing the Sox2 promoter regions using rVista 2.0 (http://rvista.dcode.org/). Two consensus LEF1 sites (CTTTGTT, between -1484 and -1490bp, and TACAAAG, between -214 and -220bp) were found in approximately 2kb of the human and mouse Sox2 promoter regions. To test whether Lgr4 trans-activates Sox2 through those LEF1-binding sites, we constructed vectors for either overexpression or knockdown of human Lgr4, and performed Sox2 luciferase reporter assays in MCF10A cells. As shown in Fig 6A, overexpression of Lgr4 caused an 8-fold increase in luciferase reporter activity compared to the control vector. On the other hand, knockdown of Lgr4 led to a 3-fold decrease in luciferase reporter activity (Fig 6A). These data suggest that Sox2 transcriptionally responds to Lgr4 expression. To test whether the putative LEF1-binding sites in the Sox2 promoter are indeed occupied by LEF1, we performed a chromatin immunoprecipitation (ChIP) of primary MEC lysates. In WT cells, LEF1 bound to the -220 - -214bp site (but not to the -1484 - -1490bp site), and this binding was significantly reduced in Lgr4−/− MECs (Fig 6B). Together, these data suggest that Sox2 is a direct target of Wnt signaling and that Lgr4 regulates Sox2 expression through Wnt signaling in the mammary gland.
Figure 6. Lgr4 regulates mammary stem cell self-renewal through Sox2.

(A) Sox2 luciferase reporter assay (*p<0.01).
(B) ChIP assay of LEF1 binding sites in the Sox2 promoter.
(C) Matrigel colony formation of primary MECs infected as indicated. *p<0.05.
(D) Ductal outgrowth of Lgr4−/− MECs infected as indicated and transplanted into cleared mammary fat pads. ● = 100% filled ◕ = 80 − 99% ◑ = 50 − 79%
 = 5 − 29%. * Lgr4+/+ MEC data repeated from Table 1.
= 5 − 29%. * Lgr4+/+ MEC data repeated from Table 1.
To investigate whether Sox2 is the crucial target gene whose protein product mediates Lgr4 and Wnt signaling in regulating mammary stem cells, we constructed a lentiviral vector overexpressing mouse Sox2 cDNA, used it to infect Lgr4−/− primary MECs, and performed in vitro colony assays and in vivo regeneration assays. In Lgr4−/− MECs, overexpression of Sox2 significantly enhanced colony formation relative to GFP-infected MECs (p=0.018; Fig 6C), effectively restoring the colony formation potential to the level of wild-type MECs that also overexpressed Sox2 (p=0.139; Fig 6C). To further confirm the role of Sox2 in regulation of mammary stem cell self-renewal, we injected 10,000 Lgr4−/− MECs infected with lentivirus overexpressing Sox2 or control GFP into the cleared fat pads of syngeneic mice. Eight weeks after transplantation, we quantified the ductal outgrowths. In 11 mice transplanted with Lgr4−/− MECs infected by control virus, only 3 exhibited ductal outgrowths (Fig 6D). However, of 11 mice transplanted with Lgr4−/− MECs infected by Sox2-overexpressing virus, 10 displayed ductal outgrowths (p=0.0075; Fig 6D). This Sox2-mediated rescue of ductal growth potential was robust since the rescued cell outgrowth frequency was similar to that of transplantation of 10,000 WT MECs (p=1.0; Fig. 6D). Taken together, these observations strongly suggest that Sox2 mediates Lgr4 and Wnt signaling to control mammary stem cell self-renewal and functions.
Discussion
We report here that genetic ablation of Lgr4 causes mammary stem cell depletion and functional impairment. There was an 80% decrease in the number of functional mammary stem cells in Lgr4−/− mammary glands as determined by mammary regeneration following transplantation, and the mammosphere formation ability of Lgr4−/− MECs diminished rapidly after three passages, indicating a crucial role for Lgr4 in mammary stem cell self-renewal. Branching morphogenesis was impaired in the absence of Lgr4, resulting in a persistent decrease in ductal tree side branching in Lgr4−/− mice. Mechanistically, Lgr4 loss resulted in decreased expression of Wnt target genes, especially Sox2, which encodes a transcriptional factor that is also a key factor in cell reprogramming. Sox2 overexpression in Lgr4−/− mammary cells restored mammary regenerative potential, demonstrating Sox2 as a critical effector of Lgr4 in mammary stem cell self-renewal. We also found that LEF binding to the Sox2 promoter was decreased in Lgr4−/− mammary cells, and that treatment with Wnt3a rescued the impaired organoid branching morphogenesis of Lgr4−/− mammary cells. Altogether, our data support a model where R-spondin binding to Lgr4 activates Wnt/β-catenin signaling, resulting in Sox2 transcriptional activation, which controls mammary stem cell self-renewal and functions (Fig S8).
A role for Lgr4 in stem cell maintenance fits well with the existing literature on developmental consequences of Lgr4 loss. Several groups including ours have reported that Lgr4 regulates embryonic growth 16, postnatal male reproductive duct system development 12-14, gall bladder formation 11, hair follicle development 15, early eye development 19, 20, midgestational erythropoiesis 21, bone formation 22, and homeostasis of electrolytes and blood pressure 38. The breadth of organs affected by Lgr4 loss, and their varying origins from all three germ layers, is initial evidence for a potential role of Lgr4 in stem cell function. Furthermore, the Lgr4 homologues Lgr5 and Lgr6 have established roles regulating stem cells of various tissues, including Lgr5 in small intestine 4, 5, stomach and hair follicle stem cells 6, 7, and Lgr6 as a marker for the most primitive type of epidermal stem cells responsible for both hair follicle and skin renewal 8. Finally, several groups have identified the R-spondin family of Wnt potentiators as ligands for LGRs 4-6 10, 17, 18, with increased Wnt-induced LRP6 phosphorylation and downstream β-catenin and PCP signaling 18 or decreased ZNRF3 ubiquitination of Frizzled and LRP6 39 following R-spondin binding to LGRs. These findings provide a mechanistic model connecting Lgr4 to Wnt signaling, and make plausible a role for Lgr4 in mammary stem cell regulation.
Extensive data support a critical role for Wnt signaling in mammary stem cells 40-43 and branching morphogenesis 44. Exogenous Wnt3a treatment expanded the mammary stem cell pool in vitro and maintained their functional capacity to reconstitute mammary glands upon transplantation 1. Sox2 is a key pluripotency transcription factor regulating embryonic and neuronal development, and is implicated in adult stem and progenitor cells in a range of epithelial tissues, including mammary epithelium 45. Sox2-expressing cells are essential for tissue renewal throughout life 46. A considerable body of literature describes connections between Wnt signaling and Sox2 in various systems, with complementary or opposing effects depending on the tissue examined 37, 47-51. Our finding that Lgr4 regulates Sox2 expression through R-spondin/Wnt/β-catenin builds upon the emerging theme of context- and tissue-dependent interactions between Wnt and Sox2. While we have not ruled out alternative pathways as mediators of Lgr4 signaling in mammary stem cells (e.g. Gα/PKA/CREB), to our knowledge, this is the first report of Lgr4 as a regulator of mammary stem cells through Sox2.
A widely accepted model of the mammary cell hierarchy posits that a rare population of mammary stem cells is able to generate multipotent progenitor cells that individually have more limited lineage generation capability, whereas larger populations of lineage-committed progenitor cells are able to divide and produce daughter cells of only that specific lineage 52. Corresponding to this hierarchy is the replicative potential of different cell populations, with mammary stem cells having potentially unlimited replicative potential, whereas committed progenitors are able to divide a limited number of times before differentiating. The gold standard for mammary stem cell function is ductal outgrowth formation following transplantation; in contrast, assays such as primary mammosphere formation likely measure a combined effect of stem cell and progenitor cell division (at least until progenitor cells cease dividing after several passages). Our data suggest that Lgr4 is required for mammary stem cell self-renewal, as illustrated by both decreased outgrowth formation and diminished late-passage mammosphere formation, whereas the lack of effect of Lgr4 loss in primary mammosphere formation argues that Lgr4 plays a different role in basal progenitor cells, perhaps contributing to preventing a luminal cell fate in this population.
Apart from impaired mammary stem cells, we found several additional defects in Lgr4−/− mammary glands. These included delayed mammary duct branching morphogenesis, frequent deficiencies in the TEB cap cell layer (Fig 2A), persistence of K14+K8+ epithelial cells (Fig 2B), and occasional luminal filling of mammary ducts (Supplemental Fig S4). The delayed branching and the defects in TEB cap cells may reflect the diminished mammary stem cell pool resulting from Lgr4 loss, while the persistence of double K14+K8+ cells suggests a potential role for Lgr4 in the lineage commitment of multipotent progenitor cells. Several defects (fewer TEBs, diminished ductal branching, and decreased mammary stem cell pool) were similar to those reported in Lrp5−/− 41 and Lrp6−/− 43 mice, emphasizing the connection between Lgr4 and Wnt signaling in mammary stem cell function. A recent report described Lgr4K5KO mice having a similar delay in branching morphogenesis 53, but otherwise normal mammary glands, perhaps due to loss of Lgr4 exclusively in cells expressing keratin 5 in Lgr4K5KO mice. Our more severe phenotype in germline knockout mice may result from loss of Lgr4 expression in a broader range of mammary epithelial cells and basal cells and especially in the stem cell pool. At least some of the mammary stem cells lack the expression of keratin markers such as K533, 54. At any rate, to our knowledge, this is the first report of Lgr4 as a regulator of mammary stem cell function.
Summary
In conclusion, we here identify Lgr4 as a key protein in maintenance of mammary stem cell self-renewal, with a significant role in mammary gland development and branching morphogenesis. Furthermore, we characterize the pluripotency transcription factor Sox2 as a target of Lgr4 signaling, and identify Wnt/β-catenin signaling as a mediator of Lgr4 signal transduction.
Supplementary Material
Supplemental Figure S1. Lgr4 is predominantly expressed in basal cells of mature ducts. (A) Mammary glands of 13 week old Lgr4+/− mice were fixed, sectioned, and stained for β-galactosidase activity. No β-galactosidase activity seen in Lgr4+/+ ducts (A, inset). (B) Immunohistochemical staining for Lgr4 in ducts of 13-week-old Lgr4+/+ mammary glands. Inset shows negative staining of Lgr4−/− mammary ducts. (C, E) Representative image of whole mount staining of 13 week old or 21 week old Lgr4+/+ or Lgr4−/− mammary glands. (D, F) Quantification of side branches in 13 week old or 21 week old Lgr4+/+ or Lgr4−/− mammary glands (N=5).
Supplemental Figure S2. Lgr6 expression in adult mammary glands not affected by loss of Lgr4. Immunohistochemistry for Lgr6 in sections from 8-9 week old wild type (left) and Lgr4−/− (right) mouse mammary glands. Scale bar = 50 μm, Representative photomicrographs of n=5 mice per genotype.
Supplemental Figure S3. Lgr4−/− mammary TEBs and ducts display decreased proliferation. (A) Proliferation as determined by immunohistochemical staining for BrdU in TEBs (A) and Ki67 in ducts (B).
Supplemental Figure S4. Lgr4−/− mammary TEBs exhibit increased apoptosis. (A) TUNEL assay showing apoptosis in TEBs of 6-week-old Lgr4+/+ and Lgr4−/− mice. (B) Quantification of apoptosis rate in TEBs from TUNEL staining (*p=0.002, n=5).
Supplemental Figure S5. Lgr4 loss disrupts mammary duct development and cell differentiation. (A-B) K5 (green) and K8 (red) co-immunofluorescence staining from 4- to 6-week-old Lgr4+/+ and Lgr4−/− mammary ducts (A) or from transplant outgrowths (B). Nuclei are stained with DAPI (n=8).
Supplemental Figure S6. Lgr4+ cells have increased solid colony formation. Lgr4+/− mouse mammary glands were digested to single cells, stained for β-galactosidase activity and FACS sorted to separate Lgr4-expressing (LacZ+) and cells lacking Lgr4 expression (LacZ−). 500 cells per group were plated on separate Matrigel-coated plates, and colonies were quantitated 10 days after plating. (A) Picture showing LacZ+ cell plate with both solid (arrowhead) and acinar-like (arrow) colonies. (B) Quantitation of colony formation. n=4, *p<0.05. Pictures taken using a 20× objective.
Supplemental Figure S7. The impact of Lgr4 loss on Hedgehog and Notch signaling target genes. (A-B) q-RT-PCR analysis of genes from mammosphere culture. Gene expression in Lgr4−/− samples was graphed relative to wild-type (*p<0.05, n=3-5).
Supplemental Figure S8. Model showing that Lgr4 regulates Sox2 and mammary stem cells through the canonical Wnt pathway. R-spondin binding to Lgr4 both prevents ZNRF3-mediated ubiquitination of Frizzled and LRP5/6, and also induces Lgr4 binding to LRP5/6, resulting in potentiation of Wnt signaling, increased Sox2 expression, and retention of stem cell activity.
Acknowledgments
The authors thank Sung-Gook Cho and Weijia Luo for invaluable technical assistance, Ms. Svasti Haricharan for statistical analysis, and Ms. Vidya Sinha for stimulating discussion and critical review of this manuscript. The authors acknowledge the assistance of the BCM Cytometry and Cell Sorting Core. We thank Dr. Qingyun Liu for the antibody against Lgr6. This work was supported in part by grants from the National Basic Research Program of China (2012CB910402 to M.L.), the National Natural Science Foundation of China (30930055 to M.L.), the DOD (BC085050 to Y.L. and PC093061 to M.L.) and NIH (R01CA124820 to Y.L., and R01CA106479 to M.L.). J. D. was supported by a SPORE career development award (P50-CA058183).
This work was supported in part by grants from the National Basic Research Program of China (2012CB910402 to M.L.), the National Natural Science Foundation of China (30930055 to M.L.), the DOD (BC085050 to Y.L. and PC093061 to M.L.) and NIH (R01CA124820 to Y.L., and R01CA106479 to M.L.). J. D. was supported by a SPORE career development award (P50-CA058183). Ying Wang is a Ph.D. student in the Graudate Program of College of Life Sciences, Hunan Normal University, Changsha, Hunan 410081, China
Footnotes
Author Contributions: Ying Wang: data collection, data analysis, manuscript writing
Jie Dong: data collection, data analysis
Dali Li: data collection, data analysis
Li Lai: data collection
Stefan Siwko: data analysis, manuscript writing
Yi Li: conception and design, data analysis, financial support
Mingyao Liu: conception and design, data analysis, financial support, final approval
The authors declare no conflicts of interest.
References
- 1.Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell stem cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouras T, Pal B, Vaillant F, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell stem cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer research. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 5.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 6.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell stem cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Jaks V, Barker N, Kasper M, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature genetics. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 8.Snippert HJ, Haegebarth A, Kasper M, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 9.Mustata RC, Van Loy T, Lefort A, et al. Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO reports. 2011;12:558–564. doi: 10.1038/embor.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lau W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita R, Takegawa Y, Sakumoto M, et al. Defective development of the gall bladder and cystic duct in Lgr4- hypomorphic mice. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:993–1000. doi: 10.1002/dvdy.21900. [DOI] [PubMed] [Google Scholar]
- 12.Li XY, Lu Y, Sun HY, et al. G protein-coupled receptor 48 upregulates estrogen receptor alpha expression via cAMP/PKA signaling in the male reproductive tract. Development. 2010;137:151–157. doi: 10.1242/dev.040659. [DOI] [PubMed] [Google Scholar]
- 13.Hoshii T, Takeo T, Nakagata N, et al. LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biology of reproduction. 2007;76:303–313. doi: 10.1095/biolreprod.106.054619. [DOI] [PubMed] [Google Scholar]
- 14.Mendive F, Laurent P, Van Schoore G, et al. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Developmental biology. 2006;290:421–434. doi: 10.1016/j.ydbio.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Mohri Y, Kato S, Umezawa A, et al. Impaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking Lgr4. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:2235–2242. doi: 10.1002/dvdy.21639. [DOI] [PubMed] [Google Scholar]
- 16.Mazerbourg S, Bouley DM, Sudo S, et al. Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Molecular endocrinology. 2004;18:2241–2254. doi: 10.1210/me.2004-0133. [DOI] [PubMed] [Google Scholar]
- 17.Carmon KS, Gong X, Lin Q, et al. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glinka A, Dolde C, Kirsch N, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO reports. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin C, Yin F, Lin M, et al. GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Investigative ophthalmology & visual science. 2008;49:4245–4253. doi: 10.1167/iovs.08-1860. [DOI] [PubMed] [Google Scholar]
- 20.Weng J, Luo J, Cheng X, et al. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6081–6086. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song H, Luo J, Luo W, et al. Inactivation of G-protein-coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathway. The Journal of biological chemistry. 2008;283:36687–36697. doi: 10.1074/jbc.M800721200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J, Zhou W, Zhou X, et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136:2747–2756. doi: 10.1242/dev.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, Liu S, Guan Y, et al. Lgr4-mediated Wnt/beta-catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development. 2013;140:1751–1761. doi: 10.1242/dev.093641. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Qian Y, Li L, et al. Lgr4 Gene Deficiency Increases Susceptibility and Severity of Dextran Sodium Sulfate-induced Inflammatory Bowel Disease in Mice. The Journal of biological chemistry. 2013;288:8794–8803. doi: 10.1074/jbc.M112.436204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SG, Wang Y, Rodriguez M, et al. Haploinsufficiency in the prometastasis Kiss1 receptor Gpr54 delays breast tumor initiation, progression, and lung metastasis. Cancer research. 2011;71:6535–6546. doi: 10.1158/0008-5472.CAN-11-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brisken C, Kaur S, Chavarria TE, et al. Prolactin controls mammary gland development via direct and indirect mechanisms. Developmental biology. 1999;210:96–106. doi: 10.1006/dbio.1999.9271. [DOI] [PubMed] [Google Scholar]
- 27.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 28.Ewald AJ, Brenot A, Duong M, et al. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Developmental cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee GY, Kenny PA, Lee EH, et al. Three-dimensional culture models of normal and malignant breast epithelial cells. Nature methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. Journal of immunological methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 33.de Visser KE, Ciampricotti M, Michalak EM, et al. Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. The Journal of pathology. 2012;228:300–309. doi: 10.1002/path.4096. [DOI] [PubMed] [Google Scholar]
- 34.Plaks V, Brenot A, Lawson DA, et al. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell reports. 2013;3:70–78. doi: 10.1016/j.celrep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dey D, Saxena M, Paranjape AN, et al. Phenotypic and functional characterization of human mammary stem/progenitor cells in long term culture. PloS one. 2009;4:e5329. doi: 10.1371/journal.pone.0005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holliday DL, Brouilette KT, Markert A, et al. Novel multicellular organotypic models of normal and malignant breast: tools for dissecting the role of the microenvironment in breast cancer progression. Breast cancer research : BCR. 2009;11:R3. doi: 10.1186/bcr2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Raay TJ, Moore KB, Iordanova I, et al. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Li X, Ke Y, et al. GPR48 increases mineralocorticoid receptor gene expression. Journal of the American Society of Nephrology : JASN. 2012;23:281–293. doi: 10.1681/ASN.2011040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao HX, Xie Y, Zhang Y, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 40.van Genderen C, Okamura RM, Farinas I, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes & development. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 41.Lindvall C, Evans NC, Zylstra CR, et al. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. The Journal of biological chemistry. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 42.Badders NM, Goel S, Clark RJ, et al. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PloS one. 2009;4:e6594. doi: 10.1371/journal.pone.0006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindvall C, Zylstra CR, Evans N, et al. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PloS one. 2009;4:e5813. doi: 10.1371/journal.pone.0005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brisken C, Heineman A, Chavarria T, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes & development. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 45.Simoes BM, Piva M, Iriondo O, et al. Effects of estrogen on the proportion of stem cells in the breast. Breast cancer research and treatment. 2011;129:23–35. doi: 10.1007/s10549-010-1169-4. [DOI] [PubMed] [Google Scholar]
- 46.Arnold K, Sarkar A, Yram MA, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell stem cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takemoto T, Uchikawa M, Kamachi Y, et al. Convergence of Wnt and FGF signals in the genesis of posterior neural plate through activation of the Sox2 enhancer N-1. Development. 2006;133:297–306. doi: 10.1242/dev.02196. [DOI] [PubMed] [Google Scholar]
- 48.Mansukhani A, Ambrosetti D, Holmes G, et al. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. The Journal of cell biology. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agathocleous M, Iordanova I, Willardsen MI, et al. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee MY, Lim HW, Lee SH, et al. Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem cells. 2009;27:1858–1868. doi: 10.1002/stem.124. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto S, Chen H, Que J, et al. beta-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. Journal of cell science. 2012;125:932–942. doi: 10.1242/jcs.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stingl J, Raouf A, Eirew P, et al. Deciphering the mammary epithelial cell hierarchy. Cell cycle. 2006;5:1519–1522. doi: 10.4161/cc.5.14.2983. [DOI] [PubMed] [Google Scholar]
- 53.Oyama K, Mohri Y, Sone M, et al. Conditional knockout of Lgr4 leads to impaired ductal elongation and branching morphogenesis in mouse mammary glands. Sexual development : genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation. 2011;5:205–212. doi: 10.1159/000329476. [DOI] [PubMed] [Google Scholar]
- 54.Spike BT, Engle DD, Lin JC, et al. A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell stem cell. 2012;10:183–197. doi: 10.1016/j.stem.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Lgr4 is predominantly expressed in basal cells of mature ducts. (A) Mammary glands of 13 week old Lgr4+/− mice were fixed, sectioned, and stained for β-galactosidase activity. No β-galactosidase activity seen in Lgr4+/+ ducts (A, inset). (B) Immunohistochemical staining for Lgr4 in ducts of 13-week-old Lgr4+/+ mammary glands. Inset shows negative staining of Lgr4−/− mammary ducts. (C, E) Representative image of whole mount staining of 13 week old or 21 week old Lgr4+/+ or Lgr4−/− mammary glands. (D, F) Quantification of side branches in 13 week old or 21 week old Lgr4+/+ or Lgr4−/− mammary glands (N=5).
Supplemental Figure S2. Lgr6 expression in adult mammary glands not affected by loss of Lgr4. Immunohistochemistry for Lgr6 in sections from 8-9 week old wild type (left) and Lgr4−/− (right) mouse mammary glands. Scale bar = 50 μm, Representative photomicrographs of n=5 mice per genotype.
Supplemental Figure S3. Lgr4−/− mammary TEBs and ducts display decreased proliferation. (A) Proliferation as determined by immunohistochemical staining for BrdU in TEBs (A) and Ki67 in ducts (B).
Supplemental Figure S4. Lgr4−/− mammary TEBs exhibit increased apoptosis. (A) TUNEL assay showing apoptosis in TEBs of 6-week-old Lgr4+/+ and Lgr4−/− mice. (B) Quantification of apoptosis rate in TEBs from TUNEL staining (*p=0.002, n=5).
Supplemental Figure S5. Lgr4 loss disrupts mammary duct development and cell differentiation. (A-B) K5 (green) and K8 (red) co-immunofluorescence staining from 4- to 6-week-old Lgr4+/+ and Lgr4−/− mammary ducts (A) or from transplant outgrowths (B). Nuclei are stained with DAPI (n=8).
Supplemental Figure S6. Lgr4+ cells have increased solid colony formation. Lgr4+/− mouse mammary glands were digested to single cells, stained for β-galactosidase activity and FACS sorted to separate Lgr4-expressing (LacZ+) and cells lacking Lgr4 expression (LacZ−). 500 cells per group were plated on separate Matrigel-coated plates, and colonies were quantitated 10 days after plating. (A) Picture showing LacZ+ cell plate with both solid (arrowhead) and acinar-like (arrow) colonies. (B) Quantitation of colony formation. n=4, *p<0.05. Pictures taken using a 20× objective.
Supplemental Figure S7. The impact of Lgr4 loss on Hedgehog and Notch signaling target genes. (A-B) q-RT-PCR analysis of genes from mammosphere culture. Gene expression in Lgr4−/− samples was graphed relative to wild-type (*p<0.05, n=3-5).
Supplemental Figure S8. Model showing that Lgr4 regulates Sox2 and mammary stem cells through the canonical Wnt pathway. R-spondin binding to Lgr4 both prevents ZNRF3-mediated ubiquitination of Frizzled and LRP5/6, and also induces Lgr4 binding to LRP5/6, resulting in potentiation of Wnt signaling, increased Sox2 expression, and retention of stem cell activity.


