Abstract
Exposure to di-(2-ethylhexyl) phthalate (DEHP) has been reported to induce spermatogenic disturbance through oxidant stress and affect the immune system as an adjuvant. However, the effect of DEHP on the testicular immune microenvironment has not yet been investigated. In the present study, we examined the testicular immune microenvironment after exposure to doses of DEHP, previously identified as no-observed-adverse-effect levels. Adult male mice were administered food containing 0%, 0.01% or 0.1% DEHP and then testes were analyzed. The results showed that a slight but significant spermatogenic disturbance appeared in the 0.1% DEHP group but not in the 0.01% DEHP group at 8 weeks. It was also demonstrated that lymphocytes and F4/80- and MHC class II- positive cells were significantly increased with the elevation of IL-10 and IFN-γ mRNA expressions in the testes of not only the 0.1% DEHP group but also the 0.01% DEHP group at 8 weeks. Histochemical analyses involving horseradish peroxidase (HRP) as a tracer showed that a little blood-borne HRP had infiltrated into the lumen of a few seminiferous tubules beyond the blood-testis-barrier in both the 0.1% and 0.01% DEHP groups at 8 weeks. This indicates that a dose of DEHP that has little effects on spermatogenesis can change the testicular immune microenvironment with functional damage of the blood-testis barrier.
Keywords: Di-(2-ethylhexyl) phthalate, Immune microenvironment, Testis
Exposure to di-(2-ethylhexyl) phthalate (DEHP), widely used as a plastic plasticizer for synthetic polymers, causes testicular atrophy with spermatogenesis disturbance in mice and rats [1,2,3]. Some studies have shown that DEHP is rapidly metabolized into mono-2-ethylhexyl phthalate and induces spermatogenetic disturbance by oxidative stress [1,2,3]. Others have reported that small vacuoles in the cytoplasm of Sertoli cells begin to appear and that morphological changes in the blood-testis barrier (BTB) can occur [4, 5].
On the other hand, it was reported that DEHP constitutes a group of xenobiotics that promote the development of an immune response [6]. DEHP itself is not an immunogenic antigen, but it enhances immunogenic activity in mice, which means it has an adjuvant effect [7,8,9,10]. However, the effects on the testicular immune microenvironment from exposure to DEHP have not previously been investigated.
It is well known that DEHP has dose-dependent effects on spermatogenesis, and a previous study reported that a diet of 500 ppm (0.0985–0.1168 mg/g/day) DEHP was identified as a no-observed-adverse-effect level (NOAEL) of DEHP in mice [11]. Here, mice fed diets of 0.01% DEHP (0.0113–0.0133 mg/g/day) and 0.1% DEHP (0.1152–0.1357 mg/g/day) were examined for immunologic changes in the testicular microenvironment.
Materials and Methods
Animals
Male A/J mice (7 weeks old, n=105) were purchased from Charles River (Kanagawa, Japan) and kept in the Laboratory Animal Center of Tokyo Medical University for 1 week. They were kept at 22–24 C and 50–60% relative humidity, on a 12 h light-12 h dark cycle. Approval from the Tokyo Medical University Animal Committee was obtained for this study.
Chemicals
DEHP was purchased from Tokyo Chemical Industry (Tokyo, Japan). The chemical purity of the DEHP was found to be >98% by gas-liquid chromatography. The standard CE-2 diet was purchased from Clea Japan (Tokyo, Japan), and CE-2 diets containing 0.01% DEHP and 0.1% DEHP, respectively, were prepared by Oriental Yeast (Chiba, Japan). We used the doses of 0.01% DEHP (0.0113–0.0133 mg/g/day) and 0.1% DEHP (0.1152–0.1357 mg/g/day) because a dose level of 500 ppm (0.0985–0.1168 mg/g/day) was identified as a NOAEL of DEHP in mice [11].
Experimental design
Mice were randomly allocated into Control (n=35), 0.01% DEHP (n=35) and 0.1% DEHP (n=35) groups. Control, 0.01% DEHP and 0.1% DEHP groups were fed the standard CE-2 diet, CE-2 diet containing 0.01% DEHP and CE-2 diet containing 0.1% DEHP, respectively. Mice of each group fed the diets for 2 (n=10), 4 (n=10) and 8 (n=15) weeks. Food and water were freely available. On average, the mice ate 4.84 ± 0.43, 4.72 ± 0.23 and 4.63 ± 0.36 g/mouse/day (mean ± standard divisions (SD)) of food in the Control, 0.01% DEHP and 0.1% DEHP groups, respectively. The mice of all three groups drank approximately 7 ml of water a day. The mice were deeply anesthetized with pentobarbital, and their body weights were recorded. Then the animals were euthanized by cervical dislocation, and the testes of each animal were weighed and analyzed. Histological samples were taken from the right testes, and total RNA and immunohistological samples were collected from the left testes of each group.
Light microscopy
The right testes were removed from mice in the Control (n=10), 0.01% DEHP (n=10), and 0.1% DEHP (n=10) groups, and fixed in Bouin’s solution for 3 days, washed, dehydrated in an ethanol series and embedded in plastic (Technovit 7100; Heraeus Kuizer, Wehrheim, Germany). The testes were sectioned at 5 μm with a microtome (HN360; Microm, Walldorf, Germany) after which sections were stained with Gill’s hematoxylin V and eosin Y for light microscope observation.
The degree of spermatogenic disturbance was determined according to Johnsen’s scoring system, ranging from a score of 1 (no cells in the seminiferous tubules) to a score of 10 (complete spermatogenesis), as we have previously done [12,13,14]. At least 100 round or oval seminiferous tubules were examined, and the mean score was calculated for each group.
The numbers of seminiferous tubules with vacuoles in the cytoplasm of Sertoli cells were counted from 100 round or oval seminiferous tubules per testis, and the mean score was calculated for each group.
The number of lymphocytes / mm2 in the testicular interstium was counted in 10 sections from each testis, and the mean score was calculated for each group.
RNA extraction
Control (n=5), 0.01% DEHP (n=5), and 0.1% DEHP (n=5) mice were anesthetized with pentobarbital and then euthanized by cervical dislocation. Total RNA was isolated from whole left testis using an RNA extraction kit, TRIzol (Invitrogen, Tokyo, Japan), according to the manufacturer’s instructions. The RNA pellet was ultimately dissolved in 10 μl of RNase-free distilled water. Total RNA was measured by 260/280 UV spectrophotometry and stored at –80 C prior to use. cDNA was prepared from 10 μg of total RNA in a 100 μl reaction mixture using random primers according to a standard protocol (High-Capacity cDNA Archive Kit; PE Applied Biosystems, Foster City, CA, USA). PCR reactions were carried out on an iCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA), and mixtures were stored at –80 C before analysis.
Real-time RT-PCR
Real-time RT-PCR (TaqMan; PE Applied Biosystems) analysis for relative determination of the mRNA level of interferon (IFN)-γ, interleukin (IL)-6, IL-10 and tumor necrosis factor (TNF)-α was performed using the standard curve method [15]. Levels of β-actin were used to normalize the data. Cytokine mRNAs were detected by using proprietary pre-developed TaqMan primers (IFN-γ (ID: Mm00801778-m1), IL-6 (ID: Mm00446190-m1), IL-10 (ID: Mm00439616), TNF-α (ID: Mm00443258-m1) and β-actin (ID: Mm00607939-s1) (PE Applied Biosystems). TaqMan reactions (total: 50 μl) were performed in optical 96-well reaction plates by adding 40 μl of a mixture comprising 25 μl of TaqMan Universal PCR Master Mix 2×, 2.5 μl of TaqMan Gene Expression Assays primers (PE Applied Biosystems) and 12.5 μl of autoclaved water. A 10 μl aliquot of RT-PCR sample was subsequently added to each well. Reactions were performed in triplicate. Real-time fluorescence-monitored PCR reactions were done with a PE Applied Biosystems model 7500 Sequence Detection System. The temperature profile was 50 C for 2 min and 95 C for 10 min, followed by 95 C for 15 sec and 60 C for 1 min for 40 cycles. Using the manufacturer’s software, a threshold above the noise was chosen, and the cycle number (CT) at which fluorescence generated by cleavage of the probe exceeded the threshold was determined for each well. For each real-time PCR analysis, a standard curve was generated using 6 twofold serial dilutions in water of the RT-PCR samples corresponding to testicular cells with IFN-γ, IL-6, IL-10, TNF-α and β-actin mRNA primers, respectively. CT values were compared using a standard curve after linear regression analysis. A no-RT-PCR sample template control was performed for each reaction in duplicate.
Immunohistochemistry
The left testes were removed from mice in the Control (n=5), 0.01% DEHP (n=5), and 0.1% DEHP (n=5) groups and embedded in Optimal Cutting Temperature compound (O.C.T.) (Sakura Finetek, Torrance, CA, USA). The testes in O.C.T. compound were immediately frozen in liquid nitrogen and stored at –80 C until used. Sections were cut ant a thickness of 5 μm with a cryostat (HM550; Microm) and dried in air. They were fixed in 95% ethanol for 10 min at –20 C to stain the F4/80 and in acetone for the 2 min at –20 C to stain the major histocompatibility complex (MHC) class II, CD3 and CD45R/B220 and were then rinsed in phosphate-buffered saline (PBS, pH 7.4) and 0.3% H2O2 in methanol for 30 min at room temperature. Sections were then incubated with Protein Block, Serum-Free (Dako, Glostrup, Denmark), and after this treatment, serial sections were incubated with the primary antibodies for 2 h at room temperature and then with the secondary antibodies for 30 min at room temperature. Subsequently, the streptavidin-biotinylated peroxidase complex technique (Vectastain Elite ABC Reagent, Vector Laboratories, Burlingame, CA, USA) was used to incubate sections with ABC complexes for 30 min at room temperature. Positively stained cells were identified as cells that had black-colored cytoplasm at the light microscopic level. Immunoreactivity was visualized using 0.05% 3,3’-diaminobenzidine 4HCl (DAB), 0.01% H2O2 and NiSO4 (NH4) 2SO4 6H2O. Primary antibodies were purified rat anti-mouse F4/80 monoclonal antibodies (1:500 dilution, macrophages; Abcam, Cambridge, UK), rat anti-mouse MHC class II monoclonal antibodies (1:200 dilution, macrophages; Abcam), rat anti-mouse CD3 monoclonal antibodies (1:100 dilution, T-cell marker; BioLegend, San Diego, CA, USA), rat anti-mouse CD45R/B220 monoclonal antibodies (1:200 dilution, B-cell marker; BD, Franklin Lakes, NJ, USA), goat anti-mouse IL-10 monoclonal antibodies (1:100 dilution, BD) and rat anti-mouse IFN-γ monoclonal antibodies (1:100 dilution, Calbiochem, Darmstadt, Germany). Secondary antibodies were biotinylated rabbit anti-rat IgG (Vectastain Elite ABC kit, Vector Laboratories) and biotinylated rabbit anti-goat IgG (Vectastain Elite ABC kit, Vector Laboratories). Sections processed with PBS instead of primary antibodies were used as negative controls. Stained sections were counterstained with methyl green (Vector Laboratories).
The positive cytoplasmic area, but not the positive cell number per interstitium, was determined in each region using the National Institutes of Health ImageJ software, because cytoplasmic volume rather than nuclei number is crucial for activation of macrophages. An area of at least 1 mm2 of the interstitium was examined in each testicular interstitial region in the 10 sections from each testis. Data were expressed as means ± SD.
Histochemical evaluation of the integrity of the BTB
To evaluate the integrity of the BTB, horseradish peroxidase (HRP) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as a tracer [16, 17]. The mice (n=5) from each group were evaluated at 8 weeks. The mice received an intraperitoneal injection of HRP (10 mg/kg bw) dissolved in 0.2 ml PBS at 30 min before euthanization. The mice were euthanized 30 min later under deep anesthesia with pentobarbital, followed by transcardiac perfusion with 2% paraformaldehyde (Merck, Darmstadt, Germany) in 0.1 M phosphate buffer, pH 7.4, at room temperature, and then their testes were immediately removed. After washing with 10% sucrose in PBS, the samples were embedded in O. C. T. compound (Miles Laboratories, Naperville, IL, USA), and then frozen in liquid nitrogen and stored at −80 C until use. Sections were cut at a thickness of 6 μm were cut with a cryostat (HM550; Microm), dried in air, and then transferred to an incubation medium comprising PBS (pH 7.4) containing 0.05% 3,3’-diaminobenzidine 4HCl and 0.01% hydrogen peroxide to detect HRP-localizing sites. Stained sections were counterstained with methyl green (Vector Laboratories). The numbers of seminiferous tubules infiltrated by HRP beyond the BTB were counted from 100 round or oval seminiferous tubules per testis of each mouse, and the mean score was calculated for each group.
Statistical analysis
Values are presented as means ± SD. Significance of differences was determined by ANOVA with the Tukey-Kramer test as a post hoc test. All statistical tests were performed as two-sided tests using a level of significance of 0.05.
Results
Differences in body and testis weights between controls and DEHP-treated mice were not significant (P>0.05 by ANOVA, Table 1). Histopathological examinations revealed a few seminiferous tubules with germ cell sloughing at 2, 4 and 8 weeks in the 0.01% DEHP group. In the 0.1% DEHP group, there were also a few pathological changes at 2 weeks and foci of some seminiferous tubules with mild germ cell sloughing in the lumen, intermingled with normal seminiferous tubules at 4 and 8 weeks. On the assessments of Johnsen’s scores, no significant pathological change was observed in any groups at 2 and 4 weeks. On the other hand, significant spermatogenic disturbance occurred in the 0.1% DEHP group (8.8 ± 2.1) but not in the 0.01% DEHP group (9.8 ± 0.5) compared with the Control group (10 ± 0) at 8 weeks. Few small vacuoles (1.0 ± 1.2 at 2 weeks, 1.3 ± 1.0 at 4 weeks and 1.2 ± 1.1 at 8 weeks) were observed in cytoplasm of Sertoli cells in the Control group. And the numbers of them were significantly increased not only in the 0.1% DEHP group (16.3 ± 2.1 at 2 weeks, 20.2 ± 3.1 at 4 weeks and 22.7 ± 2.0 at 8 weeks) but also in the 0.01% DEHP group (14.5 ± 2.2 at 2 weeks, 19.8 ± 2.3 at 4 weeks and 20.0 ± 2.4 at 8 weeks). Lymphocytic infiltrations appeared at 8 weeks in both the 0.01% DEHP group (19.2 ± 2.0) and 0.1% DEHP groups (22.6 ± 1.4) (Table 2).
Table 1. The body and testis weights in the Control-, 0.01% DEHP- and 0.1% DEHP-goups.
| Parameters | Experimental groups | ||
| Control (n=10) | 0.01% DEHP (n=10) | 0.1% DEHP (n=10) | |
| Body weight | 28.54 ± 0.51 g | 27.36 ± 1.79 g | 28.74 ± 1.13 g |
| Testis weight | 0.11 ± 0.01 g | 0.12 ± 0.02 g | 0.11 ± 0.02 g |
The means ± SD are plotted in each group. There were no significant differences among the Control-, 0.01% DEHP- and 0.1% DEHP-groups in the body and testis weights.
Table 2. Johnsen’s score, Sertoli cell vacuolation and lymphovytic infiltraion in 2, 4 and 8 weeks of the Control, 0.01% DEHP and 0.1% DEHP groups.

Regarding the interstitium of each group at 8 weeks, CD3-positive and CD45R/B220-positive lymphocytes were not seen in testes of the Control group, whereas quite a few lymphocytes appeared in both the 0.01% DEHP and 0.1% DEHP groups (Fig. 1, Fig. 2, Table 2). Furthermore, the F4/80- and MHC class II-positive areas in the interstitium of both the 0.01% DEHP and the 0.1% DEHP groups were significantly increased compared with the Control group when evaluated by the immunohistochemical method (Fig. 3).
Fig. 1.
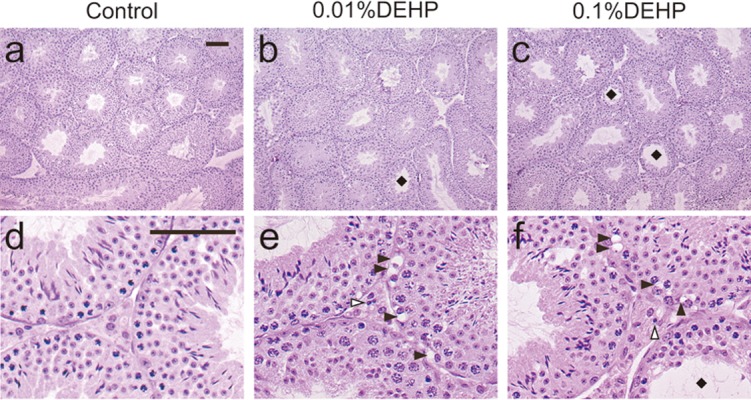
Images of testicular sections stained with hematoxylin and eosin (a, b, c) and magnified views of them (d, e, f) in the Control (a, d), 0.01% DEHP (b, e) and 0.1% DEHP (c, f) groups. Black squares indicate seminiferous tubules with spermatogenic disturbances. Black arrowheads indicate vacuoles in cytoplasm of Sertoli cells. White arrowheads indicate lymphocytes in the interstitium. Scale bar = 50 μm.
Fig. 2.
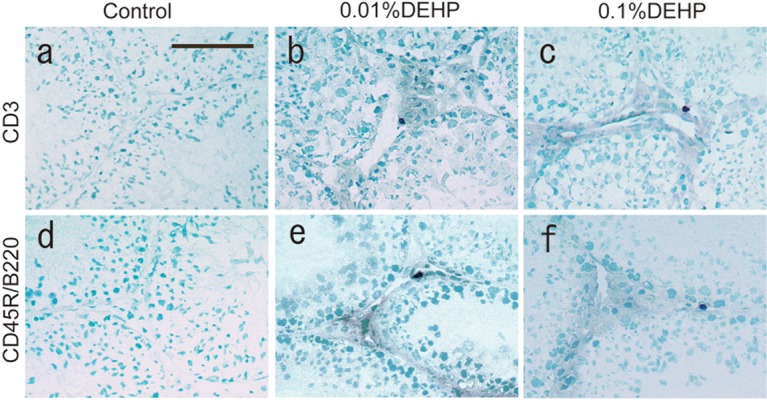
CD3 and CD45R/B220 staining in the Control (a, d), 0.01% DEHP (b, e) and 0.1% DEHP (c, f) groups. CD3-positive and CD45R/B220-positive cells appear as black cells. (a, d), Control group. (b, e), 0.01% DEHP group. (c, f), 0.1% DEHP group. Scale bar = 50 μm.
Fig. 3.
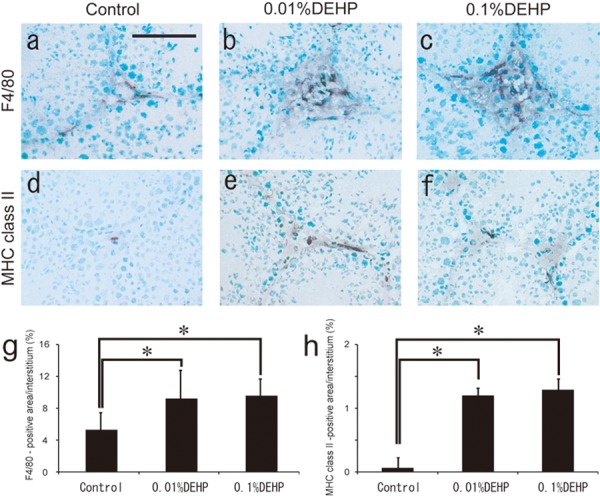
F4/80 and MHC class II staining in the Control, 0.01% DEHP and 0.1% DEHP groups. The F4/80-positive macrophages and MHC class II-positive cells appear as irregularly shaped black areas. (a, d) Control group. (b, e) 0.01% DEHP group. (c, f) 0.1% DEHP group. Scale bar = 50 μm. The F4/80-positive area/interstitial area (g) and MHC class II-positive area/interstitial area (h) ratios are given for each section for the Control, 0.01% DEHP and 0.1% DEHP groups. Each bar represents the mean ± SD. *P<0.05.
Real-time RT-PCR analyses revealed that the mRNA expressions of IFN-γ and IL-10, but not TNF-α and IL-6, were prominently increased in testes of both the 0.01% DEHP and 0.1% DEHP groups at 8 weeks (Fig. 4). Immunohistochemically, IFN-γ was detected in Sertoli cells and interstitial cells, and IL-10 was detected in spermatids, endothelial cells and interstitial cells in the Control, 0.01% DEHP and 0.1% DEHP groups. These localizations were the same in the Control, 0.01% DEHP and 0.1% DEHP groups. Moreover, both IFN-γ and IL-10 were extensively detected with many inflammatory cells in the testicular interstitium in the 0.01% DEHP and 0.1% DEHP groups (Fig. 5).
Fig. 4.
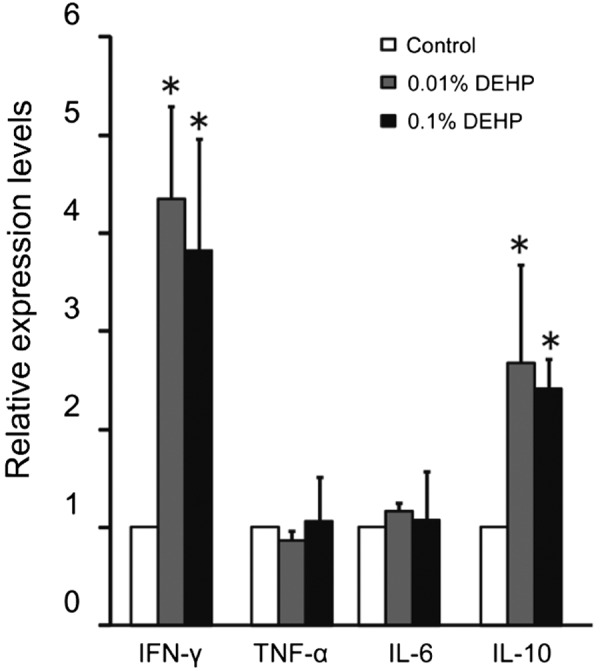
Testicular mRNA expression of IFN-γ, IL-6, IL-10 and TNF-α in the Control, 0.01% DEHP and 0.1% DEHP groups. Relative intensity was calculated after expression in the control for each point had been normalized as 1. Each bar represents the mean ± S.D. * P<0.05.
Fig. 5.
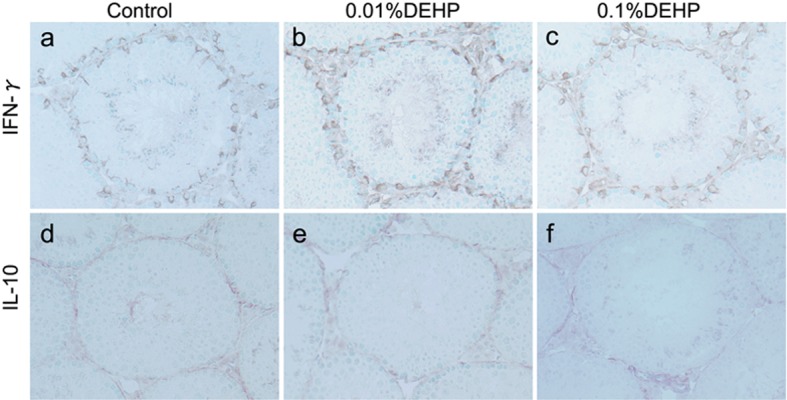
IFN-γ and IL-10 staining in the Control, 0.01% DEHP and 0.1% DEHP groups. The IFN-γ- and IL-10-positive cells appear as irregularly shaped brown areas. (a, d) Control group. (b, e) 0.01% DEHP group. (c, f) 0.1% DEHP group. Scale bar = 50 μm.
Histochemical analyses involving HRP as a tracer showed that HRP stains were not detected inside the lumen of seminiferous tubules in the Control group. On the other hand, a little blood-borne HRP had infiltrated into the lumen of a few seminiferous tubules beyond the BTB in both the 0.1% DEHP and 0.01% DEHP groups at 8 weeks (Fig. 6). The number of seminiferous tubules infiltrated by HRP beyond the BTB per 100 seminiferous tubules in the 0.1% DEHP and 0.01% DEHP groups were 2.4 ± 0.6 and 3.1 ± 0.8, respectively.
Fig. 6.
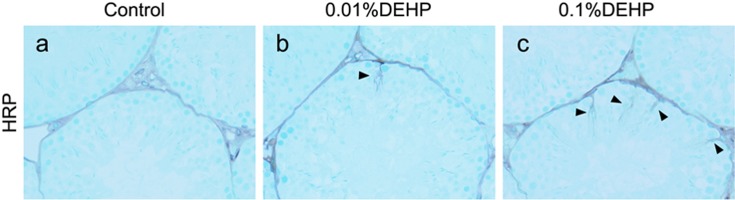
HRP detection for assessment of permeability of the blood-testis-barrier. The HRP-positive areas appear as irregularly shaped brown areas. The arrowhead shows the blood-borne HRP that infiltrated into the lumen of a few seminiferous tubules beyond the BTB in both the 0.1% and 0.01% DEHP groups. (a) Control group. (b) 0.01% DEHP group. (c) 0.1% DEHP group. Scale bar = 50 μm.
Discussion
This is the first report to examine the effect of exposure to DEHP on the testicular immune microenvironment. This study showed that small vacuoles in cytoplasm of Sertoli cells were observed from 2 weeks both in the 0.1% DEHP and the 0.01% DEHP groups. At 8 weeks, there was a slight but significant spermatogenic disturbance in the 0.1% DEHP group but not in the 0.01% DEHP group. Moreover, CD3+ and CD45R/B220+ lymphocyte infiltrations and increases of F4/80- and MHC class II-positive cells with elevated mRNA expressions of IL-10 and IFN-γ were detected at 8 weeks in both the 0.01% DEHP group and the 0.1% DEHP group. Histochemical analyses involving HRP as a tracer showed that a little blood-borne HRP had infiltrated into the lumen of a few seminiferous tubules beyond the BTB in both the 0.1% and 0.01% DEHP groups at 8 weeks.
In the testis, haploid germ cells (i.e., spermatids and spermatozoa) do not appear in seminiferous tubules until puberty when immune tolerance has already been established. Therefore, they contain various auto-immunogenic materials recognized as foreign by the autoimmune system. However, the testis is a known immunologically privileged organ. In particular, the BTB formed by tight junctions between Sertoli cells protects auto-immunogenic spermatozoa from attack by the self-immune system [18, 19]. Testicular interstitial cells containing testicular macrophages and MHC class II-positive cells also contribute to the immunologically privileged microenvironment [20, 21]. It was previously demonstrated that DEHP enhances immune responses [7, 22]. Previous studies also showed that bronchitis induced by ovalbumin as an immunogen was deteriorated by DEHP administration, although exposure to DEHP alone did not induce any significant bronchial change leading to inflammatory cell infiltration [9, 23]. In the present study, the lymphocytic infiltrations and significant increases in F4/80- and MHC class II-positive cells with elevated mRNA expressions of IL-10 and IFN-γ were detected with exposure to not only 0.1% DEHP but also 0.01% DEHP, which had little effects on spermatogenesis at 8 weeks. Moreover, it was also demonstrated that a little blood-borne HRP had infiltrated into the lumen of a few seminiferous tubules beyond the BTB in both the 0.1% and 0.01% DEHP groups at 8 weeks. These results implied that the small amounts of DEHP cause a very slight spermatogenic disturbance with a little functional damage of the BTB in the testis and leakage of the auto-immunogenic materials in haploid germ cell antigens beyond the BTB, successively inducing pathological changes in the testicular immune microenvironment.
Previous reports have shown that DEHP expresses different effects on T helper cell type 1 and type 2 cytokines under different activated conditions and in different organs [6,7,8, 24]. This study demonstrated that lymphocytes infiltrated into the testicular interstitium mRNA expressions of IFN-γ and IL-10 but not TNF-α and IL-6 were prominently increased in the testes of DEHP-treated groups. IFN-γ plays roles in macrophage activation, induction of major histocompatibility antigens, T cell homing and regulation of cell proliferation [25, 26]. Therefore, secretion of IFN-γ in the testis after DEHP exposure might be one of the factors that induce CD3+ lymphocytic infiltration and increase F4/80- and MHC class II-positive cells. In experiments with local injection of a cytokine into the testes in vivo, IFN-γ exhibited a direct cytotoxic effect on spermatogenic cells and induced spermatogenesis disturbance [27]. IL-10 is a known immunesuppressive cytokine with a complementary relation to IFN-γ [28]. Also, IL-10 has pro-inflammatory functions, upregulating testicular immunoinflammatory processes by its systemic administration [29]. Therefore, it is possible that changes in cytokines in the testis after exposure to DEHP may induce spermatogenic disturbance.
Here, we have demonstrated that exposure to DEHP at a dose that has little effect on spermatogenesis can induce changes in the testicular immune microenvironment with a little functional damage of the BTB. In the future, we intend to further investigate autoimmune responses in DEHP-treated testes in mice.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (Young Scientists [B]: 24791673 and 24791234) from the Ministry of Education, Culture, Sports, Science and Technology and a Follow-up Grant for the Tokyo Medical University Research Project in Japan. The authors wish to thank Maimaiti Kuerban PhD, Muhetaerjiang Musha PhD, Y Ogawa PhD, and Ms A Abo for technical and secretarial support.
References
- 1.Albro PW, Thomas RO. Enzymatic hydrolysis of di-(2-ethylhexly) phthalate by lipases. Biochim Biophys Acta 1973; 306: 380–390 [DOI] [PubMed] [Google Scholar]
- 2.Daniel JW, Bratt H. The absorption, metabolism and tissue distribution of di(2–ethylhexyl) phthalate in rats. Toxicology 1974; 2: 51–65 [DOI] [PubMed] [Google Scholar]
- 3.Gray TJ, Gangolli SD. Aspects of the testicular toxicity of phthalate esters. Environ Health Perspect 1986; 65: 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitoh Y, Usami K, Nagata T, Marumo H, Imai K, Katoh M. Early changes in the rat testis induced by di-(2-ethylhexyl) phthalate and 2,5-hexanedione. –Ultrastructure and lanthanum trace study–. J Toxicol Pathol 1997; 10: 51–57 [Google Scholar]
- 5.Tay TW, Andriana BB, Ishii M, Choi EK, Zhu XB, Alam MS, Tsunekawa N, Kanai Y, Kurohmaru M. An ultrastructural study on the effects of mono (2-ethylhexyl) phthalate on mice testes: cell death and sloughing of spermatogenic cells. Okajimas Folia Anat Jpn 2007; 83: 123–130 [DOI] [PubMed] [Google Scholar]
- 6.Thomas JA, Darby TD, Wallin RF, Garvin PJ, Martis L. A review of the biological effects of di-(2-ethyhlexyl) phthalate. Toxicol Appl Pharmacol 1978; 45: 1–27 [DOI] [PubMed] [Google Scholar]
- 7.Hansen JS, Larsen ST, Poulsen LK, Nielsen GD. Adjuvant effects of inhaled mono-2-ethylhexyl phthalate in BALB/cJ mice. Toxicology 2007; 232: 79–88 [DOI] [PubMed] [Google Scholar]
- 8.Larsen ST, Hansen JS, Thygesen P, Begtrup M, Poulsen OM, Nielsen GD. Adjuvant and immuno-suppressive effect of six monophthalates in a subcutaneous injection model with BALB/c mice. Toxicology 2001; 169: 37–51 [DOI] [PubMed] [Google Scholar]
- 9.Larsen ST, Hansen JS, Hansen EW, Clausen PA, Nielsen GD. Airway inflammation and adjuvant effect after repeated airborne exposures to di-(2-ethylhexyl) phthalate and ovalbumin in BALB/c mice. Toxicology 2007; 235: 119–129 [DOI] [PubMed] [Google Scholar]
- 10.Takano H, Yanagisawa R, Inoue K, Ichinose T, Sadakane K, Yoshikawa T. Di-(2-ethylhexyl) phthalate enhances atopic dermatitis-like skin lesions in mice. Environ Health Perspect 2006; 114: 1266–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David RM, Moore MR, Finney D, Guest D. Chronic toxicity of di(2-ethylhexyl) phthalate in mice. Toxicol Sci 2000; 58: 377–385 [DOI] [PubMed] [Google Scholar]
- 12.Ablake M, Itoh M, Terayama H, Hayashi S, Shoji S, Naito M, Takahashi K, Suna S, Jitsunari F. Di-(2-ethylhexyl) phthalate induces severe aspermatogenesis in mice, however, subsequent antioxidant vitamins supplementation accelerates regeneration of the seminiferous epithelium. Int J Androl 2004; 27: 274–281 [DOI] [PubMed] [Google Scholar]
- 13.Ishihara M, Itoh M, Miyamoto K, Suna S, Takeuchi Y, Takenaka I, Jitsunari F. Spermatogenic disturbance induced by di-(2-ethylhexyl) phthalate is significantly prevented by treatment with antioxidant vitamins in the rat. Int J Androl 2000; 23: 85–94 [DOI] [PubMed] [Google Scholar]
- 14.Johnsen SG. Testicular biopsy score count-A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970; 1: 2–25 [DOI] [PubMed] [Google Scholar]
- 15.Qu N, Terayama H, Naito M, Ogawa Y, Hirai S, Kitaoka M, Yi SQ, Itoh M. Caput epididymitis but not orchitis was induced by vasectomy in murine model of experimental autoimmune orchitis. Reproduction 2008; 135: 859–866 [DOI] [PubMed] [Google Scholar]
- 16.Itoh M, Ueno M, Li XQ, Satriotomo I, Takeuchi Y. Topographical uptake of blood-borne horseradish peroxidase (HRP) in the murine testis at the light microscopic level. Int J Androl 1998; 21: 74–80 [DOI] [PubMed] [Google Scholar]
- 17.Naito M, Hirai S, Terayama H, Qu N, Kuerban M, Musha M, Kitaoka M, Ogawa Y, Itoh M. Postinflammation stage of autoimmune orchitis induced by immunization with syngeneic testicular germ cells alone in mice. Med Mol Morphol 2012; 45: 35–44 [DOI] [PubMed] [Google Scholar]
- 18.Anton E. Preservation of the rat blood-testis barrier after ligation of the ductuli efferentes, as demonstrated by intra-arterial perfusion with peroxidase. J Reprod Fertil 1982; 66: 227–230 [DOI] [PubMed] [Google Scholar]
- 19.Dym M, Fawcett DW. The blood-testis barrier in the rat and physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3: 308–326 [DOI] [PubMed] [Google Scholar]
- 20.Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev 2006; 213: 66–81 [DOI] [PubMed] [Google Scholar]
- 21.Hedger MP. Testicular leukocytes: what are they doing? Rev Reprod 1997; 2: 38–47 [DOI] [PubMed] [Google Scholar]
- 22.Matsuda T, Kurohane K, Imai Y. Di-(2-ethylhexyl) phthalate enhances skin sensitization to isocyanate haptens in mice. Toxicol Lett 2010; 192: 97–100 [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Qiao Y, Li B, Yang J, Liu D, Yao H, Xu D, Yang X. Adjuvant effect of di-(2-ethylhexyl) phthalate on asthma-like pathological changes in ovalbumin-immunised rats. Food Agric Immunol 2008; 19: 351–362 [Google Scholar]
- 24.Nielsen GD, Larsen ST, Olsen O, Løvik M, Poulsen LK, Glue C, Wolkff P. Do indoor chemicals promote development of airway allergy? Indoor Air 2007; 17: 236–255 [DOI] [PubMed] [Google Scholar]
- 25.Balkwill FR. Interferons. Lancet 1989; 1: 1060–1063 [DOI] [PubMed] [Google Scholar]
- 26.Beller DI. Functional significance of the regulation of macrophage Ia expression. Eur J Immunol 1984; 14: 138–143 [DOI] [PubMed] [Google Scholar]
- 27.Natwar RK, Mann A, Sharma RK, Aulitzky W, Frick J. Effect on human gamma interferon on mice testis: a quantitative analysis of the spermatogenic cells. Acta Eur Fertil 1995; 26: 45–49 [PubMed] [Google Scholar]
- 28.de Waal Malefyt R, Haanen J, Spits H, Roncarolo M, te Velde A, Figdor C, Johnson K, Kastelein R, Ysse H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 1991; 174: 915–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko T, Itoh M, Nakamura Y, Iimura A, Hayashi S, Takahashi K, Stivala F, Bendtzen K, Nicoletti F. Proinflammatory effects of exogenously administered IL-10 in experimental autoimmune orchitis. Cytokine 2003; 22: 50–53 [DOI] [PubMed] [Google Scholar]


