Abstract
Poor reproductive efficiency is a worldwide problem that has affected the dairy industry during the last several decades. In an attempt to explain the changes in reproductive physiology caused by high milk production, a model of elevated steroid metabolism in lactating dairy cows has been proposed. A slow increase in levels and low peak levels of estradiol (E2) and progesterone (P4) characterize endocrine changes in high producing cows. Similar changes have been reported in the repeat breeder cows. The abnormal changes in E2 and P4 concentrations of these cows may cause an improper uterine environment due to disturbed expression of growth factors and cytokines in the endometrium. This review focuses on the alteration in epidermal growth factor (EGF) profile in the endometrium during the estrous cycle. The normal cow has two peaks of EGF concentrations on days 2–4 and 13–14. Low concentrations of EGF on these days distinguished both high-producing and repeat breeder cows from normal cows. Alteration of the EGF profile could be found in 70 and 40% of the repeat breeder and high-producing cows, respectively. Treatment with a high dose of estradiol benzoate and an intravaginal progesterone-releasing device restored the normal EGF profile in about 70% of the affected cows. The cows having a normal EGF profile after treatment showed a higher pregnancy rate than the cows with the altered profile. Further studies to understand the etiology of the alteration in the EGF profile are needed to develop another treatment option and preventive management for this problem.
Keywords: Embryonic loss, Endometrial biopsy, Estrogen, Infertility, Progesterone
Decreased Calving Rate and Increased Early Embryonic Loss in Modern High-producing Dairy Cows
Fertilization rate is apparently similar in modern high-producing and traditional moderate-producing cows (more than 90%); nevertheless the average calving rate for a single service is significantly lower in modern high-producing cows (40%) than in traditional moderate-producing cows (55%) [1]. Substantial evidence indicates that the difference in the calving rate between the 2 groups of cows may reflect an increased frequency of early embryonic loss occurring before day 16 of pregnancy [1]. Frequency of early embryonic loss is about 30 and 45% in the traditional and modern dairy cows, respectively. Studies using embryo transfer in the cow have demonstrated the critical importance of the state of the uterine environment for the viability and development of the embryo [2]. A considerable proportion of early embryonic loss could be attributed to inadequate circulating progesterone (P4) concentrations. The relationship between circulating P4 levels during the early luteal phase and the likelihood of an animal remaining pregnant has been reported [3,4,5,6,7]. The cows showing early rise and higher peak levels of P4 produce larger embryos with higher capacity to secrete interferon-τ by day 16 of pregnancy. The effect of early rise in P4 concentrations could be partly attributed to advanced or optimized endometrial gene expression [8] and enhanced histotroph secretion into the uterine lumen [9, 10], changes that ensure survival and development of the embryo.
Potential Mechanism by Which Alterations in the Ovarian Steroid Hormone Profile Increases Embryonic Loss in High-producing and Repeat Breeder Cows
In an attempt to explain some of the changes in reproductive physiology related to decreased fertility caused by high milk production, a model of elevated steroid metabolism in lactating dairy cows has been proposed [11, 12]. Lactating cows have greater energy requirements than non-lactating cows. The high feed consumption required to meet these energy requirements leads to a dramatic increase in liver blood flow, which leads to elevated metabolism of both estradiol (E2) and P4. This would cause a reduction in E2 and P4 concentrations in the circulation, even in the midst of high production of steroid hormones by the follicle or corpus luteum (CL).
Frequency of embryonic loss increases in the repeat breeder cows [13,14,15]. Repeat breeder cows may not necessarily be high producers but exhibit the similar alterations in the ovarian steroid hormone profiles to those found in high-producing cows. The reported alterations in the repeat breeder cows include a late postovulatory rise [16,17,18] and subsequent low concentrations [16, 17, 19, 20] of progesterone. The alterations also include decreased concentrations of estrogen before and after ovulation [21] on the day of estrus. These alterations have been linked to causes of early embryonic loss, including premature termination of the CL [15] and asynchrony between the uterus and embryonic development during the early luteal phase [14].
Importantly, data on steroid hormone concentrations in the subfertile cows are inconsistent among reports [2, 15], partly because the alterations in circulating E2 and P4 levels are modest and, thus, often indistinguishable from the normal changes. However, the alterations in the ovarian steroid hormone profile in high-producing and repeat breeder cows could be amplified and may become detectable in the endometrium as an alteration of growth factor and cytokine expression, since ovarian steroid hormones primarily regulate timing and levels of expression of these local factors [22, 23]. Growth factors and cytokines form a central regulatory network of the uterine environment to which developing embryos are exposed. The alteration of growth factor and cytokine expression would, in turn, increases embryonic loss in the cows having the altered steroid hormone profile. This hypothesis seems to be consistent with the mechanism by which embryonic loss increases in superovulated immature rats. Elevated insulin-like growth factor-I (IGF-I) activity was found in the uterus of immature rats as a result of elevation in circulating E2 concentrations after treatment with a superovulatory dose of equine chorionic gonadotropin [24]. The increased IGF-I activity in the superovulated immature rats has been found to alter the uterine environment and increase early embryonic loss [24, 25]. Normalization of IGF-I activity in the uterus resulted in an improvement in fertility [25]. The change in electrolyte composition of the uterine luminal fluid is an example of the alterations in the uterine environment for embryonic development after superovulation [26]. The electrolyte composition of the uterine fluid is controlled by ovarian steroid hormones [2]. This effect of steroid hormones seems to be mediated by growth factors that include IGF-I in the rats [26]. An alteration in electrolyte composition has also been reported and linked to the increase in embryonic loss in repeat breeder cows [2]. Together, the alteration in the cyclic change in growth factors and cytokines could be a potential mechanism by which the abnormal ovarian steroid hormone profile increases embryonic loss in high-producing and repeat breeder cows (Fig. 1). This review focuses on the role of the alterations in the endometrial epidermal growth factor (EGF) profile in high-producing and repeat breeder cows.
Fig. 1.
Hypothesis by which the abnormal changes in steroid hormones increases embryonic loss in the high-producing and repeat breeder cows.
Approach to Seek Hidden Causes of Repeat Breeding in Dairy Cows
The reasons for the decline in fertility of modern dairy cows are multifactorial; thus, it is most important to exclude animals with any known causes of infertility when seeking hidden causes of infertility in repeat breeder cows. The study should exclude cows with any sign of abnormality in the reproductive organs and function. Repeat breeder cows should be examined for inflammation of the uterus by using ultrasonography and endometrial cytology and for oviductal patency. Cows showing extended estrous periods and delayed ovulation and those that fail to show clear estrous behaviors should also be excluded from the study. In addition, cows conceived by either 1 or 2 artificial inseminations (AI) immediately after the study might be used as the normal (fertile) control cows. A strict selection policy for recruitment of animals for a study may be essential to enabling the detection of a hidden difference between the repeat breeder and normal cows.
Changes in the Endometrial EGF Concentrations during the Estrous Cycle of Dairy Cows and Potential Relationship between the Endometrial EGF Profile and Fertility
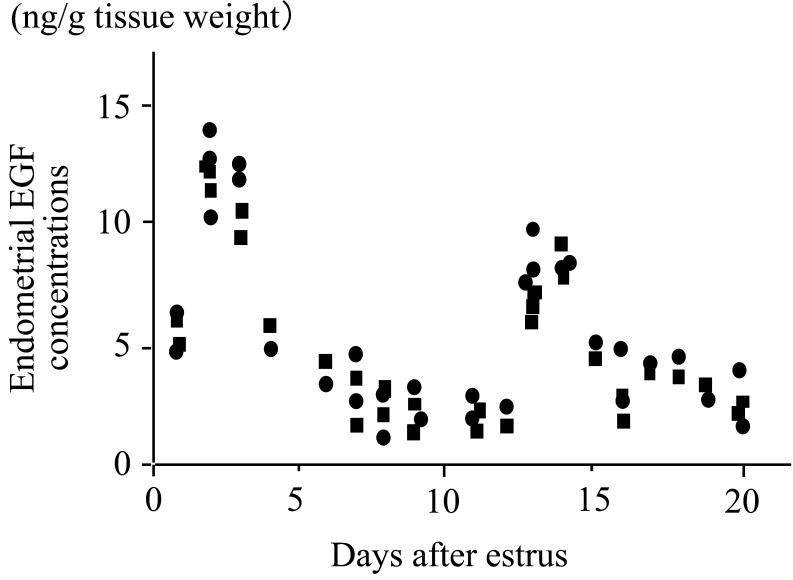
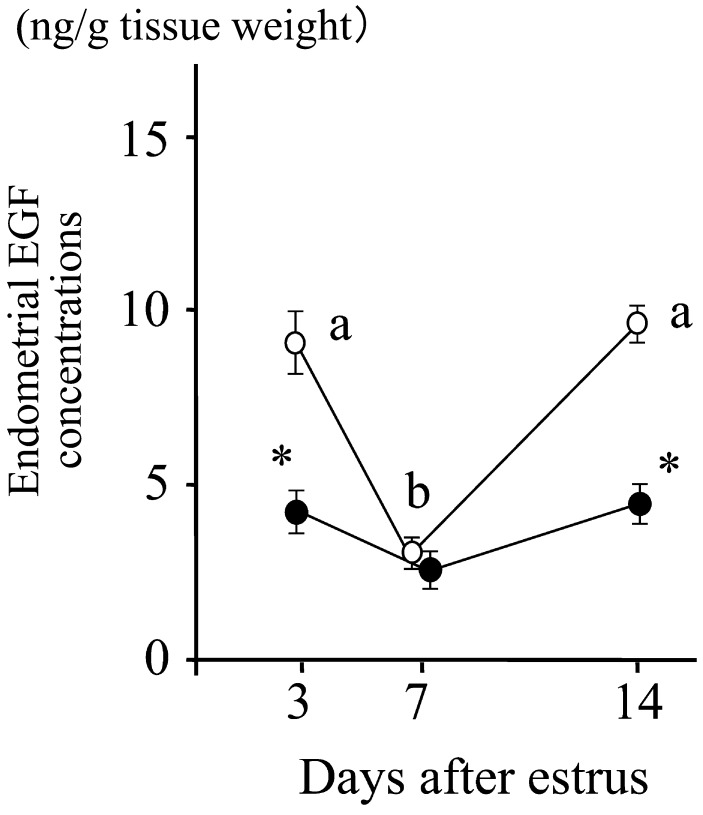
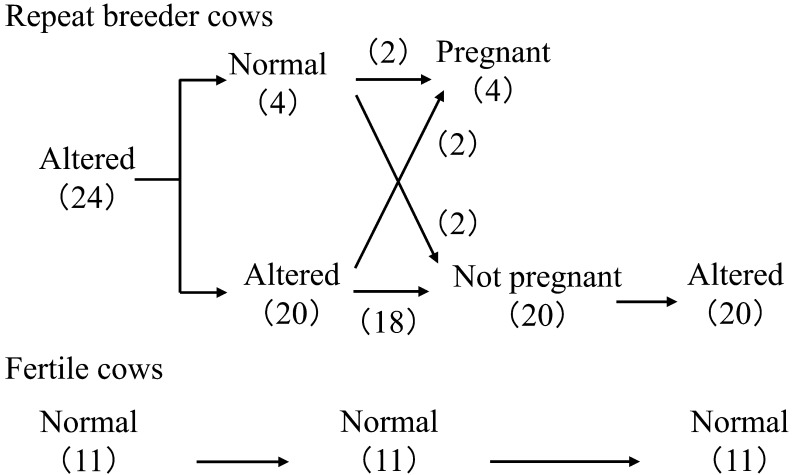
In normal cows, the endometrial EGF concentrations peak twice, on days 2–4 and 13–14, with lesser concentrations of EGF around day 7 (Fig. 2) [27]. The change in endometrial EGF concentrations has been confirmed by using repeated biopsy of the uterine endometrial tissues on days 3, 7 and 14 of the estrous cycle (Fig. 3) [28]. These studies also found [27] and confirmed [28] an alteration in the EGF profile in repeat breeder cows selected with the abovementioned strict criteria (Fig. 3). Decreased EGF concentrations on days 3 and 14 clearly distinguished the repeat breeder cows from the controls, and about 70% of the repeat breeder cows showed this alteration [28]. More importantly, the patterns of the EGF profile persisted between the estrous cycles in both fertile and repeat breeder cows (Fig. 4). In the fertile controls, the endometrial EGF concentrations exhibited a normal pattern throughout the 3 consecutive estrous cycles. In the repeat breeder cows, the alteration in the endometrial EGF concentrations persisted between the estrous cycles when the cows were not treated.
Fig. 2.
Concentrations of endometrial epidermal growth factor (EGF) during the estrous cycle. Thirty-one Holstein cows were killed at slaughterhouse between days 2 and 20 of the estrous cycle. Circles and squares indicate EGF concentrations (means from the 3 pieces of tissues) of the uterine horns ipsilateral and contralateral, respectively, to the ovary with the corpus luteum. Adopted from Katagiri and Takahashi (2004) [27].
Fig. 3.
Patterns of EGF concentrations in the endometrial tissues obtained by biopsy from 76 repeat breeder cows (closed circle) and 99 control cows (open circle). Values with different letters (a, b) differ (P<0.01). Values with an asterisk differ from controls (P<0.01). Adopted from Katagiri and Takahashi (2006) [28].
Fig. 4.
Changes in the pattern of endometrial EGF profiles and consequence of AI service in the repeat breeder and fertile cows. Numbers in parentheses indicate the number of cows. Twenty-four repeat breeder cows with low EGF concentrations on days 3 and 14 were examined a second time for the EGF profile during the next estrous cycle and, then, subjected to artificial insemination (AI). Twenty cows that showed estrus between 18 and 24 days after AI were again examined for the EGF profile. Eleven apparently normal cows were examined for the EGF profile during the 3 consecutive estrous cycles. All 11 cows conceived immediately after the study. Modified from Katagiri and Takahashi (2006) [28].
An alteration in the endometrial EGF profile was found in about 40 (55 out of 144 cows) and 20% (14 out of 71 cows) of the apparently normal high-producing cows (>10,000 kg of milk) during the periods of 60 to 90 and 90 to 120 days postpartum, respectively. Most of the cows producing less than 30 kg of milk per day showed the EGF concentrations within the normal range (8.7 ± 1.5 ng/g tissue weight, mean ± SD), while the cows producing more than 45 kg of milk per day showed the EGF concentrations near or below the lower limit of the normal range for lactating cows on day 3 (5.1 ± 0.4 ng/g tissue weight) (unpublished data). These findings indicate that high milk production or physiological changes associated with it may play a key role in the etiology of this alteration.
When repeat breeder cows having an altered EGF profile were treated and inseminated up to 2 times, the pregnancy rate of the cows with a normalized EGF profile after treatment was greater than that of the cows with an altered EGF profile (about 80 vs. 25%, P<0.05) [28]. Among those that became pregnant, about 65% of the pregnancies were obtained by the first AI after the endometrial EGF profile had been normalized by treatment. This preliminary study used a small number of cows (n=26) but clearly indicated a potential relationship between the endometrial EGF profile and fertility in cows.
Further evidence supporting the potential relationship between the EGF profile and fertility comes from a study using a total of 437 recipient cows for embryo transfer (Table 1) [29]. All cows were subjected to determination of the endometrial EGF concentration on day 3 and transferred an embryo on day 7. The recipient cows having the EGF concentrations below the normal range on day 3 showed a lower conception rate than those having the EGF concentrations within the normal range (33.3 vs. 76.9%, P<0.01). In contrast to AI, ET bypasses the critical steps for the establishment of pregnancy: fertilization and early embryonic development to day 7. Thus, the difference in conception rate after ET between the EGF concentration groups (i.e., the normal and low concentrations on day 3) may better reflect the state of the uterine environment for embryonic development between days 7 and 16 (timings of embryo transfer and pregnancy recognition, respectively).
Table 1. Conception rate of embryo transfer recipients* at different levels of endometrial EGF concentrations.
| Endometrial EGF concentrations (ng/g tissue weight) |
No. (%) of cows | No. (%) of cows conceived |
| < 2.5 | 23 (5.3) | 11 (47.8)a |
| 2.5–5.0 | 64 (14.6) | 18 (28.1)a |
| 5.0–7.5 | 109 (24.9) | 66 (60.6)ab |
| 7.5–10.0 | 110 (25.2) | 94 (85.5)b |
| 10.0–12.5 | 97 (22.2) | 82 (84.5)b |
| 12.5< | 34 (7.8) | 27 (79.4)b |
| Total | 437 (100) | 298 (68.2) |
* Japanese black (310), Holstein (87), F1 and unknown (40). a,b Values with different letters differ significantly (P<0.05). Produced by using data of Katagiri (2006) [29].
Treatment of Repeat Breeder and High-producing Cows Having an Altered EGF Profile
As mentioned above, the alteration of the endometrial EGF profile has been linked to reduced fertility and could be found in 70 and 40% of the repeat breeder and high-producing cows, respectively. Like most of health problems in dairy cows, the fundamental resolution of this problem could only be achieved by optimization of herd management, including nutrition and cow comfort. However, development of a treatment for this alteration may contribute to improve fertility of modern dairy cows and profitability of the dairy industry.
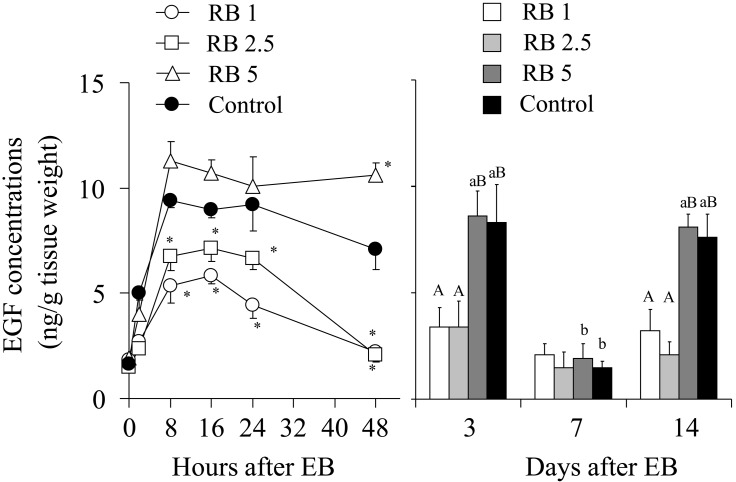
Use of steroid hormones to correct the alteration of the EGF profile may be a logical approach if the alteration of the endometrial EGF profile is caused by the abnormal steroid hormone profile in high-producing and repeat breeder cows. We have developed a hormonal treatment with a high dose of estradiol benzoate (EB) and a controlled internal drug release (CIDR) device [30]. First, repeat breeder cows having low EGF concentrations on days 3 and 14 were treated with 1, 2.5 or 5 mg of EB and prostaglandin F2α on the day of insertion and withdrawal of a CIDR device, respectively. Treatment of repeat breeder cows with 5 mg of EB produced a similar change in the endometrial EGF concentrations during the first 48 h after EB treatment to the fertile controls treated with 1 mg of EB and restored the normal EGF profile. On the other hand, treatment with 1 and 2.5 mg of EB produced a suppressed increase in the EGF concentrations and failed to restore the normal EGF profile (Fig. 5) [30]. A fertility study using the CIDR protocol with 1 and 5 mg of EB showed that the protocol with 5 mg of EB efficiently restored the normal EGF profile (66.7%) and fertility (66.7% with 2 AIs) (Table 2). Importantly, both in the 1 and 5 mg EB groups, cows having the normal EGF profile after treatment showed a greater pregnancy rate than those having an abnormal profile [30]. This finding again confirmed the relationship between the EGF profile and fertility (Fig. 6).
Fig. 5.
Response of endometrial EGF concentrations to treatment with different doses of estradiol benzoate (EB) with a controlled internal drug release (CIDR) device. Groups of 5 repeat breeder cows having an altered EGF profile were treated with 1, 2.5 and 5 mg of EB (RB1, RB2.5 and RB5, respectively). Five apparently normal cows were treated with 1 mg of EB as fertile controls. Adopted from Katagiri and Takahashi (2008) [30].
Table 2. Effect of treatment on the normalization of endometrial EGF concentrations and fertility of repeat breeder cowsa.

Fig. 6.
Relationship between the endometrial EGF profile and fertility. The endometrial EGF concentrations peak twice on days 2–4 and 13–14, with lesser concentrations of EGF around day 7 in the normal cows. Decreased EGF concentrations on days 3 and 14 clearly distinguished the repeat breeder and high-producing cows from the normal cows. About 70 and 40% of the repeat breeder and high-producing cows, respectively, showed this alteration. Treatment with a high dose (5 mg) of EB with a CIDR device restored the normal EGF profile and fertility in about 70 and 60%, respectively, of the repeat breeder cows having an altered EGF profile.
Future Studies
At present, the EGF profile is determined by measuring the EGF concentrations in the endometrial tissues obtained by biopsy on day 3 (and days 7 and 14 if necessary) of the estrous cycle. This procedure may be technically demanding, to some extent, and requires a special tool to obtain the endometrial tissues. These are obvious limitations that preclude diagnosis of the alteration from a widespread use in the field. It is necessary to establish markers that are easy and sensitive enough to detect an alteration in the EGF profile or a physiological change associated with the alteration in the EGF profile under field conditions. Studies to enhance our understanding of the etiology of this problem may provide information to select potential markers. Further, as our understanding of this problem improves, practical manipulations of the ovarian steroid hormone profile or other factors that predispose or complicate alteration of the EGF profile could be developed in the future to prevent this problem in high-producing cows.
There may be great interest from practitioners regarding a new treatment option for this alteration. Currently, use of a protein factor(s) in the seminal plasma to restore the normal EGF profile is under investigation. Treatment with a high dose of EB with a CIDR device could be a solution for those living in an area of the world where use of E2 products is allowed in the commercial dairy herd. The new treatment may provide an additional treatment option for those with E2 products and become the first practical solution for those without E2 products in the list of approved drugs for dairy cows.
Acknowledgments
The studies on endometrial EGF and its alterations were supported by Grants-in-Aid for Scientific Research (No. 11760188, 15580284, 20580350 and 23580446) from the Japan Society for the Promotion of Science.
References
- 1.Diskin MG, Murphy JJ, Sreenan JM. Embryo survival in dairy cows managed under pastoral conditions. Anim Reprod Sci 2006; 96: 297–311 [DOI] [PubMed] [Google Scholar]
- 2.Ayalon N. A review of embryonic mortality in cattle. J Reprod Fertil 1978; 54: 483–493 [DOI] [PubMed] [Google Scholar]
- 3.Forde N, Beltman ME, Duffy P, Mehta JP, O’Gaora P, Roche JF, Lonergan P, Crowe MA. Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol Reprod 2011; 84: 266–278 [DOI] [PubMed] [Google Scholar]
- 4.Inskeep EK. Preovulatory, postovulatory, and postmaternal recognition effects of concentrations of progesterone on embryonic survival in the cows. J Anim Sci 2004; 82(E-Suppl): E24–E39 [DOI] [PubMed] [Google Scholar]
- 5.Lonergan P, Woods A, Fair T, Carter F, Rizos D, Ward F, Quinn K, Evans A. Effect of embryo source and recipient progesterone environment on embryo development in cattle. Reprod Fertil Dev 2007; 19: 861–868 [DOI] [PubMed] [Google Scholar]
- 6.Mann GE, Lamming GE. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction 2001; 121: 175–180 [DOI] [PubMed] [Google Scholar]
- 7.Mann GE, Fray MD, Lamming GE. Effects of time of progesterone supplementation on embryo development and interferon-τ production. Vet J 2006; 171: 500–503 [DOI] [PubMed] [Google Scholar]
- 8.Forde N, Lonergan P. Transcriptomic analysis of the bovine endometrium: what is required to establish uterine receptivity to implantation in cattle? J Reprod Dev 2012; 58: 189–195 [DOI] [PubMed] [Google Scholar]
- 9.Bazer FW, Vallet JL, Roberts RM, Sharp DC, Thatcher WW. Role of conceptus secretory products in establishment of pregnancy. J Reprod Fertil 1986; 76: 841–850 [DOI] [PubMed] [Google Scholar]
- 10.Geisert RD, Morgan GL, Short EC, Zavy MT. Endocrine events associated with endometrial function and conceptus development in cattle. Reprod Fertil Dev 1992; 4: 301–305 [DOI] [PubMed] [Google Scholar]
- 11.Sangsritavong S, Combs DK, Sartori R, Armentano LE, Wiltbank MC. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17β in dairy cattle. J Dairy Sci 2002; 85: 2831–2842 [DOI] [PubMed] [Google Scholar]
- 12.Wiltbank M, Lopez H, Sartori R, Sangsritavong S, Gumen A. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology 2006; 65: 17–29 [DOI] [PubMed] [Google Scholar]
- 13.Casida LE. Present status of the repeat-breeder cow problem. J Dairy Sci 1961; 44: 2323–2329 [Google Scholar]
- 14.Levine AR. The repeat breeder cow. Bov Prac 1999; 33: 97–105 [Google Scholar]
- 15.Peters AR. Embryonic mortality in the cow. Animal Breeding Abstr 1996; 64: 587–598 [Google Scholar]
- 16.Båge R, Gustafsson H, Larsson B, Forsberg M, Rodríguez-Martínez H. Repeat breeding in dairy heifers: follicular dynamics and estrous cycle characteristics in relation to sexual hormone patterns. Theriogenology 2002; 57: 2257–2269 [DOI] [PubMed] [Google Scholar]
- 17.Kimura M, Nakao T, Moriyoshi M, Kawata K. Luteal phase deficiency as a possible cause of repeat breeding in dairy cows. Br Vet J 1987; 143: 560–566 [DOI] [PubMed] [Google Scholar]
- 18.Shelton K, Gayerie de Abreu MF, Hunter MG, Parkinson TJ, Lamming GE. Luteal inadequacy during the early luteal phase of subfertile cows. J Reprod Fertil 1990; 90: 1–10 [DOI] [PubMed] [Google Scholar]
- 19.Hansel W. Plasma hormone concentrations associated with early embryo mortality in heifers. J Reprod Fertil Suppl 1981; 30(Suppl): 231–239 [PubMed] [Google Scholar]
- 20.Lamming GE, Darwash AO, Back HL. Corpus luteum function in dairy cows and embryo mortality. J Reprod Fertil Suppl 1989; 37(Suppl): 245–252 [PubMed] [Google Scholar]
- 21.Erb RE, Gaverick HA, Randel RD, Brown BL, Callahan CJ. Profile of reproductive hormones associated with fertile and non-fertile inseminations of dairy cows. Theriogenology 1976; 5: 227–242 [DOI] [PubMed] [Google Scholar]
- 22.Brigstock DR. Growth factors in the uterus: steroidal regulation and biological actions. Baillieres Clin Endocrinol Metab 1991; 5: 791–808 [DOI] [PubMed] [Google Scholar]
- 23.Paria BC, Song H, Dey SK. Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol 2001; 45: 597–605 [PubMed] [Google Scholar]
- 24.Katagiri S, Ho Yuen B, Moon YS. The role for the uterine insulin-like growth factor I in early embryonic loss after superovulation in the rat. Fertil Steril 1996; 65: 426–436 [DOI] [PubMed] [Google Scholar]
- 25.Katagiri S, Moon YS, Ho Yuen B. A somatostatin analog decreases early embryonic loss following superovulation in rats by normalising insulin-like growth factor-I in the uterus. Hum Reprod 1997; 12: 671–676 [DOI] [PubMed] [Google Scholar]
- 26.Katagiri S, Ma S, Ho Yuen B, Moon YS. Role for insulin-like growth factor I in the regulation of electrolyte composition of uterine luminal fluid. J Reprod Fertil 1997; 109: 115–120 [DOI] [PubMed] [Google Scholar]
- 27.Katagiri S, Takahashi Y. Changes in EGF concentrations during estrous cycle in bovine endometrium and their alterations in repeat breeder cows. Theriogenology 2004; 62: 103–112 [DOI] [PubMed] [Google Scholar]
- 28.Katagiri S, Takahashi Y. Potential relationship between normalization of endometrial epidermal growth factor profile and restoration of fertility in repeat breeder cows. Anim Reprod Sci 2006; 95: 54–66 [DOI] [PubMed] [Google Scholar]
- 29.Katagiri S. Relationship between endometrial epidermal growth factor and fertility after embryo transfer. J Reprod Dev 2006; 52(Suppl): S133–S137 [Google Scholar]
- 30.Katagiri S, Takahashi Y. A progestin-based treatment with a high dose of estradiol benzoate normalizes cyclic changes in endometrial EGF concentrations and restores fertility in repeat breeder cows. J Reprod Dev 2008; 54: 473–479 [DOI] [PubMed] [Google Scholar]