Abstract
Puberty in mammals is timed by an increase in gonadotropin-releasing hormone (GnRH) secretion. Previous studies have shown involvement of the two neuropeptides, kisspeptin and neurokinin B (NKB), in controlling puberty onset. Little is known about the role of the other key neuropeptide, dynorphin, in controlling puberty onset, although these three neuropeptides colocalize in the arcuate kisspeptin neurons. The arcuate kisspeptin neuron, which is also referred to as the KNDy neuron, has recently been considered to play a role as an intrinsic source of the GnRH pulse generator. The present study aimed to determine if attenuation of inhibitory dynorphin-kappa-opioid receptor (KOR) signaling triggers the initiation of puberty in normal developing female rats. The present study also determined if stimulatory NKB-neurokinin 3 receptor (NK3R) signaling advances puberty onset. Female Wistar-Imamichi rats were weaned and intraperitoneally implanted with osmotic minipumps filled with nor-binaltorphimine (nor-BNI), a KOR antagonist, or senktide, a NK3R agonist, at 20 days of age. Fourteen days of intraperitoneal infusion of nor-BNI or senktide advanced puberty onset, manifested as vaginal opening and the first vaginal estrus in female rats. Frequent blood sampling showed that nor-BNI significantly increased luteinizing hormone (LH) pulse frequency at 29 days of age compared with vehicle-treated controls. Senktide tended to increase this frequency, but its effect was not statistically significant. The present results suggest that the inhibitory input of dynorphin-KOR signaling plays a role in the prepubertal restraint of GnRH/LH secretion in normal developing female rats and that attenuation of dynorphin-KOR signaling and increase in NKB-NK3R signaling trigger the onset of puberty in female rats.
Keywords: GPR54, Metastin, Sexual maturation
Puberty, a complex sequence of maturational events, is driven by the activation of the hypothalamic-pituitary-gonadal axis. The secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus represents the first well-known step in the initiation of puberty [1, 2]. GnRH-induced gonadotropin secretion from the anterior pituitary stimulates gametogenesis and gonadal steroidogenesis in the ovary [3]. An increase in the circulating level of estrogen leads to opening of the vaginal cavity as a sign of puberty onset in female rodents, and then female rodents consequently show estrous cyclicity. The potential mechanism underlying pubertal increase in GnRH secretion has been sought by pinpointing the loss-of-functional mutations of genes coding kisspeptin, neurokinin B (NKB) or their cognate receptors, GPR54 and neurokinin 3 receptor (NK3R), in patients with hypogonadotropic hypogonadism [4,5,6,7,8]. Previous rodent studies showed pubertal increases in kisspeptin and NKB expression in the hypothalamus [9,10,11,12], and central administration of kisspeptin advanced puberty onset in normal developing female rats [13]. Central administration of senktide, an NK3R agonist, induced puberty onset in 5 of 11 underfed female rats [12], suggesting that NKB-NK3R signaling at least partly contributes to the pubertal increase in GnRH secretion in underfed rats. On the other hand, central administration of SB222200, an NK3R antagonist, failed to affect puberty onset in normally fed rats [12]. This inconsistency in the involvement of NKB-NK3R signaling in puberty onset led us to evaluate the roles of NKB-NK3R signaling in puberty onset in normally fed developing female rats.
Dynorphin, a member of the endogenous opioids derived from the prodynorphin gene [14], has emerged as a key inhibitory neuropeptide in controlling reproductive function [15,16,17,18]. Dynorphin neurons are abundantly distributed in several regions of the hypothalamus [19, 20] including the ARC, where dynorphin is coexpressed in a group of neurons with kisspeptin and NKB in mice [21], rats [22], sheep [23] and goats [16]. The group of ARC neurons, referred to as KNDy neurons [23], was recently considered to play a critical role in pulsatile GnRH secretion. A series of studies recording the chronic multiple unit activity (MUA) in close proximity to KNDy neurons in the ARC of goats demonstrated that periodic bursts (MUA volleys) were accompanied by luteinizing hormone (LH) pulses with regular intervals [16, 24], suggesting that ARC KNDy neurons may be the intrinsic source of the GnRH pulse generator and that the MUA volleys may represent the rhythmic release of kisspeptin from the KNDy neurons. Central administration of NKB or a synthetic antagonist for kappa-opioid receptor (KOR), the preferred receptor for dynorphin, facilitated the MUA volleys [16]. Combined with the fact that kisspeptin governs GnRH/LH secretion in a variety of mammals [24,25,26,27,28,29], these findings suggest that dynorphin and NKB expressed in ARC KNDy neurons are involved in the process of generating the rhythmic discharge of kisspeptin that drives pulsatile GnRH/LH secretion. More specifically, dynorphin and NKB have an inhibitory and stimulatory role, respectively, in generating pulsatile GnRH/LH secretion [16].
To date, no mutation of a gene coding dynorphin or KOR has been reported in human. Little is known about the involvement of dynorphin-KOR signaling in the regulation of the pubertal increase in GnRH/LH pulses. Our recent study indicated that central administration of the KOR antagonist facilitates pulsatile LH secretion in estrogen-treated ovariectomized rats but not ovariectomized animals, suggesting that dynorphin is involved in mediating the negative feedback action of estrogen on GnRH/LH secretion in female rats [18]. Taken together with estrogen-dependent suppression of LH secretion in prepubertal rats [10], we hypothesized that dynorphin-KOR signaling plays a central and critical role in the negative regulation of the prepubertal restraint GnRH/LH secretion in rats. A single intracerebroventricular injection of KOR antagonist had no effect on LH secretion in prepubertal female rats [30], suggesting that dynorphin-KOR signaling had lower importance with regard to the prepubertal restraint of LH secretion in rats. Chronic KOR-antagonizing action, however, may be required for advancement of puberty onset in female rats.
The present study aimed to determine the roles of dynorphin-KOR and NKB-NK3R signaling in initiation of puberty in normal developing female rats. To this end, we examined the effects of chronic peripheral administration of KOR antagonist and NK3R agonist on puberty onset and LH pulses in peripubertal female rats to evaluate if attenuation of dynorphin-KOR signaling triggers puberty onset and if an increase in NKB-NK3R signaling advances puberty onset. Peripheral infusion was chosen because of the possibility that these treatments in patients with a disruption of normal pulsatile GnRH/LH secretion may be therapeutic.
Materials and Methods
Animals
Wistar-Imamichi strain rats were kept under a 1410 h light/dark cycle (lights on at 0500 h) at 22 ± 2 C with free access to food (CE-2; CLEA Japan, Tokyo, Japan) and water. Female rats (8–9 weeks old of age) having at least two consecutive regular 4-day estrus cycles were mated with males overnight on the day of proestrus, and then the pregnant females were housed individually. The day on which a newborn litter was found at noon was designated postnatal day 0. The litter size was adjusted to eight on day 1 to minimize the growth variation within litters. The pups were weaned on day 20. Surgical procedures were performed under anesthesia with inhaled isoflurane or a ketamine-xylazine mixture.
The care of the animals and all of the experimental procedures used in these experiments were approved by the Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University.
Effects of chronic intraperitoneal administration of KOR antagonist or NK3R agonist on puberty onset
Female rats (n = 6 per treatment group) were weaned on day 20 and randomly assigned to one of three treatment groups. Alzet osmotic minipumps (model 2002; flow rate 0.5 μl/h, 200 μl capacity; Durect, Cupertino, CA, USA) filled with nor-binaltorphimine (nor-BNI; 800 nmol/100 μl; Sigma-Aldrich, St. Louis, MO, USA), a KOR antagonist, or senktide (800 nmol/100 μl; synthesized at Kyoto University, Japan), an NK3R agonist, were surgically implanted into the abdominal cavity of female rats on day 20. Control animals were implanted with the pump filled with a vehicle (17 mM NaHCO3) in the same manner. The dose of senktide (4 nmol/h/40–110 g BW) was chosen in accordance with a previous study, in which continuous subcutaneous infusion of senktide (2400 nmol/h/20–35 kg BW) produced intermittent bursts of the GnRH pulse generator with corresponding LH pulses in goats [31]. An equimolar dose of nor-BNI was used. Female rats were housed in groups of three in plastic cages, and their body weights and vaginal openings were monitored daily. After vaginal opening, vaginal smears were examined daily to monitor estrous cyclicity. The first day when the cornified cells were dominant was designated as the day of the first vaginal estrous.
Effects of chronic intraperitoneal administration of KOR antagonist or NK3R agonist on pulsatile LH secretion in peripubertal rats
Female rats were weaned at day 20 and randomly assigned to one of three treatment groups: nor-BNI (n=5), senktide (n=4) and vehicle (n=5). On day 29, blood samples (50 µl) were collected from the free-moving animals for 3 h at 6-min intervals, starting at 1300 h, through a silicone catheter inserted into the right atrium through the jugular vein on the previous day of blood sampling. An equivalent volume of rat red blood cells, taken from donor rats and diluted with heparinized saline, was replaced through the cannula after each blood collection to keep the hematocrit constant. Plasma was separated by immediate centrifugation and stored at –20 C until assayed for LH.
At the end of blood sampling, rats were sacrificed and ovaries and uteri were collected and weighed. Five hundred-microliter blood samples were also collected to determine plasma estradiol-17β levels.
LH and estradiol-17β assay
LH contents in 25-μl plasma samples were measured by a double antibody radioimmunoassay (RIA) using a rat LH RIA kit provided by the National Hormone and Peptide Program (Baltimore, MD, USA). Plasma LH concentrations were expressed in terms of the NIDDK rat LH RP-3. Values below the level of detectability for the assay were assigned the minimum detectable concentration of the assay. The least detectable level was 7.8 pg/tube, and the intra- and inter-assay coefficients of variation were 6.2% and 7.2% at 45 pg/tube, respectively.
Estradiol-17β contents in 35-µl plasma samples were measured by electrochemiluminescence using ECLusys E2II kit (Roche Diagnostics Japan, Tokyo, Japan) in a commercial laboratory (SRL, Tokyo, Japan). Values below the level of detectability for the assay were assigned the minimum detectable concentration of the assay. The least detectable level was 0.35 pg/tube, and the intra- and inter-assay coefficients of variation were 4.5% and 3.1% at 1.26 pg/tube, respectively.
Data analysis
LH pulses were identified by the PULSAR computer program [32]. Mean LH concentrations and the frequency and amplitude of LH pulses were calculated for the 3-h sampling period in each individual. Averages of these parameters were then calculated for each group. Before statistical analysis, frequency of LH pulses was square-root transformed to normalize variability across a range of values. Statistical differences in the mean LH concentrations, in the frequency of LH pulses and in the age and body weight at vaginal opening and first estrus were determined by one-way ANOVA followed by the post hoc Tukey’s Honestly Significant Difference (HSD) test. Statistical differences in amplitude of LH pulses were not determined because only one vehicle-treated animal showed an LH pulse.
Results
Effects of chronic intraperitoneal administration of KOR antagonist or NK3R agonist on puberty onset
Chronic intraperitoneal infusion of nor-BNI, a KOR antagonist, between days 20 and 34 advanced vaginal opening and the first estrus. Half of the nor-BNI-treated animals showed the vaginal opening on day 29, whereas no vehicle-treated controls showed vaginal opening at this time (Fig. 1A). In addition, all individuals showed the first estrus by day 37, whereas no vehicle-treated controls showed first estrus at this time (Fig. 1B). The average age at vaginal opening tended to be younger in nor-BNI-treated animals (P=0.052, Tukey’s HSD test) than in the vehicle-treated controls (Fig. 1C), and the average age at first estrus was significantly younger in nor-BNI-treated animals (P<0.05, Tukey’s HSD test) than in the vehicle-treated controls (Fig. 1D). The nor-BNI treatment had no significant effect on the body weight at any age (Fig. 2A). The average body weight at vaginal opening (Fig. 2B) and first estrus (Fig. 2C) were significantly lower (P<0.05, Tukey’s HSD test) in nor-BNI-treated animals than in vehicle-treated controls.
Fig. 1.
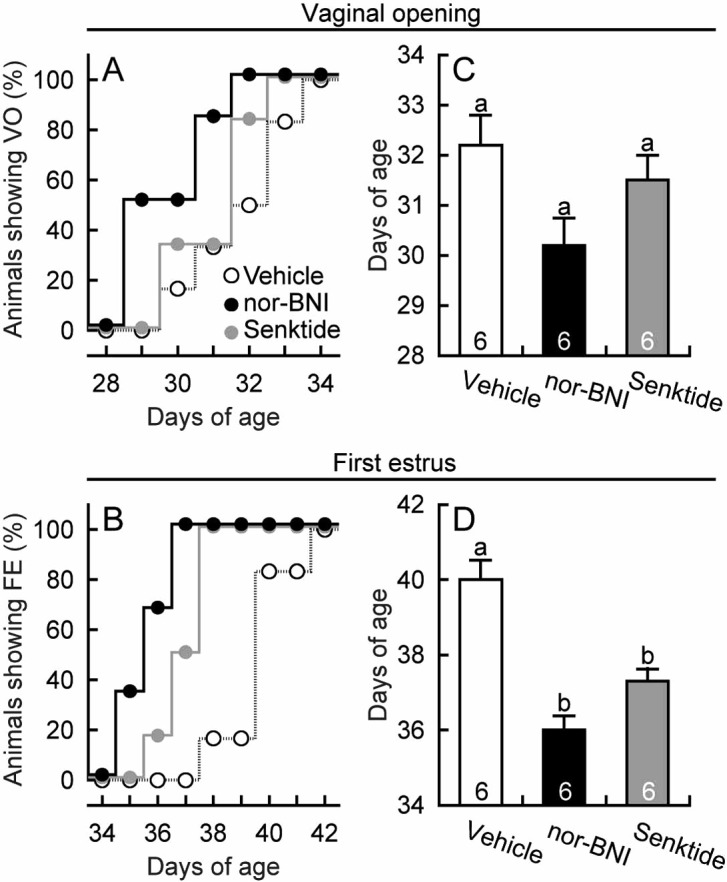
Advanced puberty onset in female rats treated with nor-binaltorphimine (nor-BNI), a kappa-opioid receptor antagonist, or senktide, a neurokinin 3 receptor agonist. Vaginal openings, vaginal smears and body weights were monitored daily. Ages at vaginal opening and first estrus (expressed as a percentage of the total number of animals per experimental group) are shown in panels A and B. Ages at vaginal opening and first estrus are shown in panels C and D. Numbers in each column indicate numbers of animals used. Values are means ± SEM. Values with different letters are significantly different (P < 0.05, one-way ANOVA followed by Tukey’s HSD test) from each other in each panel.
Fig. 2.
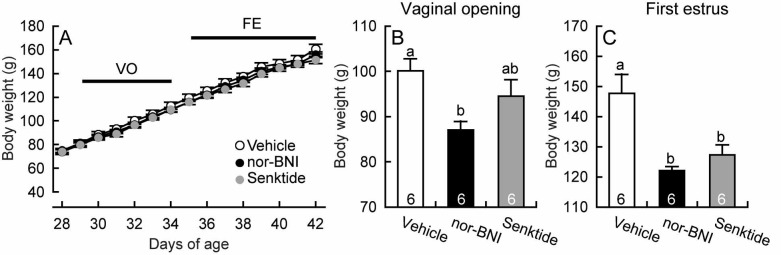
Body size at puberty onset in female rats treated with nor-binaltorphimine (nor-BNI), a kappa-opioid receptor antagonist, or senktide, a neurokinin 3 receptor agonist. Vaginal openings, vaginal smears and body weights were monitored daily. Growth curves and timing of vaginal opening and of first estrus in female rats are shown in panel A. Body weights at vaginal opening and first estrus are shown in panels B and C. Numbers in each column indicate numbers of animals used. Values are means ± SEM. Values with different letters are significantly different (P < 0.05, one-way ANOVA followed by Tukey’s HSD test) from each other in each panel.
Chronic intraperitoneal infusion of senktide, an NK3R agonist, also advanced the first estrus, but was slightly less effective than nor-BNI. Half of the senktide-treated animals showed the first estrus on day 37, when no vehicle-treated controls showed the first estrus (Fig. 1B). The average age (Fig. 1D) and body weight (Fig. 2C) at the first vaginal estrus were significantly (P<0.05, Tukey’s HSD test) younger and lower in senktide-treated animals than in vehicle-treated controls, respectively, but the age at vaginal opening was comparable to that in the vehicle-treated animals.
Effects of chronic intraperitoneal administration of KOR antagonist or NK3R agonist on pulsatile LH secretion in peripubertal rats
Intraperitoneal chronic infusion of nor-BNI or senktide between days 20 and 29 enhanced LH secretion at 29 days of age as shown in the representative animals in Fig. 3A. All five nor-BNI-treated animals and three out of four senktide-treated animals showed pulsatile LH secretion, while four out of five vehicle-treated animals showed no LH pulse. The frequency of LH pulses in the nor-BNI-treated rats was significantly higher (P<0.05, Tukey’s HSD test) compared with the vehicle-treated controls, while the frequency in the senktide-treated rats was comparable to that in the vehicle-treated rats (Fig. 3B). There was no significant difference in mean LH concentrations at 29 days of age between experimental and vehicle-treated animals (Fig. 3B).
Fig. 3.
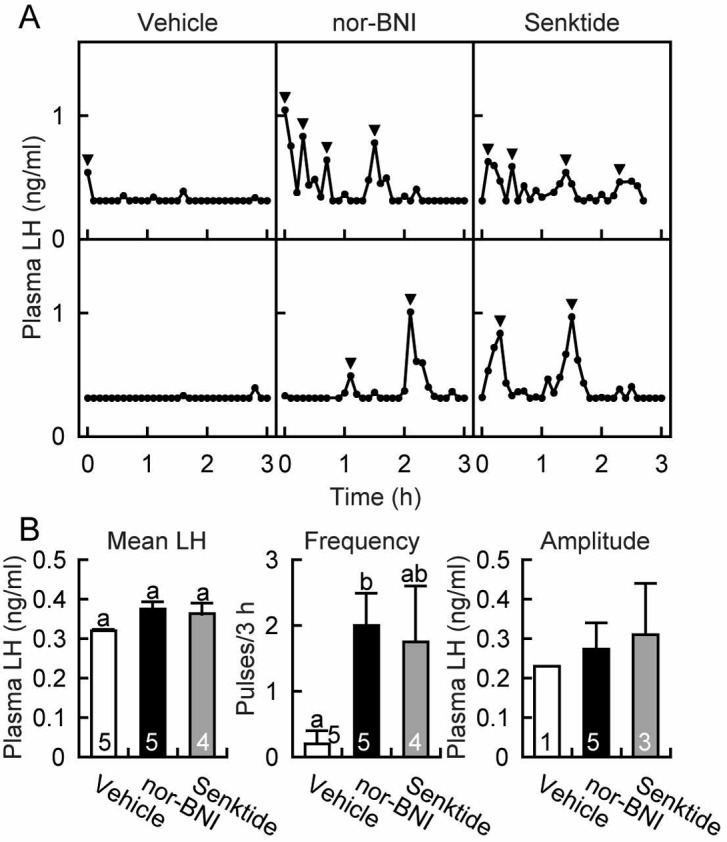
Advanced increase in plasma luteinizing hormone (LH) levels in female rats treated with a kappa-opioid receptor antagonist, nor-binaltorphimine (nor-BNI), or a neurokinin 3 receptor agonist, senktide. Plasma LH profiles in representative animals are shown in panel A. Arrowheads indicate peaks of LH pulses identified by the PULSAR computer program. Mean LH concentrations and the frequency and amplitude of LH pulses are shown in panel B. The numbers in each column of mean LH concentrations and the frequency of LH pulses indicate the numbers of animals used, and the numbers in each column of the amplitude of LH pulses indicate the number of animals showing LH pulses. Values are means ± SEM. Values with different letters are significantly different (P < 0.05, one-way ANOVA followed by Tukey’s HSD test) from each other. Statistical differences in amplitude of LH pulses were not determined because only one vehicle-treated animal showed an LH pulse.
Chronic administration of nor-BNI tended to increase plasma estradiol levels and uterine weight, but both the nor-BNI and senktide treatments failed to show a significant effect on plasma estradiol levels and weights of ovaries and uteri at 29 days of age (Fig. 4).
Fig. 4.
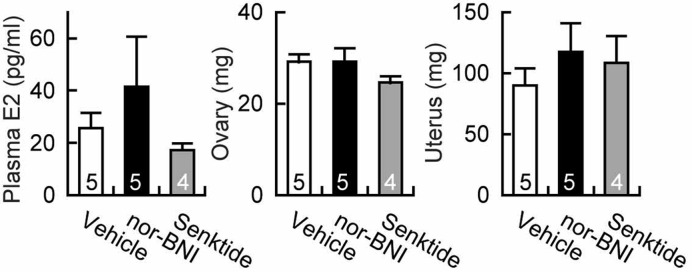
Effect of a kappa-opioid receptor antagonist, nor-binaltorphimine (nor-BNI), or a neurokinin 3 receptor agonist, senktide, on plasma estradiol concentrations and weights of ovaries and uteri at 29 days of age. The numbers in each column indicate the numbers of animals used. Values are means ± SEM. Values were not significantly different from each other in each panel.
Discussion
The present study demonstrates that dynorphin-KOR signaling plays a critical inhibitory role in the pubertal restraint of GnRH/LH secretion in female rats, because chronic peripheral administration of nor-BNI, a KOR antagonist, advanced pulsatile LH secretion and hence puberty onset in developing female rats. Dynorphin neurons are abundantly distributed in several regions of the hypothalamus [19, 20] including the ARC, wherein dynorphin is coexpressed in a group of neurons with kisspeptin and NKB in mammals [16, 21,22,23]. Increasing evidence indicates that a group of ARC neurons, referred to as KNDy neurons [23], may play a critical role in a pulsatile GnRH secretion [16, 24]. Navarro et al. [21] showed that KOR mRNA was found in 20% of KNDy neurons in the ARC of female mice, suggesting that recurrent collaterals of KNDy neurons could signal through a dynorphin-KOR signaling pathway. Thus, dynorphin-KOR signaling in the ARC KNDy neurons, a putative intrinsic source for driving the GnRH pulse generator, may play a critical role in prepubertal restraint of GnRH/LH secretion. Recently, Navarro et al. [35] suggested that some other KOR-expressing interneurons (not KNDy neurons) may mediate the action of dynorphin on GnRH pulse generation. Therefore, the current KOR antagonist may directly or indirectly act on ARC KNDy neurons to enhance GnRH/LH pulses in prepubertal rats. It is unlikely that the KOR antagonist directly acts on GnRH neurons, because previous studies showed few KOR expressions in rat GnRH neurons [33, 34]. Further studies are needed to clarify the site(s) of KOR antagonism, which advanced puberty onset in the present study.
The present study demonstrates that NKB-NK3R signaling also plays a role in regulating the pubertal increase in GnRH/LH secretion in normal developing female rats, because the administration of senktide, a NK3R agonist, advanced pulsatile LH secretion in 75% of animals and hence puberty onset in normal developing female rats. Navarro et al. [12] showed that repeated central administration of senktide advanced puberty onset in underfed female rats. Taken together with the facilitatory effect we found with senktide on LH pulses, the stimulatory role of NKB-NK3R signaling was at least partly involved in the increase in pubertal GnRH/LH secretion, and thus puberty onset, in normal developing rats as well as underfed rats. KNDy neurons have been reported to express KN3R in mammals including rats [20, 21, 36], suggesting that the current senktide may directly act KNDy neurons to advance puberty onset in female rats.
Based on the roles of dynorphin and NKB in gating pubertal initiation of GnRH/LH pulsatile secretion, we propose a possible mechanism for the pubertal activation of the GnRH/LH pulse generator in rats. The present study showed that the KOR antagonist had a more potent effect on relieving LH pulses from prepubertal restraint in female rats, compared with the NK3R agonist. This leads us to an assumption that the GnRH pulse generator may be mainly downregulated by inhibitory dynorphin-KOR signaling during the prepubertal period, rid of this inhibition at the onset of puberty and then upregulated by stimulatory input of NKB. This assumption is consistent with the previous finding that NK3R antagonist SB222200 administration had no effect on puberty onset in intact developing peripubertal female rats [12]. The present study used only a single dose of nor-BNI and senktide to evaluate the role of dynorphin and NKB signaling on the onset of puberty. A higher dose of senktide or other more potent NK3R agonists may overcome prepubertal restraint of GnRH/LH secretion. Several other issues need to be addressed, such as the effective doses, stability in circulation and binding affinity for its receptors. Future investigations are required to examine these issues.
The present results may lead to a chance to apply KOR antagonism and/or NK3R agonism to restore GnRH/LH secretion for patients bearing a hypogonadotropic hypogonadism. Exogenous gonadotropin therapy, the current major approach for human infertility, bears the risk of hyperstimulation of gonadal function, such as multiple follicular development leading to cycle cancellation, ovarian hyperstimulation syndrome and multiple pregnancies, all of which require intense monitoring of hormonal profiles. Administration of pulsatile GnRH is another therapeutic method for human infertility with a low risk of a multiple pregnancies. However, this method could be a burden on patients because of its methodological complexity, such as maintenance of an infusion pump attached to an intravenous or subcutaneous catheter. Thus, chronic administration of a KOR antagonist or NK3R agonist with a sustained-release capsule could be an alternative therapeutic approach for stimulating gonadal function based on the physiological function of GnRH/LH pulse generation.
In conclusion, the present study suggests that the inhibitory input of dynorphin on the ARC KNDy neurons plays a role in the prepubertal restraint of GnRH/LH secretion in normal developing female rats. The present study also suggests that attenuation of dynorphin-KOR signaling and an increase in NKB-NK3R signaling trigger the onset of puberty in normal developing female rats. This study expands our understanding of the mechanism controlling pubertal changes in GnRH/LH pulse generation and suggests a possible therapy comprised of KOR antagonism and/or NKB agonism, which restores GnRH/LH secretion in patients with a disruption in normal pulsatile GnRH/LH secretion.
Acknowledgments
The authors are indebted to the National Hormone and Peptide Program for the rat LH assay kit, and to Drs GR Merriam and KW Wachter for the PULSAR computer program. The RIA and LH pulse analyses were performed at the Nagoya University Radioisotope Center and the Information Technology Center, respectively. This study was supported in part by a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences (to KM), by the Research Program on Innovative Technologies for Animal Breeding, Reproduction and Vaccine Development (to HT) and by Grants-in Aid from the Japan Society for the Promotion of Science No. 23380163 (to HT) and No. 23580402 (to YU).
References
- 1.Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 1989; 125: 92–99 [DOI] [PubMed] [Google Scholar]
- 2.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 2001; 142: 2929–2936 [DOI] [PubMed] [Google Scholar]
- 3.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci 2004; 7: 1040–1047 [DOI] [PubMed] [Google Scholar]
- 4.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627 [DOI] [PubMed] [Google Scholar]
- 5.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 2009; 41: 354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonca BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 2010; 95: 2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 2010; 95: 2287–2295 [DOI] [PubMed] [Google Scholar]
- 9.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 2004; 145: 4565–4574 [DOI] [PubMed] [Google Scholar]
- 10.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 2009; 21: 527–537 [DOI] [PubMed] [Google Scholar]
- 11.Gill JC, Navarro VM, Kwong C, Noel SD, Martin C, Xu S, Clifton DK, Carroll RS, Steiner RA, Kaiser UB. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology 2012; 153: 4883–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, Garcia-Galiano D, Hobbs SJ, Manfredi-Lozano M, Leon S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 2012; 32: 2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol 2004; 561: 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci USA 1985; 82: 4291–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 2004; 145: 2959–2967 [DOI] [PubMed] [Google Scholar]
- 16.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 2010; 1364: 103–115 [DOI] [PubMed] [Google Scholar]
- 18.Mostari MP, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda KI, Tsukamura H. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev 2013; 59: 266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J Comp Neurol 1988; 276: 442–459 [DOI] [PubMed] [Google Scholar]
- 20.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 2006; 498: 712–726 [DOI] [PubMed] [Google Scholar]
- 21.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 2012; 153: 2800–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010; 151: 3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821 [DOI] [PubMed] [Google Scholar]
- 25.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 2004; 80: 264–272 [DOI] [PubMed] [Google Scholar]
- 26.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 2004; 320: 383–388 [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436 [DOI] [PubMed] [Google Scholar]
- 28.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 2005; 102: 2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pheng V, Uenoyama Y, Homma T, Inamoto Y, Takase K, Yoshizawa-Kumagaye K, Isaka S, Watanabe TX, Ohkura S, Tomikawa J, Maeda KI, Tsukamura H. Potencies of centrally- or peripherally-injected full-length kisspeptin or its C-terminal decapeptide on LH release in intact male rats. J Reprod Dev 2009; 55: 378–382 [DOI] [PubMed] [Google Scholar]
- 30.Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, Millar RP, Lightman SL, O’Byrne KT. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One 2012; 7: e44344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakabayashi Y, Yamamura T, Ohkura S, Homma T, Sakamoto K, Mori Y, Okamura H . Senktide, a neurokinin B receptor agonist, stimulates pulsatile LH secretion through a mechanism mediated by the GnRH pulse generator in goats. In: Program of 42nd Society for Neuroscience Annual Meeting; 2012; New Orleans, USA. Abstract 90.07.
- 32.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318 [DOI] [PubMed] [Google Scholar]
- 33.Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC. Presence of mu and kappa opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport 1997; 8: 3167–3172 [DOI] [PubMed] [Google Scholar]
- 34.Sannella MI, Petersen SL. Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for mu, kappa, or delta opiate receptors. Endocrinology 1997; 138: 1667–1672 [DOI] [PubMed] [Google Scholar]
- 35.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011; 152: 4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 2010; 22: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]


