Abstract
The efficiency of cloning by somatic cell nuclear transfer (SCNT) has remained low. In most cloned embryos, epigenetic reprogramming is incomplete, and usually the genome is hypermethylated. The DNA methylation inhibitor 5-aza-2’-deoxycytidine (5-aza-dC) could improve the developmental competence of cow, pig, cat and human SCNT embryos in previous studies. However, the parameters of 5-aza-dC treatment among species are different, and whether 5-aza-dC could enhance the developmental competence of porcine cloned embryos has still not been well studied. Therefore, in this study, we treated porcine fetal fibroblasts (PFF) that then were used as donor nuclei for nuclear transfer or fibroblast-derived reconstructed embryos with 5-aza-dC, and the concentration- and time-dependent effects of 5-aza-dC on porcine cloned embryos were investigated by assessing pseudo-pronucleus formation, developmental potential and pluripotent gene expression of these reconstructed embryos. Our results showed that 5-aza-dC significantly reduced the DNA methylation level in PFF (0 nM vs. 10 nM vs. 25 nM vs. 50 nM, 58.70% vs. 37.37% vs. 45.43% vs. 39.53%, P<0.05), but did not improve the blastocyst rate of cloned embryos derived from these cells. Treating cloned embryos with 25 nM 5-aza-dC for 24 h significantly enhanced the blastocyst rate compared with that of the untreated group. Furthermore, treating cloned embryos, but not donor cells, significantly promoted pseudo-pronucleus formation at 4 h post activation (51% for cloned embryos treated, 34% for donor cells treated and 36% for control, respectively, P<0.05) and enhanced the expression levels of pluripotent genes (Oct4, Nanog and Sox2) up to those of in vitro fertilized embryos during embryo development. In conclusion, treating cloned embryos, but not donor cells, with 5-aza-dC enhanced the developmental competence of porcine cloned embryos by promotion of pseudo-pronucleus formation and improvement of pluripotent gene expression.
Keywords: 5-aza-2’-deoxycytidine, Embryo development, Pig, Somatic cell nuclear transfer
The first cloned pigs were born ten years ago [1]; however, the low cloning efficiency impedes the application of cloning technique. In most cloned embryos, epigenetic reprogramming is incomplete, such as aberrant DNA methylation and histone modification, which affects developmental potential [2]. It is generally believed that DNA methylation could reflect the epigenetic reprogramming degree of cloned embryos [3]; therefore, the mechanism of reprogramming induced by somatic cell nuclear transfer (SCNT) mainly focuses on the DNA methylation.
Compared with that of in vivo or in vitro fertilized embryos, the genome of cloned embryos is usually more highly methylated, leading to failed or disrupted reactivation of the genes that are essential for the embryo development [4, 5]. Thus, inefficient demethylation results in developmental block or lethality in cloned embryos [6].
To improve the efficiency of DNA demethylation and developmental competence of cloned embryos, various strategies have been used, and 5-aza-dC, a hypomethylating drug, is usually applied, which is incorporated into DNA during DNA synthesis, inhibits DNA methyltransferase 1 (dnmt 1) activity and results in DNA hypomethylation [7]. Hypomethylated DNA allows transcription factors to bind to gene promoter regions and regulate gene expression. It has been shown that 5-aza-dC could reactivate silenced pluripotent genes and improve nuclear reprogramming efficiency [8,9,10,11]. Thus, appropriate reactivation of genes related to early embryo development induced by 5-aza-dC would enhance the developmental competence of cloned embryos.
Previous studies have shown that 5-aza-dC could improve the developmental competence of cloned embryos [7, 12,13,14], and the treatment should be performed with the appropriate concentration and for the suitable time duration. However, the parameters of 5-aza-dC treatment among species are different, and whether 5-aza-dC could enhance the developmental competence of porcine cloned embryos has still not been well studied. During embryo development, many factors could reflect embryo developmental competence, and of these factors, remodeling of nuclear morphology and the expression patterns of pluripotent genes are closely correlated with embryo developmental competence [15, 16]. Therefore, in this study, we investigated the concentration- and time-dependent effects of 5-aza-dC on the developmental competence of cloned embryos by treating donor cells or cloned embryos with 5-aza-dC, and examined the pseudo-pronucleus formation post activation and the expression levels of pluripotent genes during the development of cloned embryos.
Materials and Methods
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), and disposable and sterile plasticware was obtained from Nunclon (Roskilde, Denmark), unless otherwise stated.
All experiments were approved by the Animal Care Commission of Northeast Agriculture University, according to animal welfare laws, guidelines and policies.
Fetal fibroblast cell collection, culture and treatment
Porcine fetal fibroblast cells (PFFs) were isolated from a 35-day-old fetus. Briefly, the fetus was recovered and rinsed five times with Dulbecco’s phosphate buffered saline (DPBS). After removal of the head, internal organs and limbs, the remaining tissues were finely minced into pieces. Then minced tissues were digested with 0.25% trypsin-0.04% ethylenediaminetetraacetic acid (EDTA) solution at 37 C for 45 min, followed by dispersal in high glucose enriched Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The dispersed cells were centrifuged, resuspended, and cultured in high glucose enriched DMEM containing 10% FBS and 1% penicillin-streptomycin at 38.5 C in 5% CO2 atmosphere and saturated humidity. Until confluence, cells were digested, centrifuged and resuspended in FBS containing 10% dimethyl sulfoxide (DMSO), and they were then stored in liquid nitrogen until use. Prior to SCNT, PFFs were thawed, cultured and subsequently used in 3–5 passages.
For donor cell treatment, PFFs in the 3rd passage were treated with various concentrations (0, 10, 25 and 50 nM) of 5-aza-dC for 72 h in DMEM supplemented with 10% FBS without any antibiotics to analyze morphology, cell cycle and DNA methylation.
Cell cycle and DNA methylation level
Flow cytometry was applied as in our previous report [17]. The effect of 5-aza-dC on the cell cycle was determined by staining with propidium iodide (PI) and measuring fluorescence intensity in a FACSCalibur flow cytometer (Becton Dickinson, New York, USA). Briefly, PFFs were digested by trypsinization, centrifuged, washed with cold DPBS and fixed in cooled 75% methanol overnight. Then PFFs were washed with DPBS containing 5 mM EDTA and incubated in DPBS containing 50 µg ml–1 RNase and 50 µg ml–1 PI for 30 min. Subsequently, PFFs were washed with DPBS containing 5 mM EDTA, filtered through a 30 µm nylon mesh and analyzed on a FACSCalibur flow cytometer. The proportion in G0/G1, S and G2/M phases was calculated using the ModFit software.
Measurement of the cellular DNA methylation level with flow cytometry has been reported previously [18]. In brief, PFFs were trypsinized, resuspended in cold saline GM and fixed in 100% ethanol at 4 C for at least 12 h. Then PFFs were incubated in anti-5-methylcytosine antibody (Santa Cruz, Texas, USA) at 4 C overnight, washed with DPBS plus 5% FBS, and resuspended in FITC-conjugated secondary antibody (Santa Cruz) at 37 C for 30 min. After PFF filtration through a 30 μm nylon mesh, a FACSCalibur flow cytometer was used to measure the fluorescence level, and the fluorescence intensity of PFFs that were not treated with the secondary antibody was considered to be zero.
Oocyte collection and in vitro maturation (IVM)
Porcine ovaries were collected from a local slaughterhouse and transported to the laboratory in physiological saline with antibiotics at 37 C. Follicles with a diameter between 3 and 8 mm were aspirated, and follicular contents were washed three times with HEPES-buffered Tyrode’s lactate (TL-HEPES). Cumulus-oocyte complexes (COCs) with at least three uniform layers of compact cumulus cells and uniform cytoplasm were recovered, washed and cultured in maturation medium (TCM-199 supplemented with 0.57 mM cysteine, 10 ng ml–1 epidermal growth factor, 0.5 µg ml–1 follicle stimulating hormone, 0.5 µg ml–1 luteinizing hormone and 100 µl ml–1 porcine follicular fluid) under mineral oil at 38.5 C in a 5% CO2 atmosphere and saturated humidity. After 42 h, COCs were vortexed in 1 mg ml–1 hyaluronidase for 3 min to remove cumulus cells. Only oocytes with a visible polar body, regular morphology and homogenous cytoplasm were used.
IVF and SCNT, embryo culture, treatment and collection
The procedures for porcine IVF and SCNT have been described previously in one of our reports [19]. Briefly, for IVF, the semen was incubated at 39 C, resuspended and washed three times in DPBS supplemented with 0.1% bovine serum albumin (BSA). The sperm concentration was measured using a hemocytometer, and the proportion of motile sperm was determined. The spermatozoa were diluted with modified Tris-buffered medium (mTBM) to the appropriate concentration. Matured oocytes were washed three times in mTBM, transferred into fertilization medium (FM) and co-incubated with spermatozoa for 6 h at the rate of 1:1000. Then embryos were washed and cultured in porcine zygote medium-3 (PZM-3) for subsequent development. For SCNT, PFFs were trypsinized, centrifuged and resuspended in manipulation medium. The IVM oocytes and donor cells were placed in manipulation medium supplemented with 7.5 µg ml–1 cytochalasin B. After enucleation by aspirating the first polar body and adjacent cytoplasm, the donor cell was placed into the perivitelline space. Fusion and activation of the cell-cytoplast complexes were induced with two direct pulses of 1.2 kv cm–1 for 30 µsec in fusion medium (0.3 M Mannitol, 0.05 mM CaCl2, 0.1 mM MgCl2, and 0.5 mM HEPES), and fusion rate was confirmed by microscopic examination. Then reconstructed embryos were cultured in PZM-3 for subsequent development, and the cleavage and blastocyst rates were evaluated at 48 and 156 h after activation, respectively.
Cloned embryos derived from PFFs treated with 5-aza-dC were cultured in PZM-3 without 5-aza-dC, and cloned embryos derived from untreated PFFs were subject to treatment with 5-aza-dC at different concentrations (0, 10, 25 and 50 nM) for the same time duration or treatment with 5-aza-dC at the same concentration for a different time duration. Embryos then were washed and transferred into PZM-3 without 5-aza-dC for further culture.
For IVF and SCNT embryos, embryos at the 1-cell, 2-cell, 4-cell, 8-cell and blastocyst stages were collected at 6, 24, 48, 72 and 156 h, respectively.
Nuclear staining
The cloned embryos (donor cells treated with 5-aza-dC only, namely AN, neither donor cells nor cloned embryos treated, namely NT, cloned embryos treated only, namely NA) at 2, 4, 6 and 156 h post activation were treated with acidic Tyrode’s solution to remove the zona pellucida, fixed in 4% paraformaldehyde for 30 min, and stained in DPBS containing 10 mg l–1 Hoechst 33342 for 5 min in the dark. After staining, cloned embryos were washed, transferred to Antifade Mounting Medium (Beyotime, Shanghai, China) on sildes and covered with cover glass, and the edges were sealed with nail polish. The nuclear status and blastocyst cell number were examined or counted under ultraviolet light of a fluorescence microscope (Nikon 80i, Tokyo, Japan).
Quantitative real-time PCR
Measurement of gene expression with quantitative real-time PCR has been applied in our previous research [19, 20]. Briefly, total RNA was extracted from 30 pooled embryos at each stage using an RNeasy Mini Kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s instructions. RNA quality was confirmed by the ratios of A260/A280 (all between 1.8 and 2.0), and only RNA samples that did not show signs of degradation were used in this study. Reverse transcription was performed using PrimeScript® RT Reagent Kit (Perfect Real Time, TaKaRa, Dalian, China). The 20 µl reaction volume contained 1 µl 100 µM random hexamer primer, 4 µl 5×RT buffer, 1 µl RT Enzyme Mix, 1 µl Total RNA (<500 ng) and 13 µl RNase Free dH2O. The reaction were performed with the following parameters: 37 C for 15 min and 85 C for 5 sec; and the cDNA was stored at –20 C until use. For quantitative real-time PCR, reactions were performed in 96-well optical reaction plates (Applied Biosystems, California, USA) using SYBR® Premix ExTaqTM II (Perfect Real Time, TaKaRa) and a 7500 Real-Time PCR System (Applied Biosystems). Each reaction mixture (50 µl) contained 1 µl (<25 ng) cDNA solution, 1 µl 10 µM of each specific primer, 1 µl 50×SYBR Green Dye, and 25 µl 2×Ex Taq. The thermal cycling conditions were 95 C for 30 sec, followed by 40 two-step cycles at 95 C for 5 sec and at 60 C for 34 sec and finally a dissociation stage consisting of 95 C for 15 sec, 60 C for 1 min and 95 C for 15 sec. The specificity of the PCR reaction was confirmed by a single peak in the dissociation curve and also by a single band in agarose gel electrophoreses. As negative controls, cDNA was omitted during the real-time reaction. For each sample, the cycle threshold (CT) values were obtained from three replicates. The primers used for amplification of target and internal reference genes are presented in Table 1. The comparative CT method was used for relative quantification of target gene expression levels. Each pair of primers was confirmed for equal amplification efficiency to primers of the endogenous control (18S ribosomal RNA). The CT value was calculated by the Sequence Detection System software (Applied Biosystems). The ∆CT value was defined as CT (target gene)-CT (18S rRNA). The ∆∆CT value was defined as ∆CT (sample)-∆CT (calibrator). The relative expression levels of target genes were analyzed using the 2−ΔΔCT method.
Table 1. Detail of primers for quantitative real-time PCR.
| Gene | Primer sequence(5’-3’) | Length (bp) | Accession number |
| Oct4 | F:GAAGGTGTTCAGCCAAACGAC | 185 | NM_001113060 |
| R:CGATACTTGTCCGCTTTC | |||
| Nanog | F:CCTCCATGGATCTGCTTATTC | 209 | NM_001129971 |
| R:CATCTGCTGGAGGCTGAGGT | |||
| Sox2 | F:AACCAGAAGAACAGCCCAGAC | 155 | NM_001123197 |
| R:TCCGACAAAAGTTTCCACTCG | |||
| 18s | F:AATCTCGGGTGGCTGAACGC | 143 | NR_002170 |
| R:CCGTTCTTAGTTGGTGGAGCGAT |
Statistical analysis
Differences of data (mean ± SEM) were analyzed with the SPSS statistical software. Statistical analysis of data regarding embryo development was performed using the general linear model (GLM). The data for cell number, cell cycle, DNA methylation, gene expression and blastocyst cell number were analyzed with one-way ANOVA. For all analyses, differences were considered to be statistically significant when P<0.05.
Results
Treating donor cells with 5-aza-dC did not improve the developmental competence of cloned embryos
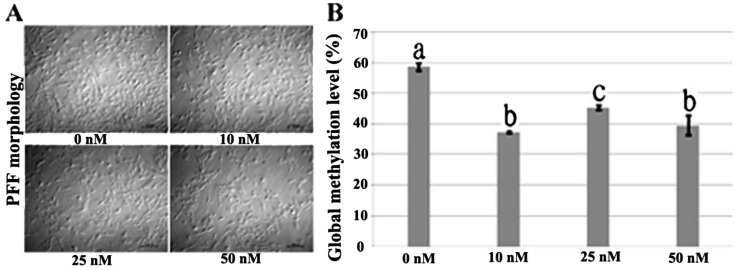
When PFFs were exposed to various concentrations of 5-aza-dC, we found that cell proliferation was affected, especially at 25 or 50 nM, showing that the cell numbers and the percentages of cells in the S phase of the cell cycle significantly decreased (Fig. 1 A and Table 2). Global DNA methylation levels of PFFs treated with 5-aza-dC were determined by flow cytometry. Compared with the DNA methylation level of PFFs that were not treated with 5-aza-dC (58.70%), those of PFFs treated at all concentrations employed in this study significantly decreased, especially in the 10 and 50 nM groups (37.37% and 39.53%, respectively) (Fig. 1 B), indicating that 5-aza-dC could enhance the cloned embryo development by causing DNA hypomethylation of donor cells. Unexpectedly, when donor cells were treated with 5-aza-dC, the developmental competence of cloned embryos decreased, showing that the cleavage rate was significantly different from that of the control group when 10 nM 5-aza-dC was employed in donor cell treatment, and as for the 25 or 50 nM 5-aza-dC treatment group, the fusion, cleavage and blastocyst rates were significantly lower than those of the untreated group (Table 3). Therefore, our results showed that 5-aza-dC could reduce the DNA methylation level of PFFs, but not improve the developmental competence of porcine cloned embryos when donor cells were treated, and taking the effects of 5-aza-dC on cell proliferation, DNA methylation and fusion rate into account, we employed 10 nM 5-aza-dC to treat PFFs for 72 h in the subsequent experiments (AN).
Fig. 1.
Morphology and global DNA methylation level of PFFs treated with 5-aza-dC for 72 h. PFF morphology was observed under a light microscope, bar = 100 μm. (A) DNA methylation levels of PFFs treated with different concentrations were determined by flow cytometry. a–c Percentages (% ± SEM) in columns with different superscripts differ significantly (P<0.05).
Table 2. Cell cycle of PFFs treated with 5-aza-dC.
| 5-aza-dC (nM) | Cell cycle stage (% ± SEM) | ||
| G0/G1 | S | G2/M | |
| 0 | 85.38 ± 0.26 | 5.77 ± 0.13a | 8.85 ± 0.37ab |
| 10 | 86.03 ± 0.19 | 5.27 ± 0.15a | 8.70 ± 0.33a |
| 25 | 85.78 ± 0.40 | 4.35 ± 0.24b | 9.87 ± 0.36ab |
| 50 | 85.85 ± 0.43 | 4.04 ± 0.07b | 10.11 ± 0.50b |
a, b Percentages at the same stage with different superscripts differ significantly (P<0.05).
Table 3. Development of cloned embryos derived from donor cells treated with 5-aza-dC.
| 5-aza-dC (nM) | No. (Rep.) | No. embryos fused (% ± SEM) |
No. embryos cleaved (% ± SEM)* |
No. blastocysts (% ± SEM)* |
Total cell numbers of blastocysts (mean ± SEM)& |
| 0 | 402 (4) | 300 (73.12 ± 4.07)a | 268 (88.19 ± 2.47)a | 64 (20.71 ± 1.55)a | 37 ± 2 (n=63)a |
| 10 | 341 (4) | 243 (70.65 ± 2.63)ab | 196 (80.98 ± 1.99)b | 44 (18.08 ± 1.04)ab | 34 ± 2 (n=42)ab |
| 25 | 387 (4) | 228 (58.37 ± 3.80)b | 176 (77.00 ± 2.04)b | 33 (14.23 ± 1.95)b | 34 ± 2 (n=28)ab |
| 50 | 364 (4) | 207 (56.54 ± 2.88)bc | 161 (77.50 ± 1.90)b | 28 (13.51 ± 1.88)b | 31 ± 2 (n=24)b |
* Cleavage and blastocyst rates were adjusted for fusion rates. & Blastocyst cell numbers of less than 16 were not included. a–c Values in the same column with different superscripts differ significantly (P<0.05).
Treating cloned embryos with 5-aza-dC enhanced embryo developmental competence
Developmental competence could be improved when cloned embryos were treated with 5-aza-dC [7]; however, at present, the concentration- and time-dependent treatment effects of 5-aza-dC on the subsequent development of porcine cloned embryos have not been determined. Therefore, we investigated the developmental competency of cloned embryos treated with 5-aza-dC at different concentrations or exposure times. As shown in Table 4, compared with the untreated group, treating cloned embryos with 5-aza-dC resulted in higher developmental competence, and 25 nM 5-aza-dC significantly increased the proportion of embryos developing to blastocysts. These results show that there was a dosage-dependent effect and that 25 nM 5-aza-dC was possibly the optimal concentration for improving cloned embryo development. When embryos were exposed to 25 nM 5-aza-dC for different time durations (Table 5), the results showed that the 24 h group had a significantly higher blastocyst rate than the control group, while when the time duration was extended to 48 h, the developmental competence of cloned embryos did not improve, suggesting that there was also a time-dependent effect. These results indicated that treating porcine cloned embryos with 25 nM 5-aza-dC for 24 h (NA) was probably the optimal treatment manner.
Table 4. Development of cloned embryos treated with 5-aza-dC at different concentrations.
| 5-aza-dC (nM) | No. embryos cultured (Rep.) |
No. embryos cleaved (% ± SEM) |
No. blastocysts (% ± SEM) |
Total cell numbers of blastocysts (mean ± SEM) & |
| 0 | 198 (4) | 170 (86.72 ± 3.11) | 40 (19.99 ± 2.16)a | 37 ± 2 (n=37) |
| 10 | 171 (4) | 150 (87.34 ± 2.03) | 37 (21.82 ± 1.19)a | 39 ± 2 (n=35) |
| 25 | 175 (4) | 159 (91.32 ± 2.04) | 50 (28.61 ± 1.44)b | 41 ± 3 (n=49) |
| 50 | 176 (4) | 143 (84.51 ± 2.78) | 41 (23.06 ± 2.57)ab | 37 ± 2 (n=38) |
& Blastocyst cell numbers of less than 16 were not included. a, b Values in the same column with different superscripts differ significantly (P<0.05).
Table 5. Development of cloned embryos treated with 5-aza-dC for different time durations.
| Time (h) | No. embryos fused (Rep.) |
No. embryos cleaved (% ± SEM) |
No. blastocysts (% ± SEM) |
Total cell numbers of blastocysts (mean ± SEM) & |
| 0 | 196 (4) | 173 (89.31 ± 1.33) | 40 (20.16 ± 2.04)a | 38 ± 2 (n=39) |
| 24 | 178 (4) | 161 (90.53 ± 1.53) | 52 (29.64 ± 1.65)b | 40 ± 3 (n=49) |
| 48 | 168 (4) | 144 (85.85 ± 2.25) | 41 (24.37 ± 2.26)ab | 35 ± 2 (n=38) |
& Blastocyst cell numbers of less than 16 were not included. a, b Values in the same column with different superscripts differ significantly (P<0.05).
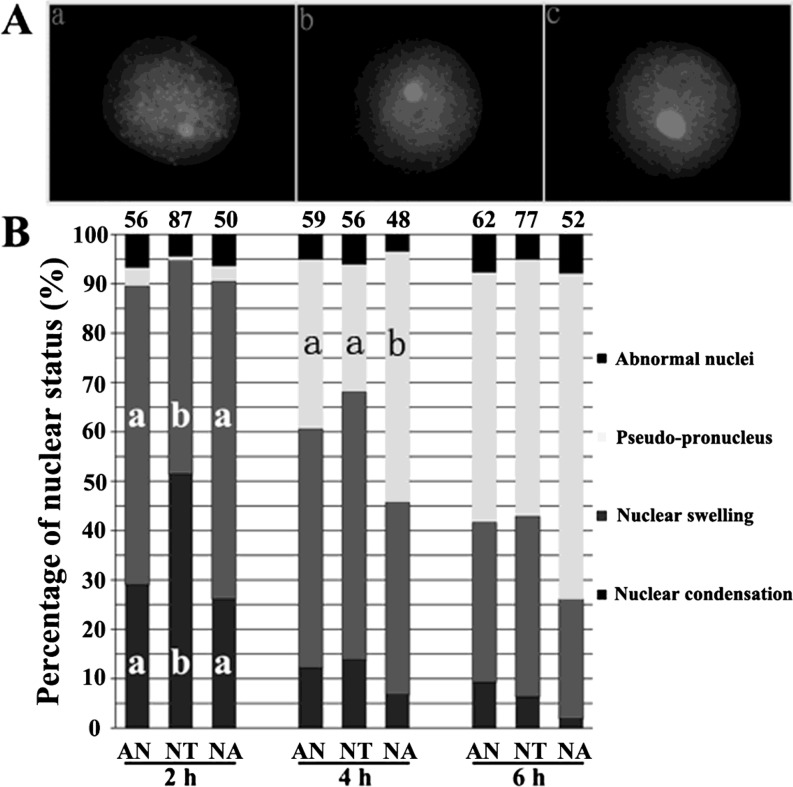
Treating cloned embryos with 5-aza-dC promoted pseudo-pronucleus formation
Morphological remodeling of the donor nucleus is fundamental to successful reprogramming, and the formation of a pseudo-pronucleus is an important event for successful development of SCNT embryos [15]. When somatic nuclei were transferred into oocytes, chromatin condensation, nuclear swelling and pseudo-pronucleus formation sequentially occurred post activation (Fig. 2 A, a, b and c, respectively). For normal cloned embryos (NT), there were 51% cloned embryos showing nuclear condensation at 2 h, 54% at the status of nuclear swelling at 4 h and 52% showing a pseudo-pronucleus at 6 h (Fig. 2 B). When cloned embryos were treated with 5-aza-dC, the progress of nuclear status remodeling was accelerated, and the proportion of embryos with a pseudo-pronucleus in the NA group was higher than that in the NT group (significantly at 4 h, 51% and 36%, respectively, and at 6 h, 65% and 52%, respectively), while there were no significant differences between the pseudo-pronucleus proportions in the AN and NT groups (at 4 h, 34% and 36%, respectively, and at 6 h, 51% and 52%, respectively). Overall, compared with the NT group, treating cloned embryos but not donor cells accelerated the nuclear remodeling progress and promoted pseudo-pronucleus formation; therefore, it benefited for the subsequent development of cloned embryos.
Fig. 2.
Effect of 5-aza-dC on nuclear morphology remodeling of cloned embryos post activation. (A) Nuclear morphology of cloned embryos. a, nuclear condensation; b, nuclear; c, pseudo-pronucleus. Images were magnified × 400 and desaturated. (B) The progress of nuclear morphology remodeling during 6 h post activation. The number of embryos detected is at the top of the column chart. a, b Percentages at a given time in columns with different superscripts differ significantly (P<0.05).
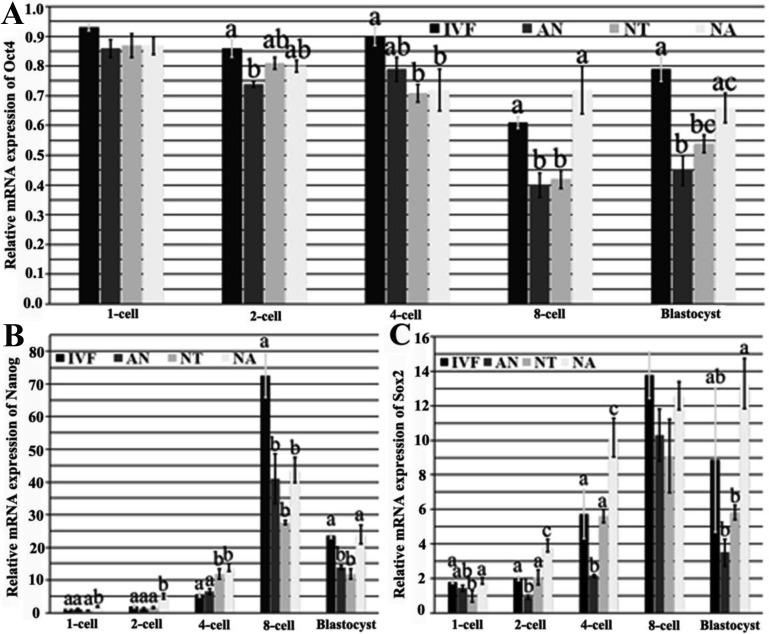
Treating cloned embryos with 5-aza-dC improved the expression levels of pluripotent genes during the development of cloned embryos
The expression pattern of pluripotent genes is correlated with the developmental potential of cloned embryos, and core transcriptional factors (Oct4, Nanog and Sox2) are crucial for nuclear reprogramming during embryo development [16]. In this study, we found that compared with those in the NT group, the mRNA expression levels of Oct4, Nanog and Sox2 in the NA group were more similar to those in IVF embryos, while the expression levels in the AN group were relatively downregulated with embryo development (Fig. 3). The overall trend of Oct4 expression level in all kinds of embryos was gradually downward from the 1-cell to 8-cell stage, and slightly upregulated at the blastocyst stage. Compared with those of the AN and NT embryos, the Oct4 expression levels of the NA embryos at the 8-cell and blastocyst stages were upregulated and closer to those in IVF embryos (Fig. 3 A). As for Nanog, the transcript level at the blastocyst stage in the NA group was significantly higher than those in the AN and NT groups, and no significant differences were observed between those in the NA and IVF groups (Fig. 3 B). The Sox2 expression levels in the NA group were not significantly different from those in the IVF group after the 4-cell stage, and were significantly higher than those in the AN and NT embryos at the 2-cell, 4-cell and blastocyst stages (Fig. 3 C). These results showed that treating cloned embryos, but not donor cells, with 5-aza-dC improved the transcription levels of Oct4, Nanog and Sox2, resulting in higher embryo developmental potential.
Fig. 3.
Effect of different 5-aza-dC treatment manners on mRNA expression of pluripotent genes during the development of cloned embryos. (A) The relative mRNA expression of Oct4 in porcine embryos derived from IVF, AN, NT and NA was quantified by quantitative real-time PCR. (B) The relative mRNA expression of Nanog in porcine embryos derived from IVF, AN, NT and NA was quantified by quantitative real-time PCR. (C) The relative mRNA expression of Sox2 in porcine embryos derived from IVF, AN, NT and NA was quantified by quantitative real-time PCR. The transcript abundance for every gene at MII was condidered to be the control. The data are expressed as means ± SEM. a–c Percentages at a given stage in columns with different superscripts differ significantly (P<0.05).
Discussion
Our study showed that treating cloned embryos, but not donor cells, with 5-aza-dC could enhance the developmental competence of porcine cloned embryos, and the results of nuclear morphology remodeling and pluripotent gene expression suggested that the mechanism underlying the improvement of porcine cloned embryo development by treatment of cloned embryos with 5-aza-dC was probably the promotion of somatic nuclear remodeling progress and improvement of pluripotent gene transcription levels.
It is generally believed that 5-aza-dC can induce DNA hypomethylation and cause the chromatin to be open, thus nuclear reprogramming would consequently be easier [12]. In this study, our results showed that treating donor cells with 5-aza-dC could reduce global DNA methylation but not improve the developmental potential of porcine cloned embryos, even at a low dose or for a short exposure time (data not shown), and the results are in agreement with the reports in pigs, cattle, cats, etc. [21,22,23]. Thus we consider that treating donor cells with 5-aza-dC may be inappropriate for porcine SCNT. However, some reports show that 5-aza-dC can induce donor cell DNA hypomethylation and enhance the cloned embryo development [7, 24]. This apparent inconsistency may be due to different species or the use of different concentrations or exposure times for 5-aza-dC, which make it difficult to compare different reports. When we applied 5-aza-dC in donor cell treatment, the results showed that 5-aza-dC could significantly reduce the DNA methylation level of PFFs, but had a detrimental effect on PFF morphology and proliferation, especially at a high concentration. Further study showed that the gene expression profiles (especially imprinting genes, which are essential to the embryo development [25, 26]) were disrupted (data not shown), even at a low concentration. These above results showed that the cytotoxic effect and disorder of gene expression maked donor cells against reprogramming by SCNT, which was reflected indirectly by the decrease in the cleavage and blastocyst rates. Previous studies also reported that treating donor cells with 5-aza-dC could cause some defects and reduce the cloned embryo development [12, 27]. Therefore, it seems that making changes in the natural status of PFFs only using 5-aza-dC might not be a proper choice for improving porcine cloning efficiency.
We demonstrated that treating cloned embryos with 5-aza-dC could enhance the developmental potential of porcine cloned embryos, in contrast to the result of donor cell treatment. It is possible that the treatment manner makes the nuclear reprogramming induced by SCNT different, and with oocyte reprogramming factors, treating cloned embryos could easily enhance the reprogramming progress from the epigenetic state of donor nuclei to that of an embryo. For cloned embryo treatment, previous studies reported that 5-aza-dC did not always enhance SCNT efficiency [7, 14, 28]. These differences might be due to the dosage- and time-dependent effects of 5-aza-dC and species. Our study showed that treating porcine cloned embryos with 25 nM 5-aza-dC for 24 h was the optimal treatment manner, as our preliminary research showed that other treatment parameters did not enhance the efficiency of SCNT (data not shown). These results show that adequate epigenetic modification to donor cells or cloned embryos is the key step for enhancing reprogramming by SCNT.
During embryo development, many factors can reflect embryo developmental competence, and of these factors, nuclear morphology remodeling and pluripotent gene expression pattern are closely correlated with embryo developmental competence. Nuclear morphology remodeling refers to the change in chromatin architecture. For SCNT embryos to exhibit successful development, the chromatin structure of the donor cell nucleus must be remodeled to that of zygote nucleus. In this study, we found that compared with the NT group, treating cloned embryos (NA) with 5-aza-dC accelerated the nuclear remodeling progress and promoted pseudo-pronucleus formation, while the AN group did not facilitate nuclear morphology remodeling. Pseudo-pronucleus formation resulting from nuclear morphology remodeling is fundamental to somatic nuclear reprogramming of cloned embryos [15]. Thus, treating cloned embryos but not donor cells with 5-aza-dC could result in the successful nuclear reprogramming and the subsequent development of cloned embryos. During nuclear morphology remodeling, many proteins are involved in pseudo-pronucleus formation, resulting in reprogramming of the gene expression pattern in the cloned embryos [29]. The molecular mechanism underlying the promotion of nuclear remodeling by treating cloned embryos with 5-aza-dC needs further study.
The expression patterns of pluripotent genes are correlated with the developmental efficiency of cloned embryos, and failure to reactivate pluripotent genes leads to embryo developmental block or lethality [16]. In this study, we found that the general transcription levels of core transcriptional factors (Oct4, Nanog and Sox2) in the NA group were higher than those in the NT group, similar to those in IVF embryos, while the transcription levels in the NA group were downregulated in comparison with those in the NT group. Thus, the appropriate expression pattern of pluripotent genes may be the cause underlying the improvement of embryo developmental potential. Previous studies reported that the transcription levels of Oct4, Nanog and Sox2 are essential for embryo development and considered the standard for evaluating embryo developmental potential [16, 30]. Our study showed that treating cloned embryos with 5-aza-dC could reactivate pluripotent gene expression effectively, naturally resulting in higher embryo developmental competence. The mechanism by which 5-aza-dC regulates and increases the pluripotent gene expression in cloned embryos has not been well elucidated. Some reports show that the restoration of pluripotent gene expression in cloned embryos treated with 5-aza-dC partly results from the loss of methylation in promoter regions of these genes [22, 31], and other mechanisms are worthy of investigation.
In summary, this study demonstrates that treating cloned embryos, but not donor cells, with 5-aza-dC improves the developmental potential of porcine cloned embryos and that the improvement is due to the enhanced epigenetic reprogramming of donor cells under the appropriate combination of 5-aza-dC and oocyte reprogramming factors following SCNT, which was revealed by promotion of pseudo-pronucleus formation and upregulation of pluripotent gene expression.
Acknowledgments
The present work was supported by the National Science Foundation of China, NSFC (30871431), and Excellent Youth Foundation of Heilongjiang Province (JC200905). The authors are gratefully thankful to their colleagues in the Laboratory of Embryo Biotechnology for their full support. The authors declare that no conflicting financial interests exist.
References
- 1.Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KH. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 2000; 407: 86–90 [DOI] [PubMed] [Google Scholar]
- 2.Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, Reik W. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci 2001; 98: 13734–13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niemann H, Carnwath JW, Herrmann D, Wieczorek G, Lemme E, Lucas-Hahn A, Olek S. DNA methylation patterns reflect epigenetic reprogramming in bovine embryos. Cell Reprogram 2010; 12: 33–42 [DOI] [PubMed] [Google Scholar]
- 4.Haaf T. Methylation dynamics in the early mammalian embryo: implications of genome reprogramming defects for development. Curr Top Microbiol Immunol 2006; 310: 13–22 [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki Y, Fujita TC, Low EW, Alarcon VB, Yanagimachi R, Marikawa Y. Gradual DNA demethylation of the Oct4 promoter in cloned mouse embryos. Mol Reprod Dev 2006; 73: 180–188 [DOI] [PubMed] [Google Scholar]
- 6.Li X, Kato Y, Tsunoda Y. Comparative analysis of development-related gene expression in mouse preimplantation embryos with different developmental potential. Mol Reprod Dev 2005; 72: 152–160 [DOI] [PubMed] [Google Scholar]
- 7.Ding X, Wang Y, Zhang D, Wang Y, Guo Z, Zhang Y. Increased pre-implantation development of cloned bovine embryos treated with 5-aza-2’-deoxycytidine and trichostatin A. Theriogenology 2008; 70: 622–630 [DOI] [PubMed] [Google Scholar]
- 8.Ruau D, Ensenat-Waser R, Dinger TC, Vallabhapurapu DS, Rolletschek A, Hacker C, Hieronymus T, Wobus AM, Muller AM, Zenke M. Pluripotency associated genes are reactivated by chromatin-modifying agents in neurosphere cells. Stem Cells 2008; 26: 920–926 [DOI] [PubMed] [Google Scholar]
- 9.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature 2010; 467: 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Noushmehr H, Han H, Andreu-Vieyra C, Liang G, Jones PA. Gene reactivation by 5-aza-2’-deoxycytidine-induced demethylation requires SRCAP-mediated H2A.Z insertion to establish nucleosome depleted regions. PLoS Genet 2012; 8: e1002604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Z, Li L, Liu X, Wang D, Tu B, Wang L, Wang H, Zhu WG. 5-Aza-2’-deoxycytidine reactivates gene expression via degradation of pRb pocket proteins. FASEB J 2012; 26: 449–459 [DOI] [PubMed] [Google Scholar]
- 12.Enright BP, Sung LY, Chang CC, Yang X, Tian XC. Methylation and acetylation characteristics of cloned bovine embryos from donor cells treated with 5-aza-2’-deoxycytidine. Biol Reprod 2005; 72: 944–948 [DOI] [PubMed] [Google Scholar]
- 13.Naruse K, Kim HR, Kim BC, Han RX, Choi SM, Kim MY, Park CS, Jin DI. Effects of 5-aza-2’-deoxyctidine on development of porcine parthenogenetic and cloned embryos. In: 41st Annual Meeting of the Society for the Study of Reproduction: 2008; Hawaii, USA. Abstract 647.
- 14.Sun L, Wu KL, Zhang D, Wang HY, Wang Y, Xu ZY, Huang XY, Chen ZJ, Liu HQ. Increased cleavage rate of human nuclear transfer embryos after 5-aza-2’-deoxycytidine treatment. Reprod Biomed Online 2012; 25: 425–433 [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Whyte J, Prather RS. Effect of epigenetic regulation during swine embryogenesis and on cloning by nuclear transfer. Cell Tissue Res 2010; 341: 13–21 [DOI] [PubMed] [Google Scholar]
- 16.Bortvin A, Eggan K, Skaletsky H, Akutsu H, Berry DL, Yanagimachi R, Page DC, Jaenisch R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 2003; 130: 1673–1680 [DOI] [PubMed] [Google Scholar]
- 17.Kong QR, Zhu J, Huang B, Huan YJ, Wang F, Shi YQ, Liu ZF, Wu ML, Liu ZH. TSA improve transgenic porcine cloned embryo development and transgene expression. Yi Chuan 2011; 33: 749–756 [DOI] [PubMed] [Google Scholar]
- 18.Mohana Kumar B, Jin HF, Kim JG, Song HJ, Hong Y, Balasubramanian S, Choe SY, Rho GJ. DNA methylation levels in porcine fetal fibroblasts induced by an inhibitor of methylation, 5-azacytidine. Cell Tissue Res 2006; 325: 445–454 [DOI] [PubMed] [Google Scholar]
- 19.Wei Y, Huan Y, Shi Y, Liu Z, Bou G, Luo Y, Zhang L, Yang C, Kong Q, Tian J, Xia P, Sun Q-Y, Liu Z. Unfaithful Maintenance of methylation imprints due to loss of maternal nuclear Dnmt1 during somatic cell nuclear Transfer. PLoS One 2011; 6: e20154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Luo YB, Bou G, Kong QR, Huan YJ, Zhu J, Wang JY, Li H, Wang F, Shi YQ, Wei YC, Liu ZH. Overexpression Nanog activates pluripotent genes in porcine fetal fibroblasts and nuclear transfer embryos. Anat Rec (Hoboken) 2011; 294: 1809–1817 [DOI] [PubMed] [Google Scholar]
- 21.Jafarpour F, Hosseini SM, Hajian M, Forouzanfar M, Ostadhosseini S, Abedi P, Gholami S, Ghaedi K, Gourabi H, Shahverdi AH, Vosough AD, Nasr-Esfahani MH. Somatic cell-induced hyperacetylation, but not hypomethylation, positively and reversibly affects the efficiency of in vitro cloned blastocyst production in cattle. Cell Reprogram 2011; 13: 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez M, Biancardi M, Jenkins J, Dumas C, Galiguis J, Wang G, Earle Pope C. Scriptaid and 5-aza-2’deoxycytidine enhanced expression of pluripotent genes and in vitro developmental competence in interspecies black-footed cat cloned embryos. Reprod Domest Anim 2012; 47(Suppl 6): 130–135 [DOI] [PubMed] [Google Scholar]
- 23.Ning SF, Li QY, Liang MM, Yang XG, Xu HY, Lu YQ, Lu SS, Lu KH. Methylation characteristics and developmental potential of Guangxi Bama minipig (Sus scrofa domestica) cloned embryos from donor cells treated with trichostatin A and 5-aza-2’-deoxycytidine. Zygote 2013; 21: 178–186 [DOI] [PubMed] [Google Scholar]
- 24.Kumar BM, Maeng GH, Lee YM, Lee JH, Jeon BG, Ock SA, Kang T, Rho GJ. Epigenetic modification of fetal fibroblasts improves developmental competency and gene expression in porcine cloned embryos. Vet Res Commun 2013; 37: 19–28 [DOI] [PubMed] [Google Scholar]
- 25.Li W, Shuai L, Wan H, Dong M, Wang M, Sang L, Feng C, Luo GZ, Li T, Li X, Wang L, Zheng QY, Sheng C, Wu HJ, Liu Z, Liu L, Wang L, Wang XJ, Zhao XY, Zhou Q. Androgenetic haploid embryonic stem cells produce live transgenic mice. Nature 2012; 490: 407–411 [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Shi L, Wang BA, Liang D, Zhong C, Liu W, Nie Y, Liu J, Zhao J, Gao X, Li D, Xu GL, Li J. Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell 2012; 149: 605–617 [DOI] [PubMed] [Google Scholar]
- 27.Oka M, Meacham AM, Hamazaki T, Rodic N, Chang LJ, Terada N. De novo DNA methyltransferases Dnmt3a and Dnmt3b primarily mediate the cytotoxic effect of 5-aza-2’-deoxycytidine. Oncogene 2005; 24: 3091–3099 [DOI] [PubMed] [Google Scholar]
- 28.Yu JN, Xue CY, Wang XG, Lin F, Liu CY, Lu FZ, Liu HL. 5-AZA-2’-deoxycytidine (5-AZA-CdR) leads to down-regulation of Dnmt1o and gene expression in preimplantation mouse embryos. Zygote 2009; 17: 137–145 [DOI] [PubMed] [Google Scholar]
- 29.Prather RS, Kuhholzer B, Lai L, Park KW. Changes in the structure of nuclei after transfer to oocytes. Cloning 2000; 2: 117–122 [DOI] [PubMed] [Google Scholar]
- 30.Theunissen TW, van Oosten AL, Castelo-Branco G, Hall J, Smith A, Silva JC. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr Biol 2011; 21: 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YS, Xiong XR, An ZX, Wang LJ, Liu J, Quan FS, Hua S, Zhang Y. Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2’-deoxycytidine and trichostatin A. Theriogenology 2011; 75: 819–825 [DOI] [PubMed] [Google Scholar]