Abstract
Recently we demonstrated an ectopic expression of the human herpesvirus 1 thymidine kinase (HHV1-TK) gene by functioning of an intrinsic endogenous promoter in the transgenic rat (TG-rat), suggesting that HHV1 infection in humans induces expression of the TK gene with the ectopic promoter in the testis and results in accumulation of HHV1-TK protein, triggering male infertility similar to that in the TG-rat. Hence, in this study, we started to investigate a relationship between infection of herpesvirus and human male infertility. Semen was donated by Chinese male infertile patients (153 men, aged 21–49 years) with informed consent, followed by DNA preparation and analysis by PCR and DNA sequencing. Semen volume, sperm number and density, and sperm motility were examined. DNAs of HHV1, HHV4, HHV5 and HHV6 were confirmed by PCR, electrophoresis and DNA sequencing. Finally, virus DNA was identified in 59 patients (39%). The number of carriers was 39 (25%) for HHV1, 6 (4%) for HHV4, 33 (22%) for HHV5 and 3 (2%) for HHV6, respectively. Moreover, double-infection was found in 22 out of 59 specimens (37%), most of which were double-infection of HHV1 and HHV5 (15 out of 22 carriers). Though slight severity was present in some of the carriers, the relationship between virus infection and sperm impairment was not conclusive. Accordingly, it is essential to examine whether the viral HHV1-TK gene is expressed in the testis of the infertile human HHV carrier.
Keywords: Human herpesvirus (HHV), Human, Male infertility, Spermatogenesis, Thymidine kinase
We recently described male infertility of the transgenic rat, in which the reporter gene HHV1-TK (human herpes virus type1-thymidine kinase; HHV1 is also the same as HSV1 (herpes simplex virus 1)) is ectopically expressed in the testis [1, 2]. Similar observations were also reported in transgenic mice harboring HHV1-TK with an abnormal nuclear morphology [3], immotile spermatozoa in these animals [4] and insufficient chromatin condensation and abnormal acrosome structures [5]. We previously demonstrated that the transgenic rats show an ectopic expression and accumulation of HHV1-TK proteins in postmeiotic germ cells with disruption of spermatogenesis by the appearance of immature and abnormal sperm and age-dependent loss of germ cells via apoptotic cell death [2]. Continuous investigation by microarray and real-time PCR analyses revealed an increase in gene expression of Testin [6], a sensitive marker for monitoring the integrity of the Sertoli-germ cell junction. These results are suggestive and may provide a clue that may help uncover a relationship between virus infection and male infertility if the viral gene product(s) is expressed in the human male testis and causes male impairment of spermatogenesis.
It is known that human male infertility accounts for 20–50% infertile couple and is often associated with genital infections [7]. In fact, several investigators have focused on analysis of the correlation between human male infertility and HHV infection [7,8,9,10,11], but conclusive elucidation has yet to be obtained. As a first step, we sought to detect herpes virus DNA (HHV1, HHV4, HHV5 and HHV6) and sperm parameters in semen from 153 human male infertile patients in China. Ultimately, we found viral infection in 59 carriers out of 153 male infertile patients. Twenty-two out of the 59 HHV carriers were infected with two types of the virus. Comparison of sperm properties between infected and noninfected infertile patients did not show any apparent difference.
Materials and Methods
Samples
Semen samples were collected by masturbation from 153 men who were attending the infertility clinic for couple fertility problems between August 2009 and February 2010 at the First Affiliated Hospital of Soochow University. Informed consent was obtained from each patient for the purposes of the current study in accordance with the guidelines, and the study was approved by the ethics committee of the First Affiliated Hospital of Soochow University. Semen parameters were assessed according to the 4th edition of the World Health Organization (WHO) Guidelines for part of the patients.
DNA extraction from semen samples
After collection, each fresh semen sample was centrifuged at 600× g for 15 min. The supernatant was transferred to a new Eppendorf tube, while the pellet containing the sperm remained in the original Eppendorf tube. Sperm pellet proteins were removed by digestion with proteinase K, and DNA extraction was performed using the standard phenol-chloroform protocol [1]. All DNA samples were subjected to spectrophotometry for the quantification of DNA at 260 nm.
PCR amplification reactions
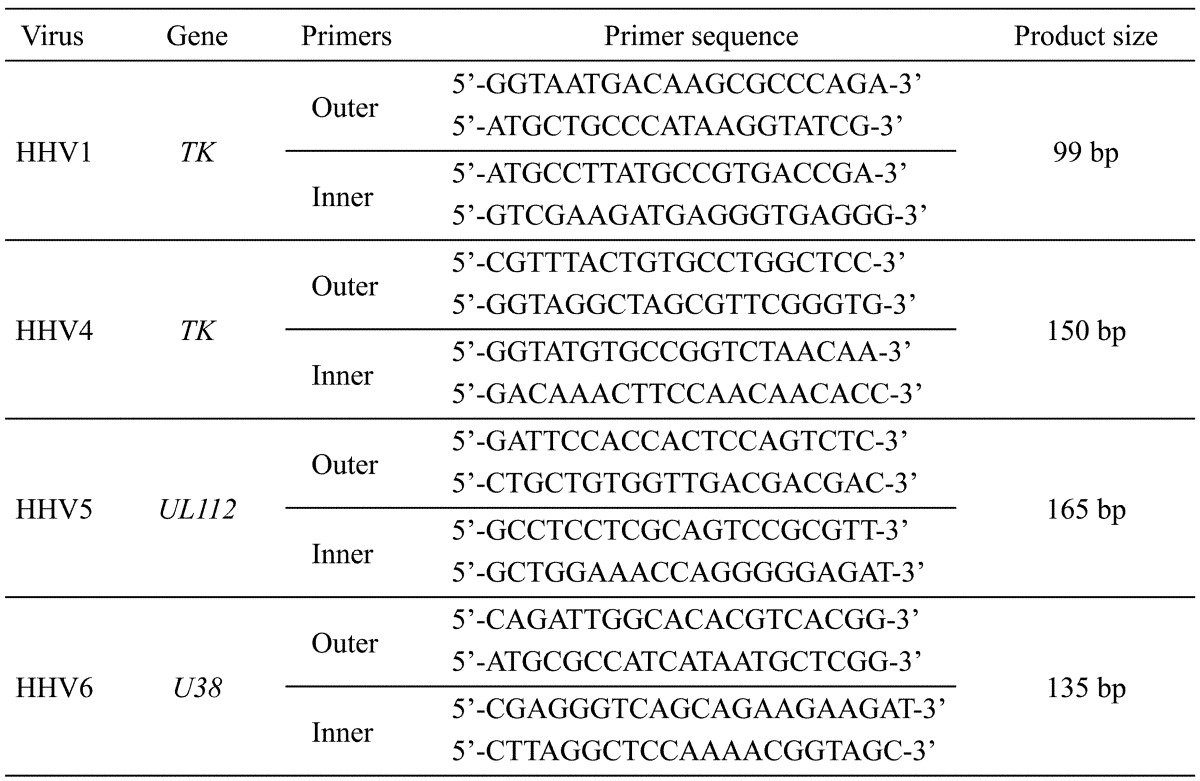
Collected sperm DNA from all participants were subjected to a nested PCR to examine the presence of HHV1 (same as HSV1), HHV4 (same as EBV), HHV5 (same as CMV) and HHV6 using two appropriate sets of primers (Table 1) in a reaction solution of 5 μl containing 0.2 mM MgSO4, 2 μM of outer primer, 2 mM of each dNTP, 0.25 mM dimethyl sulfoxide, supplier’s PCR buffer and 0.1 U KOD Taq polymerase (Toyobo, Osaka, Japan). Extracted DNA (100 ng) of each sample was initially amplified by a first-round PCR (15 cycles), and an aliquot of the dilute was used for the 2nd PCR (36 cycles).
Table 1. List of primers used for the detection of HHV1, HHV4, HHV5 and HHV6.
Agarose gel electrophoresis
PCR products were examined by electrophoresis in a 2% agarose gel. All viral PCR products were recovered from gel using a MagExtractor (Toyobo), followed by direct sequencing analysis to confirm the viral sequence.
Analysis of semen
Sperm concentrations and motility of 75 patients were examined using computer-aided sperm analysis (CASA; WLJY-9000, Beijing, PRC) according to the 4th edition of the World Health Organization Guidelines [12]. Sperm morphology was assessed by observation of eosin-thiazine-stained methanol-fixed smears of fresh ejaculate under a light microscope and evaluated according to strict Krueger criteria. Normal samples were considered to be the semen specimens that fulfilled all the criteria mentioned above. An excessive number of white blood cells alone did not render the sample abnormal. All semen specimens that had one or more altered semen parameters were characterized as abnormal.
Statistical analysis
Continuous variables are presented as the mean and standard error of the mean (mean ± SEM). The normal distribution of continuous parameters was tested by the Kolmogorov-Smirnov test. The differences of variables between two groups (e.g., virus carrier versus non-carrier, HHV positive versus HHV negative) were compared using the Student’s t-test and Mann-Whitney U-test depending on the distribution. The difference in the prevalence of abnormal semen analysis results in the various groups was examined by the chi-square test. Statistical analysis was performed using the SPSS 16.0 statistical software (SPSS, Chicago, IL, USA). Probability (P) < 0.05 was considered significant.
Results
PCR and sequence analyses
Nested PCR for 4 types of virus was performed for 153 samples using each 2 primer sets (outer and inner). After the 2nd PCR, the products were analyzed on 2% agarose gel (Fig. 1A). Amplified bands were observed at the sizes of 99, 150, 165 and 135 bp for HHV1, HHV4, HHV5 and HHV6, respectively. A positive band for two different PCR targets in a sample indicates double infection with different types of HHV virus. Further confirmation was performed by subsequent sequence analyses.
Fig. 1.
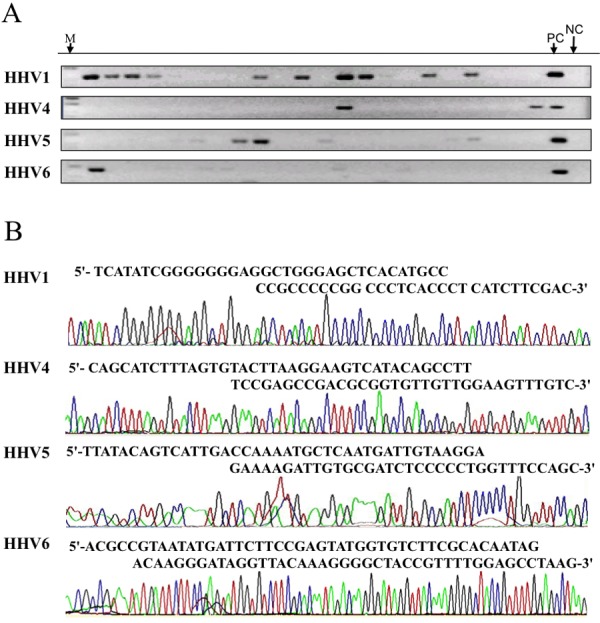
Analysis of viral DNAs by PCR and DNA sequencing. A: Agarose gel electrophoresis. All 153 samples were subjected to nested PCR for 4 viruses using primer sets listed in Table 1 and analyzed on 2% agarose gel. Electrophoresis of representative PCR products after the 2nd PCR is shown. PC (positive control) and NC (negative control) indicate reactions with control virus fragments and without template, respectively. M indicates the size marker. B: Sequence profiles of PCR products. Amplified samples corresponding to the expected length were subjected to sequencing. Representative results harboring virus sequences are shown with the nucleotide sequence determined.
A total of 103 bands were collected to further analyze the nucleotide sequence. Consequently, the nucleotide sequences corresponding to HHV1, HHV4, HHV5 and HHV6 were identified in 39, 6, 33 and 3 patients, respectively, as summarized in Table 2. At the same time, the sequence analysis confirmed the presence of double infection of the virus (Fig. 1B). There were 15, 1, 2 and 4 carriers of double infection with HHV1/HHV5, HHV1/HHV4, HHV1/HHV6 and HHV4/HHV5, respectively. HHV4 carriers accounted for 83% of cases with double infection, while the rate was about 50% for the other viruses.
Table 2. Number cases with single and double viral infection.
| Type | HHV1 | HHV4 | HHV5 | HHV6 |
| HHV1 | 21 | 1 | 15 | 2 |
| HHV4 | 1 | 1 | 4 | 0 |
| HHV5 | 15 | 4 | 14 | 0 |
| HHV6 | 2 | 0 | 0 | 1 |
| Double infection (Double infection/total) | 18 (46.1%) | 5 (83.3%) | 19 (57.6%) | 2 (66.7%) |
| Total infection (Infection/153) | 39 (25.40%) | 6 (3.90%) | 33 (21.60%) | 3 (1.90%) |
Sperm concentrations and motility
Sperm concentrations and motility were analyzed for 39 carriers and 36 noncarrier patients. Oligozoospermia diagnoses were made with an abnormality in sperm density (<20×106/ml). Asthenozoospermia diagnoses were also made by index of sperm motility with an abnormality in the percentage of rapid progressive sperms (<25%) or sum of the percentage of rapid progressive sperms and average progressive sperms (<50%), according to the WHO standard (1999).
Table 3 shows the results for the prevalence of herpes virus in normal and abnormal semen samples. Twenty-nine of 75 semen samples were classified as normal (normozoospermia) according to the 4th edition WHO guidelines. Viral DNA of HHV1, HHV5, HHV4 and HHV6 was detected in 10 (34.5%), 9 (31%), 1 (3.4%) and 0 cases of normal semen samples (Table 3). Viral DNA was also detected in the abnormal zoospermia group from 46 male subjects (Table 3). In this group, the samples were further divided into the following subgroups: 32 oligozoospermic, 33 asthenozoospermic, 19 oligoasthenozoospermic, and three teratozoospermic. Viral DNA of HHV1, HHV5, HHV4 and HHV6 was detected in 14 (30.4%), 15 (32.6%), 3 (6.5%) and 1 (2.2%) case of abnormal semen samples (Table 3). The prevalence of herpes virus in subgroups is also shown in Table 3. Statistical analysis was performed, but there was no significant statistical difference between the presence of each herpes virus and each abnormal subgroup.
Table 3. Herpes viral prevalence in normal and abnormal semen samples.
| Virus | Normozoospermia (29/75) |
Abormozoospermia (46/75) |
Oligozoospermia (32/75) |
Asthenozoospermia (33/75) |
Oligoasthenozoospermia (19/75) |
Teratozoospermia (3/75) |
| HHV1 | 10 (34.5) | 14 (30.4) | 10 (31.3) | 8 (24.2) | 6 (31.6) | 1 (33.3) |
| HHV4 | 1 (3.4) | 3 (6.5) | 3 (9.4) | 2 (6.1) | 2 (10.5) | 1 (33.3) |
| HHV5 | 9 (31.0) | 15 (32.6) | 10 (31.3) | 9 (27.3) | 6 (31.6) | 2 (66.7) |
| HHV6 | 0 (0) | 1 (2.2) | 1 (3.1) | 1 (3.0) | 1 (5.3) | 1 (33.3) |
Data in parentheses are percents.
Table 4 shows the mean sperm count of virally infected and noninfected semen samples. According to the results, the presence of HHV5 and double infection with HHV1 and HHV5 in sperm was associated with a trend toward decreasing sperm count, but there was no significant difference. In contrast, HHV1 as well as other types of HHV infection did not show any influence on sperm count. Table 5 presents the mean sperm motility and viral DNA, HHV1, HHV5 and double types of HHV infection. The mean sperm motilities of semen samples that were positive for HHV5 and those positive for two types of HHV were similar to those of negative semen samples (46.6% vs. 48.6% and 47.8% vs. 48.1%, respectively). In addition, the presence of HHV1 as well as other types of HHV infection did not show any association with poor motility.
Table 4. Mean sperm count in virally infected and noninfected semen samples.
| Viral DNA | N (%) | Mean sperm count (million/ml) |
SEM | P value |
| Viral DNA+ | 39 (52%) | 39.4 | 6.3 | 0.77 |
| Viral DNA– | 36 (48%) | 36.8 | 5.4 | |
| HHV1+ | 24 (32%) | 43.1 | 8.3 | 0.66 |
| HHV1– | 51 (68%) | 35.8 | 4.7 | |
| HHV5+ | 24 (32%) | 37.4 | 7.1 | 0.91 |
| HHV5– | 51 (68%) | 38.5 | 5.1 | |
| HHV1+/HHV5+ | 11 (14.7%) | 36.9 | 9.4 | 0.97 |
| Others | 64 (85.3%) | 38.3 | 4.6 |
Table 5. Mean sperm motility in virally infected and noninfected semen samples.
| Viral DNA | N (%) | Mean sperm motility (%) | SEM | P value |
| Viral DNA+ | 39 (52%) | 47.8 | 3.3 | 0.85 |
| Viral DNA– | 36 (48%) | 48.1 | 3.3 | |
| HHV1+ | 24 (32%) | 49.3 | 4.6 | 0.31 |
| HHV1– | 51 (68%) | 47.3 | 2.7 | |
| HHV5+ | 24 (32%) | 46.6 | 4 | 0.63 |
| HHV5– | 51 (68%) | 48.6 | 2.9 | |
| HHV1+/HHV5+ | 11 (14.7%) | 47.8 | 6.4 | 0.99 |
| Others | 64 (85.3%) | 48.1 | 2.5 |
Discussion
In the present study, we demonstrated that carriers of herpes virus show substantially high prevalence (39%) in the male infertile patients and that 37% of the carriers were diagnosed with the double infection. The semen density and number and sperm motility parameters were lower in carriers in comparison with those in noncarriers, but significant differences were not obtained in the present study. On the other hand, several earlier investigators have examined the relationship between male infertility and herpes viral infections as summarized in Table 6. In some cases, a significant difference in the prevalence of sperm number and density and sperm motility between the carriers and non-carriers was observed in some viral types. To conclude that male infertility is caused by viral infection, more data are required. Notably, similar to our present study, a high infection rate was observed in two previous studies (49.4% and 56.6%) [7, 9], though others showed a low infection rate, which may be due to the small number of virus types examined. In addition, when infectious viruses were classified, the type of infected virus likely showed a local attribute (Table 6). While the present study showed that carriers (Suzhou in China) frequently carried HHV1 (25.4%) and HHV5 (21.6%), Neofytou et al. reported that carriers in Crete (Greece) carried HHV4 (39.1%), HHV5 (56.5%) and HHV6 (66.3%) in their study [9]. In the case of the carriers in Athens, Greece, Kapranos et al. observed that 49.5% of carriers carried HHV1/2 [7]. Both studies also found carriers with double infection, similar to the present study. Another three studies did not show remarkable attributes, since two of them examined only one virus type. Although these studies demonstrated a considerably high prevalence in virus infection, it may be difficult to conclude infection with herpes viruses as an etiology for male infertility, since direct evidence is absent at present and the mechanism of the herpes virus resulting in male infertility is mostly unresolved at present.
Table 6. Different HHV infection profiles reported around the world.
| Country or Area | Carrier % (carrier/subject) | HHV1 (HSV1) | HHV2 (HSV2) | HHV3 (VZV) | HHV4 (EBV) | HHV5 (CMV) | HHV6 | HHV7 | HHV8 |
| Present study | 38.5 (59/153) | 25.4 | - | - | 3.9 | 21.6 | 1.9 | - | - |
| Crete [9] | 49.4 (85/172) | 2.1 | - | 3.2* | 39.1 | 56.5 | 66.3 | 0 | - |
| Athens [7] | (64/113) | 49.5* | - | 16.8 | 7.1 | - | - | - | |
| USA [8] | 45/241) | 3.7 | - | 0.4 | 8.7 | 3.7 | - | - | |
| Iran [10] | (16/70) | 22.9* | - | - | - | - | - | - | |
| Denmark [11] | (10/91) | - | - | - | - | 11.0 | - | - | - |
* Significant difference in sperm count or sperm motility in the infertile and fertile groups.
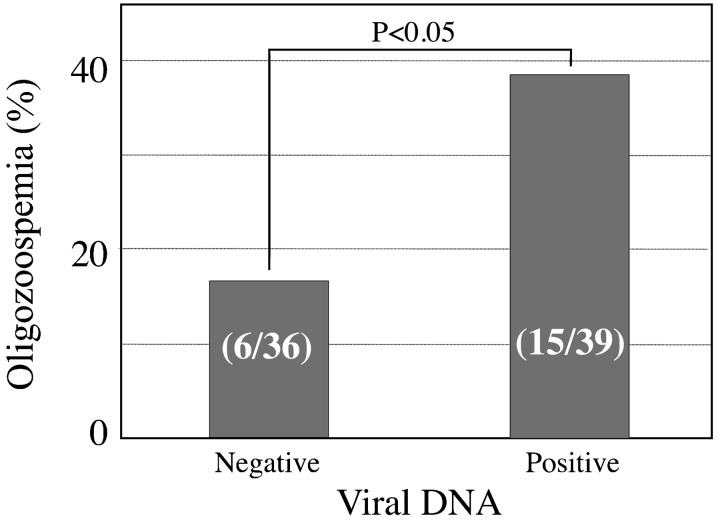
This study has some shortcomings. First, though the presence of HHV5 and double infection of sperm with HHV1 and HHV5 was associated with a trend toward decreasing sperm count and sperm motility, we found no significant association between viral infection and the change in semen parameters. This result may be due to the small sample size involved in this study. Second, semen parameters were analyzed according to the 4th edition of World Health Organization Guidelines [12] in our study. Recently, the World Health Organization (WHO) has established new reference values for semen characteristics in its 5th edition manual that are lower than those previously reported [13]. The 5th edition of the WHO Guidelines has a more strict laboratory manual and quality control system for the examinations. Therefore, our semen analysis according to the 4th edition of the WHO Guidelines in this study probably affected the semen analysis results. Though some parameters are not suitable for reanalysis according to the 5th edition WHO Guidelines, we reanalyzed and compared the rate of oligozoospermia samples between viral DNA-negative and viral DNA-positive groups. The viral DNA-positive group showed a higher incidence rate of oligozoospermia in comparison with that of the viral DNA-negative groups (38.5% vs. 16.7%, P<0.05; Fig. 2). Therefore, examination of a larger sample is strongly suggested in accordance with the 5th edition WHO Guidelines to further confirm the relationship between HHVs infection and the alteration of semen parameters in the near future.
Fig. 2.
Comparison of oligozoospermia between viral DNA-negative and viral DNA-positive groups. According to the 5th edition WHO Guidelines, reanalysis was performed for the rate of oligozoospermia samples between the viral DNA-negative (36 patients) and viral DNA-positive groups (39 patients).
Interestingly, several investigators have examined the correlation between infection with various types of viruses and male infertility. Mostly, they have focused on human papillomaviruses (HPV) [14,15,16,17,18], adenoviruses (AVs) [19], hepatitis B viruses (HBV) [20], human immunodeficiency viruses (HIV) [21] and JC polyomaviruses (JCV) [22]. Though there is no evidence to confirm that virus infections induce male infertility, impairment of sperm parameters has been reported in HPV [14,15,16] and in JCP [22]. In HIV carriers, abnormality of sperm parameters was found depending on the clinical stage and immunological status in HIV1-seropositive men [23]. Dulioust et al. also reported abnormality in these parameters, presumably due to anti-retroviral treatment [24]. Meanwhile, Comar et al. detected JCV DNA in 24.5% of semen samples and 43.4% of urine samples of male infertile patients [21]. Kapranos et al. found that HHV1 infection was significantly related to low sperm count and poor motility [7]. Neofytou et al. also reported that there was a significant association between the presence of VZV DNA and a decreased mean sperm motility [9]. Given the high prevalence of several virus infections in addition to that of herpes viruses, most people may be infected with viruses. If so, most of the investigations that have focused on the relation between the presence of virus in semen and abnormalities as discussed above might have no specific significance and be unable to prove a virus as one etiology for male infertility. Hereafter, it may be necessary to demonstrate directly the expression of virus genes, the presence of virus proteins and damage to spermatogenesis by the product(s) of virus infection.
This study started by finding that the HHV1-TK coding region has an ectopic promoter activity in the testis of the transgenic rat that produces a truncated TK protein specifically in the round spermatid independently of the fused 5’-upstream region of the porcine FSHβ gene, which can be used to verify its tissue-specific promoter activity [1, 2]. More recently, we demonstrated the possibility of Sertoli cell-germ cell disruption possibly by upregulation of Testin [6], the products of which play a crucial role in maintaining the junction between Sertoli cells and germ cells. Though the role of the HHV1-TK protein expressing thymidine kinase activity in the disruption of spermatogenesis is completely unclear at present, our cumulative data [1, 2, 6] certainly indicate a possibility that ectopic expression of infected HHV1 gene causes disruption of male gametogenesis. Elucidation of the molecular mechanism of the ectopic expression of HHV1-TK in round spermatid cells of the TG-rat is an important in vitro approach.
Moreover, there is increasing evidence that many of the interactions between the immune and reproductive systems involve the Toll-like receptors (TLRs) [25]. HHV1 genes including HHV1-TK are rich in CpG motif, which as an immunogen can initiate inflammatory programs in response to HSV1 infection through the signaling pathway of TLRs in human corneal endothelial (HCEn) cells [26]. This suggests male genital tract infection, since HHVs probably influence male reproduction through the signaling pathway of TLRs. Therefore, confirmation of the presence of HHV genes and interaction with the signaling pathway of TLRs in the human testis might be the first prerequisite to be accomplished as soon as possible.
Acknowledgments
This research was partially supported by an Institute of Science and Technology, Meiji University, Research Grant (A) to YK. This work was also supported in part by the Key Laboratory of Reproductive Genetics (Zhejiang University), Ministry of Education, P.R. China/Women’s Reproductive Health Key Laboratory of Zhejiang Province [No. 2012-RG/RH-0004] and by the Meiji University International Institute for BioResource Research (MUIIR).
References
- 1.Cai LY, Kato T, Ito K, Nakayama M, Susa T, Aikawa S, Maeda K, Tsukamura H, Ohta A, Izumi S, Kato Y. Expression of porcine FSHbeta subunit promoter-driven herpes simplex virus thymidine kinase gene in transgenic rats. J Reprod Dev 2007; 53: 201–209 [DOI] [PubMed] [Google Scholar]
- 2.Cai LY, Kato T, Nakayama M, Susa T, Murakami S, Izumi S, Kato Y. HSV type 1 thymidine kinase protein accumulation in round spermatids induces male infertility by spermatogenesis disruption and apoptotic loss of germ cells. Reprod Toxicol 2009; 27: 14–21 [DOI] [PubMed] [Google Scholar]
- 3.Al-Shawi R, Burke J, Jones CT, Simons JP, Bishop JO. A Mup promoter-thymidine kinase reporter gene shows relaxed tissue-specific expression and confers male sterility upon transgenic mice. Mol Cell Biol 1988; 8: 4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shawi R, Burke J, Wallace H, Jones C, Harrison S, Buxton D, Maley S, Chandley A, Bishop JO. The herpes simplex virus type 1 thymidine kinase is expressed in the testis of transgenic mice under the control of a cryptic promoter. Mol Cell Biol 1991; 11: 4207–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun RE, Lo D, Pinkert CA, Widera G, Flavell RA, Palmiter RD, Brinster RL. Infertility in male transgenic mice: disruption of sperm development by HSV-tk expression in postmeiotic germ cells. Biol Reprod 1990; 43: 684–693 [DOI] [PubMed] [Google Scholar]
- 6.Cai LY, Kato T, Chen M, Wang H, Sekine E, Izumi S, Kato Y. Accumulated HSV1-TK proteins interfere with spermatogenesis through a disruption of the integrity of sertoli-germ cell junctions. J Reprod Dev 2012; 58: 544–551 [DOI] [PubMed] [Google Scholar]
- 7.Kapranos N, Petrakou E, Anastasiadou C, Kotronias D. Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertil Steril 2003; 79 (Suppl 3): 1566–1570 [DOI] [PubMed] [Google Scholar]
- 8.Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril 2007; 87: 1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigiannakis A. Prevalence of human herpes virus types 1–7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil Steril 2009; 91: 2487–2494 [DOI] [PubMed] [Google Scholar]
- 10.Salehi-vaziri M, Monavari SH, Khalili M, Shamsi-Shahrabadi M, Keyvani H, Mollaei H, Fazlalipour M. Detection of HSV-1 DNA in the semen of infertile men and evaluation of its correlation with semen parameters in Iran. Iranian J Virol 2010; 4: 1–6 [Google Scholar]
- 11.Naumenko VA, Tiulenev IA, Pushkar’ DI, Segal AS, Kovalev VA, Kurilo LF, Shileĭko LV, Klimova RR, Al’khovskiĭ SV, Kushch AA. Effect of herpes simplex virus on spermatogenesis. Urologiia 2011; Nov-Dec: 32–36 [PubMed] [Google Scholar]
- 12. World Health Organization Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction, 4th ed. New York: Cambridge University Press; 1999 [Google Scholar]
- 13.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16: 231–245 [DOI] [PubMed] [Google Scholar]
- 14.Lai YM, Lee JF, Huang HY, Soong YK, Yang FP, Pao CC. The effect of human papillomavirus infection on sperm cell motility. Fertil Steril 1997; 67: 1152–1155 [DOI] [PubMed] [Google Scholar]
- 15.Foresta C, Garolla A, Zuccarello D, Pizzol D, Moretti A, Barzon L, Palù G. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil Steril 2010; 93: 802–806 [DOI] [PubMed] [Google Scholar]
- 16.Foresta C, Pizzol D, Moretti A, Barzon L, Palù G, Garolla A. Clinical and prognostic significance of human papillomavirus DNA in the sperm or exfoliated cells of infertile patients and subjects with risk factors. Fertil Steril 2010; 94: 1723–1727 [DOI] [PubMed] [Google Scholar]
- 17.Garolla A, Pizzol D, Foresta C. The role of human papillomavirus on sperm function. Curr Opin Obstet Gynecol 2011; 23: 232–237 [DOI] [PubMed] [Google Scholar]
- 18.Garolla A, Lenzi A, Palù G, Pizzol D, Bertoldo A, De Toni L, Foresta C. Human papillomavirus sperm infection and assisted reproduction: a dangerous hazard with a possible safe solution. Hum Reprod 2012; 27: 967–973 [DOI] [PubMed] [Google Scholar]
- 19.Rohde V, Erles K, Sattler HP, Derouet H, Wullich B, Schlehofer JR. Detection of adeno-associated virus in human semen: does viral infection play a role in the pathogenesis of male infertility? Fertil Steril 1999; 72: 814–816 [DOI] [PubMed] [Google Scholar]
- 20.Oger P, Yazbeck C, Gervais A, Dorphin B, Gout C, Jacquesson L, Ayel JP, Kahn V, Rougier N. Adverse effects of hepatitis B virus on sperm motility and fertilization ability during IVF. Reprod Biomed Online 2011; 23: 207–212 [DOI] [PubMed] [Google Scholar]
- 21.Santulli P, Gayet V, Fauque P, Chopin N, Dulioust E, Wolf JP, Chapron C, de Ziegler D. HIV-positive patients undertaking ART have longer infertility histories than age-matched control subjects. Fertil Steril 2011; 95: 507–512 [DOI] [PubMed] [Google Scholar]
- 22.Comar M, Zanotta N, Croci E, Murru I, Marci R, Pancaldi C, Dolcet O, Luppi S, Martinelli M, Giolo E, Ricci G, Tognon M. Association between the JC polyomavirus infection and male infertility. PLoS One 2012; 7: e42880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller CH, Coombs RW, Krieger JN. Effects of clinical stage and immunological status on semen analysis results in human immunodeficiency virus type 1-seropositive men. Andrologia 1998; 30 (Suppl 1): 15–22 [DOI] [PubMed] [Google Scholar]
- 24.Dulioust E, Du AL, Costagliola D, Guibert J, Kunstmann JM, Heard I, Juillard JC, Salmon D, Leruez-Ville M, Mandelbrot L, Rouzioux C, Sicard D, Zorn JR, Jouannet P, De Almeida M. Semen alterations in HIV-1 infected men. Hum Reprod 2002; 17: 2112–2118 [DOI] [PubMed] [Google Scholar]
- 25.Girling JE, Hedger MP. Toll-like receptors in the gonads and reproductive tract: emerging roles in reproductive physiology and pathology. Immunol Cell Biol 2007; 85: 481–489 [DOI] [PubMed] [Google Scholar]
- 26.Takeda S, Miyazaki D, Sasaki S, Yamamoto Y, Terasaka Y, Yakura K, Yamagami S, Ebihara N, Inoue Y. Roles played by toll-like receptor-9 in corneal endothelial cells after herpes simplex virus type 1 infection. Invest Ophthalmol Vis Sci 2011; 52: 6729–6736 [DOI] [PubMed] [Google Scholar]