Abstract
One of the factors that impairs in vitro produced porcine embryos is the oxidative stress that is mainly caused by the imbalance between reactive oxygen species (ROS) generation and antioxidants activity, especially that of glutathione (GSH). Here, we examined the effect of 7,8-dihydroxyflavone (7,8-DHF), a kind of flavonoid antioxidant, on porcine oocyte maturation and its developmental competence. Porcine oocytes were cultured in media supplemented with 0, 1, 5 and 10 μM 7,8-DHF during both in vitro maturation (IVM) and in vitro culture (IVC) after parthenogenetic activation. Maturation of oocytes was evaluated based on first polar body (PB) extrusion and intracellular GSH level, and developmental competence was assessed through observing cleavage and blastocyst formation. In each step, the levels of intracellular GSH and ROS were assessed by fluorescence intensity, and the apoptosis-related gene expression was examined using semiquantitative RT-PCR. The group treated with 1 μM 7,8-DHF during IVM and IVC showed increased cytoplasmic maturation and reached the blastocysts stage (36.1%) at a higher rate than the other groups (24.7, 16.0 and 10.3% for 0, 5 and 10 μM, P<0.05). In that group, the intracellular GSH level was significantly increased while ROS generation was significantly decreased after IVM and IVC (P<0.05). Moreover, it showed high expression of an anti-apoptotic gene (BCL2L1) and low expression of a pro-apoptotic gene (BAK1) (P<0.05). In conclusion, treatment with 1 μM 7,8-DHF during IVM and IVC showed an anti-apoptotic effect by increasing intracellular GSH synthesis and scavenging ROS and therefore improved the developmental competence of porcine embryos.
Keywords: 7, 8-Dihydroxyflavone; Antioxidants; Glutathione (GSH); Porcine oocytes; Reactive oxygen species (ROS)
In vitro production (IVP) of porcine embryos has been extensively studied for improving embryonic development and reproductive technologies. To date, it has also been extended to biomedical research and xenotransplantation [1]. Therefore, many researchers are investigating ways to optimize the condition of in vitro maturation (IVM) of oocytes or in vitro culture (IVC) of embryos, including temperature [2, 3], gas tension [4, 5], composition of media [6,7,8], etc. It is well known that one of the problems that impair IVP of porcine embryos is the oxidative stress [9, 10] that is mainly caused by reactive oxygen species (ROS) generation such as hydrogen peroxide (H2O2), hydroxyl radicals (•OH), superoxide anions (O2•–) and nitric oxide (NO), the highly reactive molecules formed by oxygen metabolism [11]. This can damage the cell by breaking the DNA [12] and RNA or inducing lipid peroxidation [13, 14]. Cells generate antioxidants themselves such as superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) [15] to reduce ROS levels by scavenging free radicals. However, when the level of intracellular ROS is above the threshold, intrinsic antioxidants cannot scavenge free radicals, and the cells are in an oxidative stress condition. In particular, oocytes and early stage embryos are more vulnerable to oxidative stress [16], and the developmental competence of embryos is impaired by the resulting damage. In addition, oxidative stresses accelerate cellular apoptosis, resulting in a decrease in total cell number [17].
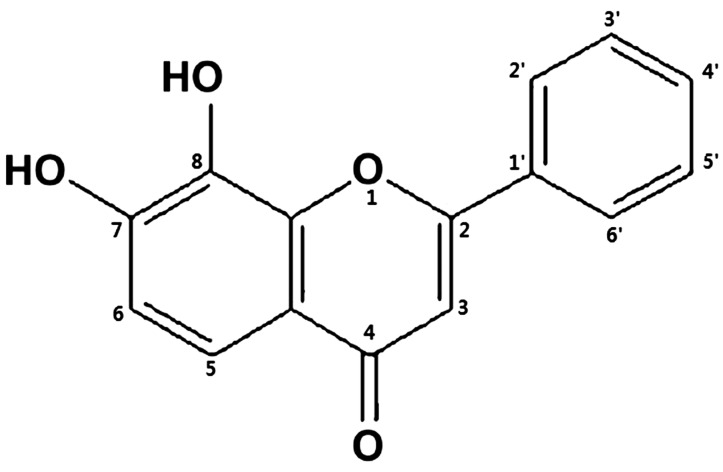
Therefore, many studies have been performed to reduce ROS using antioxidant treatments such as anthocyanin [18], L-carnitine [19], hypotaurine [20], vitamin C [21], β-mercaptoethanol [22, 23] and Selenium [24]. 7,8-Dihydroxyflavone (7,8-DHF), a kind of flavonoid (Fig. 1) present in high concentrations in fruits and vegetables and a brain-derived neurotrophic factor (BDNF), is a brain-protecting drug [25, 26]. It inhibits glutamate-triggered apoptosis induced by glutathione (GSH) depletion and ROS production and has antioxidant activity in neurons by acting as a selective tyrosine kinase receptor B agonist [27, 28]. In addition, 3,4-dihydroxyflavone (3,4-DHF) supports bovine embryo development in vitro as an antioxidant and anti-apoptotic agent [29], and 7,8-DHF appeared to have protective effect against oxidative stress [30]. However, the effects of 7,8-DHF for porcine oocytes and embryos have not been well investigated.
Fig. 1.
Structure of 7,8-dihydroxyflavone. The structure of flavonoids consists of an O-heterocyclic ring fused to a dihydoroxylated aromatic ring at C7 and C8 with a third ring system attached at C2 of the heterocyclic ring.
The purpose of this study was to determine the effect of 7,8-DHF treatment on oocyte maturation and embryo development in pigs. Also, intracellular levels of GSH, ROS and gene expression in oocytes and embryos were examined.
Materials and Methods
All chemicals and reagents used for this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated.
Collection of oocytes and IVM
Porcine ovaries were obtained at a local slaughterhouse and transported to the laboratory in 0.9% NaCl within 3 h. Cumulus oocyte complexes (COCs) were collected by slicing the 3–6 mm follicles and washed 3 times in washing media containing 9.5 g/l tissue culture medium (TCM) 199 (Invitrogen, Carlsbad, CA, USA), 5 mM sodium hydroxide, 2 mM bicarbonate, 10 mM N-[2-Hydroxyethyl] piperazine-N’-[2-ethanesulfonic acid] (HEPES), 0.3% polyvinyl alcohol (PVA) and 1%, Pen-Strep (Invitrogen). Based on the morphological features, COCs with compact, multilayered cells and homogeneous cytoplasm were selected. COCs were then transferred to IVM medium containing TCM 199 supplemented with 10 ng/ml epidermal growth factor (EGF), 0.57 mM cysteine, 5 μl/ml Insulin, Transferrin, Selenium, Sodium Pyruvate Solution (ITS-A) 100X (Invitrogen), 1% (v/v) Pen-Strep, 0.5 μg/ml porcine follicle stimulating hormone, 0.5 μg/ml human luteinizing hormone, 10% porcine follicular fluid (pFF) and 5 nM retinoic acid for 22 h at 38 C in a humidified atmosphere of 5% CO2. Subsequently, the COCs were cultured further for 22 h without hormones and retinoic acid. The COCs were untreated or treated with 1, 5 and 10 μM 7,8-DHF (Tocris Bioscience, Ellisville, MO, USA) during IVM.
Evaluation of porcine oocyte maturation
After 44 h of IVM, cultured oocytes were denuded by pipetting with 0.1% hyaluronidase in Dulbecco’s phosphate buffered saline (DPBS) (Invitrogen) supplemented with 0.1% polyvinyl alcohol, and then denuded oocytes were stained with 5 μg/ml of bisbenzimide (Hoechst 33342) in DPBS. Extrusion of the first polar body (PB) in metaphase II (MII) was used as an indicator for assessment of nuclear maturation with a fluorescence microscope using a 346 nm excitation filter. For examination of cytoplasmic maturation, the intracellular GSH level was measured using 10 μM CellTracker Blue CMF2HC (4-chloromethyl-6,8-difluoro-7-hydroxycoumarin) (Invitrogen). The staining method for observing GSH levels was fully explained in the section concerning assessment of oocyte and embryo quality in Materials and Methods.
Parthenogenetic activation of oocytes and IVC
Oocytes were denuded in the same way as above after 44 h of IVM. Denuded oocytes with homogeneous cytoplasm were selected and then gradually equilibrated in activation solution containing 0.26 M mannitol, 0.5 mM HEPES, 0.1 mM CaCl2 and 0.1 mM MgSO4. The oocytes were activated in a chamber having two electrodes 3.2 mm apart that was filled with activation medium using a single direct current (DC) pulse of 1.5 kV/cm for 60 μsec utilizing an ECM 2001 Electro Cell Manipulator (BTX Instrument Division, Havard Apparatus, Holliston, MA, USA). After washing 3 times in porcine zygote medium-5 (PZM-5) (Funakoshi, Tokyo, Japan), embryos were cultured in PZM-5 supplemented with 0, 1, 5 and 10 μM 7,8-DHF during IVC and covered with mineral oil under a humidified atmosphere of 5% CO2, 5% O2 and 90% N2 at 38.5 C for 7 days. The cleavage rate and blastocyst formation rate were checked at day 2 and day 7 of IVC, respectively. Percentage of blastocyst formation was measured using all of the embryos. To count the total cell number, blastocysts were fixed in absolute alcohol and stained with 5 μg/ml Hoechst 33342 overnight at 4 C. The number of nuclei was determined with a fluorescence microscope using a 346 nm excitation filter.
Assessment of oocytes and embryos quality
Intracellular GSH and ROS levels of all oocytes and day 2 embryos were determined, respectively. Embryonic day 2 is the decisive period in which the first cell cycle occurs, which is directly correlated with developmental potential [31]. Before treatment, 0.01 mM H2O2 was added to the maturation/culture media to increase oxidative damage [32, 33]. Oocytes were incubated for 20 min in HEPES-buffered Tyrode’s albumin lactate pyruvate (TALP) medium [5] with 10 μM carboxy-2’7’-dichlorodihydrofluorescein diacetate (H2DFFDA) and 10 μM CellTracker Blue CMF2HC. After washing 3 times in HEPES-buffered TALP medium, they were transferred into 20 μl droplets, and fluorescence was observed with ultraviolet filters (370 nm for GSH and 460 nm for ROS). The fluorescence intensities were analyzed using the ImageJ 1.42q (National Institutes of Health, Bethesda, MD, USA) software.
RNA extraction and semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from mature oocytes and day 2 parthenotes in each group using an easy-spinTM (DNA free) Total RNA Extraction Kit (iNtRON Biotechnology, Gyeonggi-do, Korea) following the manufacturer’s instructions and quantified using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). From these samples, cDNA was produced using amfiRivertIITM cDNA Synthesis Master Mix (GenDEPOT, Barker, TX, USA) in a 20 µl reaction volume. Briefly, 5–8 µl of total RNAs were placed in 0.5 ml PCR tubes, and then 10 µl of amfiRivertII cDNA reaction buffer with oligo-dT and 2 µl of amfiRivertII cDNA enzyme mix buffer (2X) were added. Diethylpyrocarbonate (DEPC)-treated water was added to the tubes to adjust the total volume to 20 µl, and the tubes were then incubated at 70 C for 5 min and then held on ice for 5 min. Reverse transcription was carried out once at 25 C for 5 min, 50 C for 60 min and then 70 C for 15 min.
PCR was done according to the manufacturer’s instructions. The sequences of the primers used for gene expression analysis and product sizes are listed in Table 1. Aliquots of PCR reaction were performed based on optimization curves of PCR cycles with denaturation at 94 C for 30 sec, annealing at 59 C for 30 sec and then extension at 72 C for 30 sec. Amplified PCR products were analyzed by gel electrophoresis (Mupid-exU; Takara Korea Biomedical, Seoul, Korea) at 100 V for 25 min in 1% agarose gel with RedSafe (iNtRON Biotechnology). After gel electrophoresis, the intensities of the bands under ultraviolet light were quantified using ImageJ 1.42q, and results were normalized to those of the control (GAPDH) to express arbitrary units of relative expression.
Table 1. Primer sequences and product sizes used for semiquantitative RT-PCR.
| Gene | Sequence (5’–3’) | Product size (bp) | Accession no. |
| BCL2L1 | F : CGTCCCAGCTCCACATCACC R : AGTGCCCCACCGAAGGAGAA |
130 | AF216205 |
| BAK1 | F : ATGACATCAACCGGCGATAC R : GGAGGCGATCTTGGTGAAGT |
107 | AJ001204 |
| GAPDH | F : ACCTGCCGTCTGGAGAAACC R : GACCATGAGGTCCACCACCCTG |
252 | AF017079.1 |
BCL2L1: B-cell lymphoma 2 like 1. BAK1: BCL2 homologue antagonist/killer 1. GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
All data were subjected to one-way ANOVA followed by Tukey’s test using Prism version 5.0 (GraphPad Software, San Diego, CA, USA) to determine differences among experimental groups. Statistical significance was determined when the P value was less than 0.05.
Results
Effects of 7,8-DHF on IVM of porcine oocytes
Nuclear maturation of porcine oocytes was evaluated by measuring the rate of first PB extrusion. Although a total 684 oocytes were assessed in four replicates, there were no significant differences among the experimental groups (Table 2).
Table 2. Effects of 7,8-dihydroxyflavone treatment during in vitro maturation on nuclear maturation.
| Treatment concentration (μM) |
No. of oocytes cultured* |
No. oocytes ofwith first polar body extrusion |
Metaphase II (% ± S.E.M.) |
| Control | 171 | 148 | 82.2 ± 5.4 |
| 1 | 171 | 152 | 84.4 ± 5.0 |
| 5 | 171 | 143 | 79.4 ± 5.8 |
| 10 | 171 | 134 | 74.4 ± 6.4 |
* At least five replications were performed.
Developmental competence of porcine parthenogenetic embryos after 7,8-DHF treatment
We examined the effect of 7,8-DHF treatment according to the concentration (0, 1, 5 and 10 μM 7,8-DHF). A total of 776 embryos were parthenogenetically activated in five replicates. Table 3 shows that the cleavage rates of parthenogenetic embryos were similar in the control group and groups treated with 1 and 5 μM 7,8-DHF and that blastocyst formation was significantly increased in the group treated with 1 μM 7,8-DHF. Parthenotes cultured in a high (5 and 10 μM) concentration of 7,8-DHF showed lower developmental competence based on the cleavage rate and blastocyst formation compared with the 1 μM treatment group. Also, there was no significant difference in the total cell numbers of blastocysts in all groups (Table 3).
Table 3. Effects of 7,8-dihydroxyflavone during in vitro development of parthenogenetic embryos.
| Treatment concentration (μM) |
No. of embryos cultured* |
Embryos developed (% ± S.E.M.) |
No. of cells in blastocyst (mean ± S.E.M.) |
|
| ≥2 cell | Blastocyst | |||
| Control | 194 | 58.8 ± 3.5a | 24.7 ± 3.1a | 62.0 ± 8.4 |
| 1 | 194 | 67.0 ± 3.4a | 36.1 ± 3.5b | 66.2 ± 3.1 |
| 5 | 194 | 56.2 ± 3.6a,b | 16.0 ± 2.6a,c | 60.9 ± 11.1 |
| 10 | 194 | 44.9 ± 3.6b | 10.3 ± 2.2c | 62.5 ± 11.5 |
* At least five replications were performed. a–c Within a column, values with different superscripts are significantly different (P<0.05).
Intracellular levels of GSH and ROS in matured oocytes and parthenotes
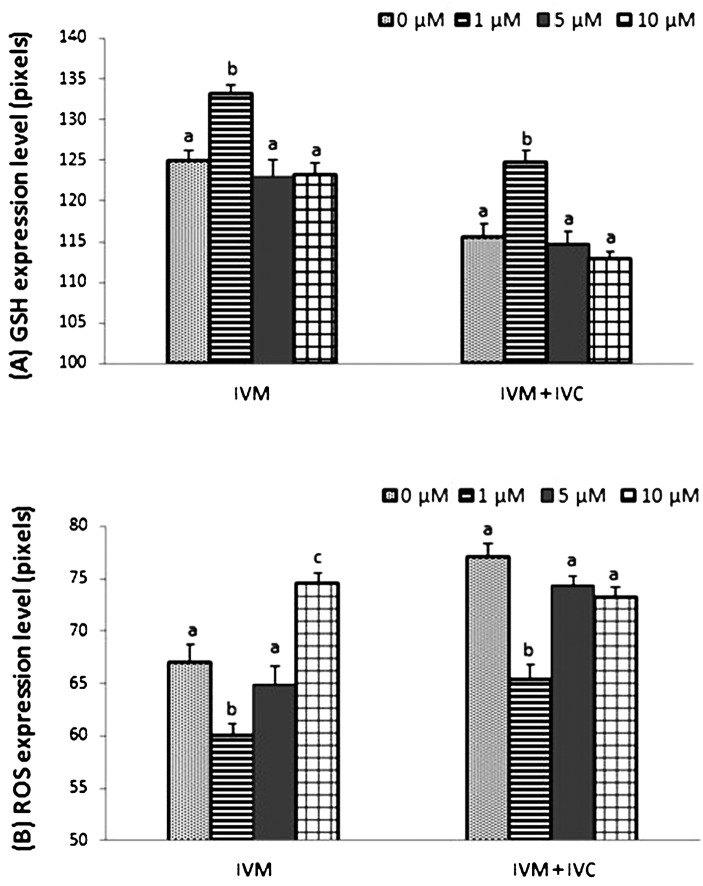
Treatment with 1 μM 7,8-DHF significantly altered the level of intracellular GSH and ROS during IVM and IVC. In total, 419 oocytes and 544 day 2 embryos were examined in three and four replicates, respectively. The GSH level was increased in both matured oocytes and parthenotes in the group treated with 1 μM 7,8-DHF, and the levels were similar in the other groups (Fig. 2 (A)). Expression of ROS was significantly reduced in the group treated with 1 μM 7,8-DHF, and there were no differences between the control group and group treated with 5 μM 7,8-DHF after IVM, but the group treated with 10 μM 7,8-DHF showed an increased expression level compared with the other experimental groups (Fig. 2 (B)). After IVC, the group treated with 1 μM 7,8-DHF showed a lower level of ROS than any other group (Fig. 2 (B)). Figure 3 shows oocytes and parthenotes in each experiment stained with CellTracker Blue (A–H) and carboxy-H2DFFDA (I–P) according to the concentration of 7,8-DHF.
Fig. 2.
Relative intracellular glutathione (GSH) (A) and reactive oxygen species (ROS) (B) levels (pixels) in matured oocytes and day 2 parthenotes, respectively. Addition of 1 μM 7,8-dihydroxyflavone significantly increased the level of GSH in both matured oocytes and parthenotes and significantly decreased the level of ROS in both matured oocytes and parthenotes. At least five replications were performed. a–c Within a column, values with different superscripts are significantly different (P<0.05).
Fig. 3.
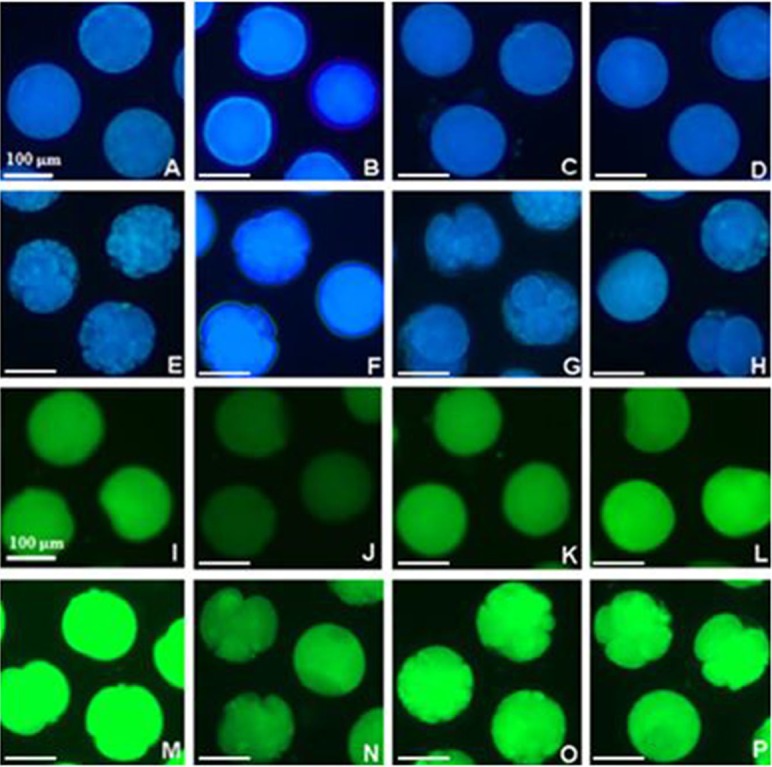
Photographic images of in vitro matured oocytes (A–D, I–L) and day 2 parthenotes (E–H, M–P) are arranged according to concentration (0, 1, 5 and 10 μM) of 7,8-dihydroxyflavone in order from left- to right-hand side. They were stained with CellTracker Blue (A–H) and carboxy-H2DFFDA (I–P) to evaluate the intracellular GSH and ROS levels, respectively.
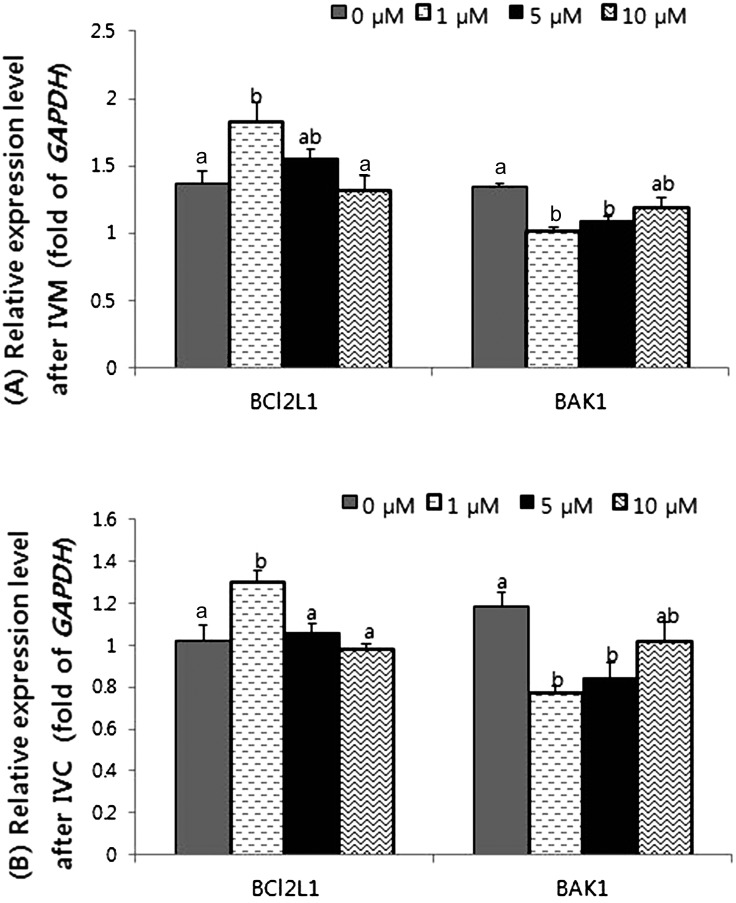
Apoptosis-related gene expression in matured oocytes and porcine embryos
The anti-apoptotic effect of 7,8-DHF was evaluated using analysis of apoptosis-related genes, and the results are shown in Fig. 4. Each sample was collected after measuring the intracellular GSH and ROS levels. After IVM, anti-apoptotic (BCL2L1) gene expression was significantly increased in the group treated with 1 μM 7,8-DHF, and pro-apoptotic (BAK1) gene expression was significantly decreased in that group and the group treated with 5 μM 7,8-DHF (Fig. 4 (A)). This pattern also appeared after IVC. The relative expression level of the anti-apoptotic gene was increased and that of the pro-apoptotic gene was decreased in the group treated with 1 μM 7,8-DHF (Fig. 4 (B)). The group treated with 5 μM 7,8-DHF also showed reduced pro-apoptotic gene expression; however, the group treated with 10 μM 7,8-DHF did not show a difference compared with control group (Fig. 4 (A), (B)).
Fig. 4.
Relative expression level of BCL2L1 and BAK1 in mature oocytes (A) and day 2 parthenotes (B) treated with 0, 1, 5 and 10 μM 7,8-dihydroxyflavone (7,8-DHF). Treatment with 1 μM 7,8-DHF significantly increased BCL2L1 and decreased BAK1 after in vitro maturation (IVM) and in day 2 parthenotes during in vitro culture (IVC). The relative gene abundance was normalized to GAPDH levels. BCL2L1 = B-cell lymphoma 2 like 1, BAK1 = BCL2 homologue antagonist/killer 1 and GAPDH = glyceraldehyde 3-phosphate dehydrogenase. At least four replicates were performed. a, b Within a column, values with different superscripts are significantly different (P<0.05).
Discussion
The ROS produced by normal metabolism during culture damages oocytes and embryos constantly [20]. Cysteine, the precursor of glutathione, has been added to IVM media to protect cells from oxidative stress; however, the beneficial effects of this is controversial with regard to cell number, GSH level and embryonic development [34,35,36]. Therefore, 7,8-DHF was examined as an antioxidant and anti-apoptotic factor. In this study, the results showed that the addition of 1 μM 7,8-DHF to the IVM/IVC media improved the competence of oocytes with regard to cytoplasmic maturation and development of embryos.
At first, we investigated the effect of 7,8-DHF on oocyte maturation. Intracellular GSH is used as a molecular marker that predicts cytoplasmic maturation in porcine oocytes [37] because the level of GSH rises as cytoplasmic maturation progresses in oocytes [38, 39], and first PB extrusion is closely related to nuclear maturation because it takes place during nuclear maturation in MII [40]. After culturing COCs in maturation media supplemented with 7,8-DHF, although there were no significant differences in PB extrusion, the cytoplasmic maturation was increased in 1 μM-treated group compared with the other groups. Nuclear and cytoplasmic maturation are normally coordinated, but some processes of cytoplasmic maturation related to successful preimplantation development probably occur without coordination with nuclear maturation [41], and cytoplasmic quality in IVM oocytes plays a major role in the reconstruction of embryonic development [42]. Moreover, the level of GSH was significantly increased and that of ROS was decreased in the group treated with 1 μM 7,8-DHF, and it is supposed that intracellular redox metabolism [43] was effectively regulated in that group. This is important during IVM because oxygen consumption of COCs increases during IVM as a result of IVM with mitochondrial oxidative phosphorylation [44]. The beneficial effects of 1 μM 7,8-DHF as an antioxidant also contribute to development of embryos, and this was reflected in the blastocyst formation rates. The level of GSH with the addition of 1 μM 7,8-DHF was higher than that in other groups, and expression of ROS was decreased. In terms of developmental competence, although the group treated with 10 μM 7,8-DHF showed poor development, the intracellular oxidative levels were similar to those of the control group. Total cell number of blastocysts was similar in all groups. This means that treatment with 1 μM 7,8-DHF increased the production efficiency of embryos without affecting blastocyst quality.
An increased level of intracellular ROS was correlated with apoptosis induced by oxidative damage [45]. The mitochondrial respiratory chain is the main oxygen consuming system in the cell and is the major source of toxic ROS [46] in cells. ROS change mitochondrial integrity with various effectors such as Ca2+, induce release of cytochrome c and thereby activate a caspase cascade [47] that then leads to apoptosis [48]. This damage was reflected in the individual gene expressions of apoptotic factors [49, 50]. In this experiment, both porcine oocytes and parthenotes supplemented with 1 μM 7,8-DHF showed a higher level of BCL2L1 and lower level of BAK1 expression. This group was significantly different compared with the other groups in terms of the intracellular GSH and ROS levels. Oocytes and parthenotes with 1 μM 7,8-DHF appeared to have an increased level of GSH and decreased level of ROS compared with the control group. This result correlated with production of GSH, which maintained the intracellular redox state and protected cells from the harmful effects of oxidative stress, resulting from treatment with 1 μM 7,8-DHF. GSH acts as a deducing agent and electron donor and oxidizes itself from GSSG [51]. Therefore, H2O2, as a major factor of ROS, is reduced to H2O in mitochondria and cytoplasm [52]. Synthesis and secretion of GSH is observed in oviductal fluid [53], which indicates that redox balance is also important for in vivo embryonic development.
It has been reported that some flavonoids generate ROS by autoxidation and that redox cycling resulted in the generation of high concentrations of O−2 and H2O2 [54, 55]. There has previously been research performed about the concentration-dependent effect of 7,8-DHF. In this study, intracellular ROS was increased in the 10 μM 7,8-DHF treatment compared with the control and 1 and 5 μM treatment groups after IVM. This was thought to be the result of activation of metabolism and respiratory chains in COCs with autoxidation of 10 μM 7,8-DHF. Although the level of ROS was not significantly increased at day 2 parthenotes, oxidative stress during culture may affect development based on the decreased rate of cleavage and blastocyst formation. However, the expression levels of BCL2L and BAK1 were similar in the 10 μM 7,8-DHF treatment group to those in the control. This suggests that other factors that involve inhibition of the glucose transporter GLUT2 [56] or estrogenic activity that mimics 17 beta-estradiol by virtue of their ability to bind to and activate the nuclear estrogen [57] receptor might exist that are related to the poor development independently or indirectly via the apoptosis pathway.
This study was performed to determine the effect of 7,8-DHF using parthenogenetic activation with an electric stimulus instead of nuclear transfer. Nuclear transfer was replaced with parthenogenesis because parthenogenesis is simple and maintains early embryonic development in vivo [58, 59]. However, further assessment using porcine embryos produced by nuclear transfer is needed in a future study.
In conclusion, treatment with 1 μM 7,8-DHF during IVM promotes cytoplasmic maturation rather than nuclear maturation and improves embryonic development during IVC by increasing the intracellular GSH level and decreasing the ROS level. Moreover, this concentration has an anti-apoptotic effect; however, a high concentration of 7,8-DHF seems to have a detrimental effect on porcine oocytes and embryos.
Acknowledgments
This study was supported by the Industrial Strategic Technology Development Program (10033839) funded by MOTIE; IPET (#311011-05-2-SB010) and the Research Institute for Veterinary Science; the BK21 Plus program; and TS Corporation. We thank to Dr B Bavister, PhD, for careful editing of the manuscripts for English grammar.
References
- 1.Yoshioka K. Development and application of a chemically defined medium for the in vitro production of porcine embryos. J Reprod Dev 2011; 57: 9–16 [DOI] [PubMed] [Google Scholar]
- 2.Isom SC, Prather RS, 3rd, Rucker EB. Heat stress-induced apoptosis in porcine in vitro fertilized and parthenogenetic preimplantation-stage embryos. Mol Reprod Dev 2007; 74: 574–581 [DOI] [PubMed] [Google Scholar]
- 3.Jin YX, Lee JY, Choi SH, Kim T, Cui XS, Kim NH. Heat shock induces apoptosis related gene expression and apoptosis in porcine parthenotes developing in vitro. Anim Reprod Sci 2007; 100: 118–127 [DOI] [PubMed] [Google Scholar]
- 4.Booth PJ, Holm P, Callesen H. The effect of oxygen tension on porcine embryonic development is dependent on embryo type. Theriogenology 2005; 63: 2040–2052 [DOI] [PubMed] [Google Scholar]
- 5.Kang JT, Atikuzzaman M, Kwon DK, Park SJ, Kim SJ, Moon JH, Koo OJ, Jang G, Lee BC. Developmental competence of porcine oocytes after in vitro maturation and in vitro culture under different oxygen concentrations. Zygote 2012; 20: 1–8 [DOI] [PubMed] [Google Scholar]
- 6.Mao J, Whitworth KM, Spate LD, Walters EM, Zhao J, Prather RS. Regulation of oocyte mitochondrial DNA copy number by follicular fluid, EGF, and neuregulin 1 during in vitro maturation affects embryo development in pigs. Theriogenology 2012; 78: 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koike T, Matsuura K, Naruse K, Funahashi H. In-vitro culture with a tilting device in chemically defined media during meiotic maturation and early development improves the quality of blastocysts derived from in-vitro matured and fertilized porcine oocytes. J Reprod Dev 2010; 56: 552–557 [DOI] [PubMed] [Google Scholar]
- 8.Hirao Y. Isolation of ovarian components essential for growth and development of mammalian in vitro. J Reprod Dev 2012; 58: 167–174 [DOI] [PubMed] [Google Scholar]
- 9.Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med 1993; 15: 69–75 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Nagai T, Okamura N, Takahashi H, Okano A. Promoting effect of beta-mercaptoethanol on in vitro development under oxidative stress and cystine uptake of bovine embryos. Biol Reprod 2002; 66: 562–567 [DOI] [PubMed] [Google Scholar]
- 11.Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction 2002; 123: 479–486 [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett 1991; 281: 9–19 [DOI] [PubMed] [Google Scholar]
- 13.Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 1990; 109: 501–507 [DOI] [PubMed] [Google Scholar]
- 14.Noda Y, Matsumoto H, Umaoka Y, Tatsumi K, Kishi J, Mori T. Involvement of superoxide radicals in the mouse two-cell block. Mol Reprod Dev 1991; 28: 356–360 [DOI] [PubMed] [Google Scholar]
- 15.Choe C, Shin YW, Kim EJ, Cho SR, Kim HJ, Choi SH, Han MH, Han J, Son DS, Kang D. Synergistic effects of glutathione and beta-mercaptoethanol treatment during in vitro maturation of porcine oocytes on early embryonic development in a culture system supplemented with L-cysteine. J Reprod Dev 2010; 56: 575–582 [DOI] [PubMed] [Google Scholar]
- 16.Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 2001; 7: 175–189 [DOI] [PubMed] [Google Scholar]
- 17.Jiménez A, Madrid-Bury N, Fernández R, Pérez-Garnelo S, Moreira P, Pintado B, de la Fuente J, Gutiérrez-Adán A. Hyperglycemia-induced apoptosis affects sex ratio of bovine and murine preimplantation embryos. Mol Reprod Dev 2003; 65: 180–187 [DOI] [PubMed] [Google Scholar]
- 18.You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 2010; 74: 777–785 [DOI] [PubMed] [Google Scholar]
- 19.Wu GQ, Jia BY, Li JJ, Fu XW, Zhou GB, Hou YP, Zhu SE. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology 2011; 76: 785–793 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki C, Yoshioka K, Sakatani M, Takahashi M. Glutamine and hypotaurine improves intracellular oxidative status and in vitro development of porcine preimplantation embryos. Zygote 2007; 15: 317–324 [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Cheng D, Gao X, Bao J, Ma X, Wang H. Vitamin C enhances the in vitro development of porcine pre-implantation embryos by reducing oxidative stress. Reprod Domest Anim 2012; 47: 873–879 [DOI] [PubMed] [Google Scholar]
- 22.Gupta MK, Uhm SJ, Lee HT. Effect of vitrification and beta-mercaptoethanol on reactive oxygen species activity and in vitro development of oocytes vitrified before or after in vitro fertilization. Fertil Steril 2010; 93: 2602–2607 [DOI] [PubMed] [Google Scholar]
- 23.Funahashi H. Effect of beta-mercaptoethanol during in vitro fertilization procedures on sperm penetration into porcine oocytes and the early development in vitro. Reproduction 2005; 130: 889–898 [DOI] [PubMed] [Google Scholar]
- 24.Tareq KM, Akter QS, Khandoker MA, Tsujii H. Selenium and vitamin E improve the in vitro maturation, fertilization and culture to blastocyst of porcine oocytes. J Reprod Dev 2012; 58: 621–628 [DOI] [PubMed] [Google Scholar]
- 25.Zeng Y, Liu Y, Wu M, Liu J, Hu Q. Activation of TrkB by 7,8-dihydroxyflavone prevents fear memory defects and facilitates amygdalar synaptic plasticity in aging. J Alzheimers Dis 2012; 31: 765–778 [DOI] [PubMed] [Google Scholar]
- 26.Johnson RA, Lam M, Punzo AM, Li H, Lin BR, Ye K, Mitchell GS, Chang Q. 7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. J Appl Physiol 2012; 112: 704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve 2012; 45: 274–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devi L, Ohno M. 7,8-Dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2012; 37: 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KS, Kim EY, Jeon K, Cho SG, Han YJ, Yang BC, Lee SS, Ko MS, Riu KJ, Lee HT, Park SP. 3,4-Dihydroxyflavone acts as an antioxidant and antiapoptotic agent to support bovine embryo development in vitro. J Reprod Dev 2011; 57: 127–134 [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Kang KA, Piao MJ, Ko DO, Wang ZH, Chang WY, You HJ, Lee IK, Kim BJ, Kang SS, Hyun JW. Preventive effect of 7,8-dihydroxyflavone against oxidative stress induced genotoxicity. Biol Pharm Bull 2009; 32: 166–171 [DOI] [PubMed] [Google Scholar]
- 31.Kawakami M, Kato Y, Tsunoda Y. The effects of time of first cleavage, developmental stage, and delipidation of nuclear-transferred porcine blastocysts on survival following vitrification. Anim Reprod Sci 2008; 106: 402–411 [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Osorio N, Kim IJ, Wang H, Kaya A, Memili E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J Pineal Res 2007; 43: 283–288 [DOI] [PubMed] [Google Scholar]
- 33.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008. 44: 280–287 [DOI] [PubMed] [Google Scholar]
- 34.Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 2003; 59: 939–949 [DOI] [PubMed] [Google Scholar]
- 35.Caamaño JN, Ryoo ZY, Youngs CR. Promotion of development of bovine embryos produced in vitro by addition of cysteine and b-mercaptoethanol to a chemically defined culture system. J Dairy Sci 1998; 81: 369–374 [DOI] [PubMed] [Google Scholar]
- 36.Katayama M, Rieke A, Cantley T, Murphy C, Dowell L, Sutovsky P, Day BN. Improved fertilization and embryo development resulting in birth of live piglets after intracytoplasmic sperm injection and in vitro culture in a cysteine-supplemented medium. Theriogenology 2007; 67: 835–847 [DOI] [PubMed] [Google Scholar]
- 37.Wang WH, Abeydeera LR, Cantley TC, Day BN. Effects of oocyte maturation media on development of pig embryos produced by in vitro fertilization. J Reprod Fertil 1997; 111: 101–108 [DOI] [PubMed] [Google Scholar]
- 38.Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod 1993; 49: 89–94 [DOI] [PubMed] [Google Scholar]
- 39.de Matos DG, Furnus CC, Moses DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod 1997; 57: 1420–1425 [DOI] [PubMed] [Google Scholar]
- 40.Ogawa B, Ueno S, Nakayama N, Matsunari H, Nakano K, Fujiwara T, Ikezawa Y, Nagashima H. Developmental ability of porcine in vitro matured oocytes at the meiosis II stage after vitrification. J Reprod Dev 2010; 56: 356–361 [DOI] [PubMed] [Google Scholar]
- 41.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996; 8: 485–489 [DOI] [PubMed] [Google Scholar]
- 42.Hu J, Ma X, Bao JC, Li W, Cheng D, Gao Z, Lei A, Yang C, Wang H. Insulin-transferrin-selenium (ITS) improves maturation of porcine oocytes in. Zygote 2011; 19: 191–197 [DOI] [PubMed] [Google Scholar]
- 43.Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol 2005; 5: 5–17 [PubMed] [Google Scholar]
- 44.Grygoruk C, Pietrewicz P, Modlinski JA, Gajda B, Greda P, Grad I, Pietrzycki B, Mrugacz G. Influence of embryo transfer on embryo preimplantation development. Fertil Steril 2012; 97: 1417–1421 [DOI] [PubMed] [Google Scholar]
- 45.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 2012; 20: 249–259 [DOI] [PubMed] [Google Scholar]
- 46.Noda Y, Goto Y, Umaoka Y, Shiotani M, Nakayama T, Mori T. Culture of human embryos in alpha modification of Eagle’s medium under low oxygen tension and low illumination. Fertil Steril 1994; 62: 1022–1027 [PubMed] [Google Scholar]
- 47.Nutt LK, Gogvadze V, Uthaisang W, Mirnikjoo B, McConkey DJ, Orrenius S. Indirect effects of Bax and Bak initiate the mitochondrial alterations that lead to cytochrome c release during arsenic trioxide-induced apoptosis. Cancer Biol Ther 2005; 4: 459–467 [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod 2000; 62: 1745–1753 [DOI] [PubMed] [Google Scholar]
- 49.Lonergan P, Rizos D, Gutierrez-Adan A, Moreira PM, Pintado B, de la Fuente J, Boland MP. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 2003; 69: 1424–1431 [DOI] [PubMed] [Google Scholar]
- 50.Guillemin Y, Lalle P, Gillet G, Guerin JF, Hamamah S, Aouacheria A. Oocytes and early embryos selectively express the survival factor BCL2L10. J Mol Med 2009; 87: 923–940 [DOI] [PubMed] [Google Scholar]
- 51.Abeydeera LR, Wang WH, Cantley TC, Prather RS, Day BN. Glutathione content and embryo development after in vitro fertilisation of pig of cysteine. Zygote 1999; 7: 203–210 [DOI] [PubMed] [Google Scholar]
- 52.Silva PF, Gadella BM, Colenbrander B, Roelen BA. Exposure of bovine sperm to pro-oxidants impairs the developmental competence of the embryo after the first cleavage. Theriogenology 2007; 67: 609–619 [DOI] [PubMed] [Google Scholar]
- 53.Meister A, Tate SS. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem 1976; 45: 559–604 [DOI] [PubMed] [Google Scholar]
- 54.Hodnick WF, Kung FS, Roettger WJ, Bohmont CW, Pardini RS. Inhibition of mitochondrial respiration and production of toxic oxygen radicals. Biochem Pharmacol 1986; 35: 2345–2357 [DOI] [PubMed] [Google Scholar]
- 55.Metodiewa D, Jaiswal AK, Cenas N, Dickancaite E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic Biol Med 1999; 26: 107–116 [DOI] [PubMed] [Google Scholar]
- 56.Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M, Levine M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J 2007; 21: 366–377 [DOI] [PubMed] [Google Scholar]
- 57.Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol 1993; 44: 37–43 [PubMed] [Google Scholar]
- 58.Kure-bayashi S, Miyake M, Okada K, Kato S. Successful implantation of in vitro-matured, electro-activated oocytes in the pig. Theriogenology 2000; 53: 1105–1119 [DOI] [PubMed] [Google Scholar]
- 59.Jolliff WJ, Prather RS. Parthenogenic development of in vitro-matured, in vivo-cultured porcine oocytes beyond blastocyst. Biol Reprod 1997; 56: 544–548 [DOI] [PubMed] [Google Scholar]