Abstract
Effects of supporting materials during vitrification procedure on the morphologies of preantral follicles of pig ovaries were assessed. Ovarian cortical sections of prepubertal pigs were randomly allocated to 5 groups. The sections were vitrified ultrarapidly with 5 different vitrification devices. The sections were put on 4 fine needles (Cryosupport), on a thin copper plate, or on a carbon graphite sheet or were sandwiched between copper plates or between carbon graphite sheets before cooling. The cooling and warming rates with the graphite sheets were significantly higher than those with the copper plates (P<0.05). A total of 3,064 follicles were analyzed following HE staining after vitrification with 5 different devices. The morphologies follicles vitrified on the Cryosupport or on the graphite sheet were well preserved compared with those vitrified on the copper plate or between copper plates (P<0.01). The morphologies of follicles vitrified between copper plates were mostly damaged (P<0.05). Taken together, good thermally conducting material supports follicle morphologies of ovaries cryopreserved with ultrarapid vitrification.
Keywords: Cryopreservation, Fertility preservation, Ovary, Ultrarapid vitrification
Cryopreservation of preantral follicles in ovarian tissues has been expected to be an effective measure for preserving fertility of young women who need to undergo cytotoxic therapy and female animals on the endangered list.
The major damaging factors, which occur during cryopreservation, are associated with chilling injury, osmotic stress, cryoprotectant toxicity and ice crystallization [1]. In general, we are trying to reduce these damages by increasing cooling and warming rates during the vitrification protocol. In the past, vitrification was based on the combination of a high cooling rate and high concentration of cryoprotectants, which caused chemical toxicity and osmotic stress [1]. The major breakthrough in the field of vitrification came when the sample volume was reduced to a level that permitted lowering of the cryoprotectants concentration [2, 3]. For ovarian tissues, rapid cooling by direct immersion into LN2 led to a better outcome [4,5,6,7,8].
Recently, an ultrarapid vitrification protocol we developed using nonhuman primates (Cryosupport vitrification) [8] supported the morphological normality of vitrified preantral follicles and oocytes compared with conventional vitrification and slow freezing [9]. Moreover, revascularization of vitrified-warmed tissues after autotransplantation was also confirmed, and normal oocytes were successfully retrieved. However, an adiabatic effect caused by nitrogen gas, which occurs on the surface of tissue, is of concerns. The Cryosupport has a size limit because it consists of 4 fine needles and the gap between needles is 2 mm.
We hypothesized that good thermally conducting material would maximize cooling and warming rates to facilitate vitrification and to prevent ice crystal injury during warming. The thermal conductivity of carbon graphite is approximately twice as high as that of copper. In this study, we assessed effects of supporting materials during cooling and warming procedures on the morphologies of preantral follicles of vitrified pig ovaries.
At the beginning, the cooling and warming rates using carbon graphite sheets and copper plates were assessed. The average values of the cooling and warming rates were 10,021 and 28,000 C/min, respectively, with the graphite sheets and 2,524 and 4,957 C/min, respectively, with the copper plates. The cooling and warming rates with the graphite sheets were significantly higher than with the copper plates (P<0.05).
A total of 3,064 follicles (1,552 primordial, 1,030 intermediary, 482 primary) were analyzed by light microscope to evaluate the morphologies of follicles vitrified ultrarapidly with the five different devices. Non-vitrified follicles (417 primordial, 204 intermediary, 77 primary) were also analyzed as the control. There were no differences in normality of follicles between vitrification on the Cryosupport (primordial, 92.2%; intermediary, 84.5%; primary, 77.2%; Table 1) and on the graphite sheet (primordial, 93.3%; intermediary, 81.1%; primary, 87.0%). Morphological normality of primordial follicles vitrified on the Cryosupport (92.2%) and on the graphite sheet (93.3%) was well preserved (P<0.01) compared with those vitrified on the copper plate (70.6%) and between the copper plates (36.4%). The morphologies of follicle vitrified between the copper plates were the most damaged compared with the follicles vitrified with the other devices (P<0.05). The architecture of the compact matrix of ovarian stromal tissue, which consists of stromal cells and bundles of collagen fibers, was damaged most in the specimens vitrified between copper sheets. A sandwich method in which an ovarian section was put between two sheets or plates was inferior to a method in which an ovarian section was put in direct contact with liquid nitrogen (putting on method), possibly due to a decrease in the cooling and warming rates.
Table 1. Proportions of morphologically normal follicles at the primordial, intermediary, and primary stages after ultrarapid vitrification.
| Ovarian sections were vitrified | Primordial follicles | Intermediary follicles | Primary follicles | |||
| Mean ± SEM | N | Mean ± SEM | N | Mean ± SEM | N | |
| On the Cryosupport | 92.2 ± 2.2a | 239 | 84.5 ± 4.4ab | 126 | 77.2 ± 7.8a | 89 |
| On the graphite sheet | 93.3 ± 2.0a | 243 | 81.1 ± 5.4ab | 153 | 87.0 ± 3.7a | 116 |
| Between graphite sheets | 84.3 ± 2.8ab | 647 | 65.8 ± 5.7b | 478 | 64.4 ± 14.2a | 138 |
| On the copper plate | 70.6 ± 4.3b | 197 | 69.5 ± 6.2ab | 65 | 64.1 ± 16.9a | 34 |
| Between copper plates | 36.4 ± 9.0c | 226 | 26.5 ± 11.6c | 208 | 23.6 ± 12.3b | 105 |
| Non vitrified | 90.9 ± 3.8a | 417 | 87.9 ± 3.9a | 204 | 70.4 ± 22.6a | 77 |
Four ovarian sections were used to assess the follicle morphology in each experimental condition. Data are shown as the mean ± SEM. N: number of examined follicles. Different superscript letters (a, b, c) in the same row denote significant difference at P<0.05 by ANOVA followed by Fisher’s PLSD test.
The data of the present study clearly indicated that good thermally-conducting material supports follicle morphologies of ovaries cryopreserved with ultrarapid vitrification. In 2010, we started clinical application of ovarian vitrification using Cryosupport vitrification [8] after receiving approval from the institutional review board of St. Marianna University.
Methods
For measurement of cooling and warming rates, an ultrafine thermocouple probe (0.1 mm diameter; JT1, Chino, Tokyo, Japan) was sandwiched between copper sheets (8 mm × 15 mm × 0.1 mm, 4940372036534, Taiho Trading, Tokyo, Japan) or between carbon graphite sheets (8 mm × 15 mm × 0.1 mm, EYGS12810, Panasonic, Osaka, Japan), immersed directly into liquid nitrogen, held briefly and then warmed in a 35 C thawing solution: Hepes-buffered 199 solution (H199, 12350-039, Invitrogen, Tokyo, Japan) [8] containing 20% SSS (SSS, 99193, Irvine Scientific, Santa Ana, CA, USA) and 0.8 M sucrose (192-00012, Wako Pure Chemical Industries, Osaka, Japan). The gap between sheets was filled with vitrification solution: H199 containing 20% SSS, 35% (v/v) ethylene glycol (054-0983, Wako Pure Chemical Industries), 5% (w/v) polyvinylpyrrolidone, a synthetic polymer (PVP; PVP360, Sigma-Aldrich, St. Louis, MO, USA), and 0.5 M sucrose. Temperature changes were measured with an electronic thermometer (Model EB22005, Chino). For each method, the time required for the temperature to drop from –20 to –100 C (or the reverse) was measured during cooling and during warming with 3 replicates each, and the average cooling and warming rates were calculated.
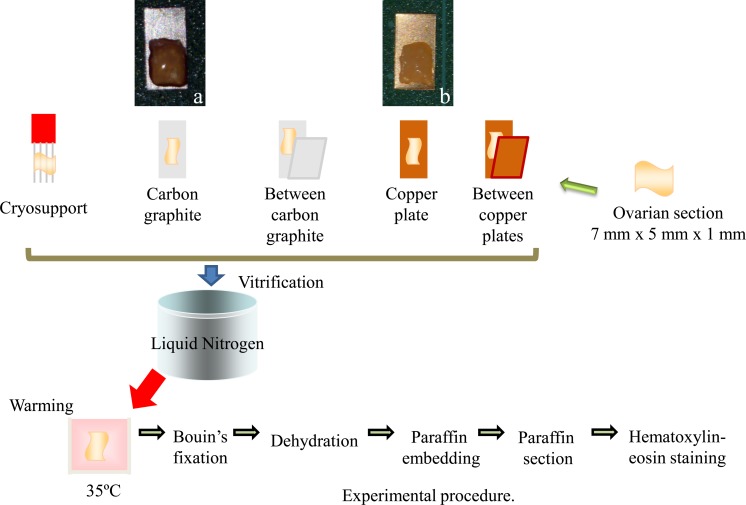
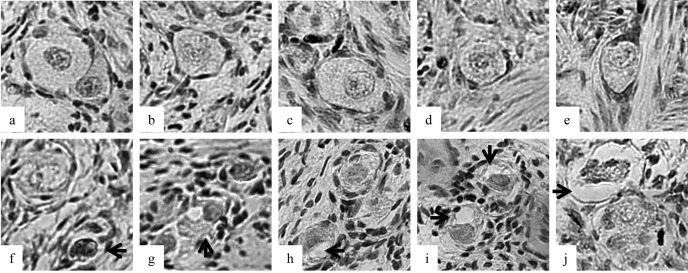
Ovarian cortical sections (7 mm × 5 mm × 1 mm) were made by removal of the 1-mm-thick outer layer of the ovarian cortex of prepubertal pig ovaries. The ovaries were obtained from a local abattoir in Osaka Prefecture. Twenty-four sections prepared from ten ovaries were randomly allocated to six groups. The sections were vitrified ultrarapidly with the 5 different vitrification devices by direct application into liquid nitrogen following exposure to vitrification solution as described above except in the non-vitrified group. The sections were put on the Cryosupport, which consisted of 4 fine stainless steel needles [8], on the copper sheet or on the graphite sheet or were sandwiched between the graphite sheets or between the copper plates before cooling (Fig. 2). The Cryosupport was designed to minimize the surface area of the stainless steel needle to support the direct contact of ovarian tissue with liquid nitrogen [8]. After warming, the morphologies of the follicles were analyzed using light microscopy (LM). Vitrified and warmed ovarian sections were fixed in Bouin’s solution. The fixed ovarian tissues were then dehydrated, embedded in paraffin wax and serially sectioned to a thickness of 5 µm. These sections were mounted and stained with hematoxylin-eosin. All sections were examined using a light microscope (Olympus, Tokyo, Japan) at a magnification of ×400. Only primordial to primary follicles that possessed oocytes surrounded by one layer of flattened or cuboidal granulosa cells were counted. Follicles with an oocyte surrounded by a single layer of flat granulosa cells were defined as primordial. Follicles with an oocyte surrounded by flat granulose cells and one or more cuboidal granulosa cells were regarded as intermediary, and those with one layer of only cuboidal granulosa cells were regarded as primary. Follicular quality was evaluated based on the morphological integrity of the oocyte, granulosa cells and basement membrane. Follicles were histologically classified as normal (1) when they contained an intact oocyte and intact granulosa cells, or degenerated (2) when they contained a pyknotic oocyte nuclei, shrunken ooplasm and/or disorganized granulose cells (e.g., enlargement in volume and/or detachment from the basement membrane, Fig. 1). Data were analyzed using the t-test or Fisher’s PLSD test following ANOVA.
Fig. 2.
Ovarian sections were put on the Cryosupport, which consisted of 4 thin needles (Cryosupport), the graphite sheet (a), or the copper plate (b) or were sandwiched between copper plates or between carbon graphite sheets before cooling. Vitrified and warmed ovarian sections were fixed in Bouin’s solution. The fixed ovarian tissues were then dehydrated, embedded in paraffin wax and serially sectioned to a thickness of 5 µm. These sections were mounted and stained with hematoxylin-eosin.
Fig. 1.
Morphologies of porcine follicles after vitrification. a–e: Follicles were categorized as normal after vitrification between the graphite sheets. f–j: Follicles were categorized as damaged after vitrification on the copper sheet. Each arrow shows a pyknotic oocyte nucleus (f), shrunken ooplasm (g–j) and disorganized granulose cells (j).
Acknowledgments
This study was supported in part by a grant from the Japan Society for the Promotion of Science (JPS-RFTF 23580397 to SH).
References
- 1.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011; 141: 1–19 [DOI] [PubMed] [Google Scholar]
- 2.Steponkus PL, Myers SP, Lynch DV, Gardner L, Bronshteyn V, Leibo SP, Rall WF, Pitt RE, Lin TT, MacIntyre RJ. Cryopreservation of Drosophilia melanogaster embryos. Nature 1990; 345: 170–172 [DOI] [PubMed] [Google Scholar]
- 3.Mazur P, Cole KW, Hall J, Schreuders PD, Mahowald AP. Cryobiological preservation of Drosophila embryos. Science 1992; 258: 1932–1935 [DOI] [PubMed] [Google Scholar]
- 4.Chen SU, Chien CL, Wu MY, Chen TH, Lai SM, Lin CW, Yang YS. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod 2006; 21: 2794–2800 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod 2008; 23: 2256–2265 [DOI] [PubMed] [Google Scholar]
- 6.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online 2009; 18: 568–577 [DOI] [PubMed] [Google Scholar]
- 7.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod 2009; 24: 1670–1683 [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto S, Suzuki N, Yamanaka M, Hosoi Y, Ishizuka B, Morimoto Y. Effects of vitrification solutions and equilibration times on the morphology of cynomolgus ovarian tissues. Reprod Biomed Online 2010; 21: 501–509 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki N, Hashimoto S, Igarashi S, Takae S, Yamanaka M, Yamochi T, Takenoshita M, Hosoi Y, Morimoto Y, Ishizuka B. Assessment of long term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod 2012; 27: 2420–2429 [DOI] [PubMed] [Google Scholar]