Abstract
It is not until accomplishment of a variety of molecular changes during the transit through the female reproductive tract that mammalian spermatozoa are capable of exhibiting highly activated motility with asymmetric whiplash beating of the flagella (hyperactivation) and undergoing acrosomal exocytosis in the head (acrosome reaction). These molecular changes of the spermatozoa are collectively termed capacitation and promoted by bicarbonate, calcium and cholesterol acceptors. Such capacitation-promoting factors can stimulate intracellular cyclic AMP (cAMP) signal transduction in the spermatozoa. Meanwhile, hyperactivation and the acrosome reaction are essential to sperm fertilization with oocytes and are apparently triggered by a sufficient increase of intracellular Ca2+ in the sperm flagellum and head, respectively. Thus, it is necessary to investigate the relationship between cAMP signal transduction and calcium signaling cascades in the spermatozoa for the purpose of understanding the molecular basis of capacitation. In this review, I cover updated insights regarding intracellular cAMP signal transduction, the acrosome reaction and flagellar motility in mammalian spermatozoa and then account for possible roles of intracellular cAMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa.
Keywords: Acrosome reaction, cAMP, Capacitation, Hyperactivation, Protein phosphorylation
It has been accepted that epithelial secretion and absorption within the epididymis produces a favorable environment for maturation and storage of luminal spermatozoa. Mammalian spermatozoa (including mouse spermatozoa) usually undergo various changes in their cellular components during their transit through the epididymis and finally acquire the full ability to move progressively and to fertilize oocytes in the terminal region (cauda) of the epididymis [1,2,3,4,5]. In boars, however, most of the spermatozoa develop the ability to penetrate into oocytes in the proximal (caput) and middle (corpus) regions [6], but progressive motility develops in the corpus and cauda epididymides [7, 8]. In particular, when immature spermatozoa from the rete testis are treated to induce the acrosome reaction in vitro, they are capable of penetrating into zona-free hamster eggs [9]. In the cauda epididymidis, the spermatozoa are maintained in a quiet state by the interaction with weakly acidic luminal fluid containing stabilizing factors to minimize possible premature membrane destabilizations that could lead to events normally occurring in the female tract, such as the acrosome reaction [10,11,12,13].
At ejaculation, mammalian spermatozoa (including mouse and boar spermatozoa) initiate flagellar beating to move intensively in a forward direction in response to the exposure to the secretions from the male accessory genital glands [14]. Specifically, bicarbonate in the secretions enters the spermatozoa through the plasma membrane via the action of a carbonic anhydrase [15, 16], sodium-bicarbonate cotransporter [17] and bicarbonate/chloride exchanger [18, 19]. The sperm acceptor for the cytoplasmic bicarbonate is adenylyl cyclase 10 (ADCY10) [also called soluble adenylyl cyclase (sAC or SACY)], which catalyzes the synthesis of cyclic adenosine 3´,5´-monophosphate (cAMP) from adenosine 5´-triphosphate (ATP) [20,21,22,23]. The synthesized cAMP is an intracellular second messenger for the protein kinase A (PKA)-mediated signaling cascades and stimulates serine/threonine phosphorylation in the flagellar proteins including dynein [24,25,26] and axokinin [27], which are required for flagellar beating.
In the female reproductive tract, a relatively higher concentration of bicarbonate in the luminal fluid also promotes a series of sperm changes that are required for the expression of fertilizing ability. The details of capacitation-associated changes in mouse spermatozoa have already been reviewed in well-known publications [e.g.,4, 28, 29]. For boar spermatozoa, there are reports available on the phospholipid changes in the plasma membrane [30, 31]; aggregation of lipid ordered microdomains (lipid rafts), probably in response to the depletion of cholesterol from the plasma membrane [32, 33]; organization of the docking SNARE complex (VAMP 3, syntaxin 1B and SNAP 23) leading to the stable docking between the plasma membrane and outer acrosomal membrane [34], followed by disassembly of this docking complex and assembly of the fusion-driving SNARE complex (syntaxin 3, SNAP 23 and VAMP 2) with an additional interacting protein (complexin 2) [35]; changes in surface glycoconjugates [36]; increases and decrease in phosphorylated proteins [37, 38]; increases in intracellular calcium [39,40,41]; activation of phospholipase A2 [42]; and changes in the expression and localization of actin [43, 44]. These capacitation-associated changes enable the spermatozoa to undergo their acrosome reaction in the heads and hyperactivation in their flagella. Moreover, boar spermatozoa also come under the unique influence of the increase in head-to-head agglutination, which is linked to the progress of capacitation and is apparently promoted by the actions of the intracellular cAMP signal transduction [45,46,47,48,49]. Nonetheless, the agglutination may reduce the number of spermatozoa available for fertilization in vitro [50].
In this review, I cover updated insights regarding intracellular cAMP signal transduction, the acrosome reaction and flagellar motility in mammalian spermatozoa and then account for possible roles of intracellular cAMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa.
Intracellular cAMP Signal Transduction of Mammalian Spermatozoa
In 1958, Southerland and his coworker discovered the role of cAMP as the second messenger in intercellular signal transduction in experiments on the hormonal regulation of metabolism in mammalian hearts and livers [51,52,53]. Specifically, in the glucose metabolism of liver cells, the hormone [adrenaline (epinephrine)] bound to its receptor and subsequently stimulated the transmembrane adenylyl cyclases, leading to the formation of cAMP from ATP. The increased cAMP stimulated unknown factors to activate glycogen phosphorylase of the key enzyme in glycogenolysis.
The adenylyl cyclases are important enzymes that convert ATP to cAMP in response to stimulation by various hormones, neurotransmitters, free ions and other molecules. Nine transmembrane adenylyl cyclase isoforms (Adcys #1-9) and one soluble adenylyl cyclase (Adcy10) have been identified in the rodent so far. All of the transmembrane adenylyl cyclases include two transmembrane domains and two cytosolic domains and are activated via the interaction between G protein-coupled receptors (GPCRs) and Gs-heterotrimeric G proteins. The other isoform (Adcy10), which is distinguished from Adcys 1-9 by G-protein-independent activation and lack of the membrane-binding domain, is abundantly present in the testis. Adcy10 in rodent male germ cells has been characterized in great detail for the purpose of examining its roles in male reproductive performance and sperm expression of fertilizing ability [e.g.,20, 21, 54,55,56,57,58,59,60]. For instance, two controversial hypotheses have been suggested concerning the mechanisms for generation of the truncated form of Adcy10. The primary report explained that this cyclase is originally synthesized mainly in the testis as a 189-kDa precursor (full-length form) with two catalytic domains in the N-terminal region and that it then changes into the 48-kDa truncated form during the sperm maturational process in the epididymis. However, it has lately been reported that both the full-length and truncated forms of Adcy10 are generated from the same Adcy10 gene in the testis by alternate splicing. In any case, the resultant truncated form consists almost exclusively of two conserved catalytic domains. The specific cyclase activity of the truncated form is approximately 20-fold higher than that of the full-length form. This cyclase is also stimulated by direct binding with not only bicarbonate but also calcium. In mature spermatozoa, Adcy10 is localized in the middle piece and involved in ATP synthesis. Moreover, it also plays a critical role in the regulation of capacitation-associated protein tyrosine phosphorylation, motility activation and hyperactivation. In fact, male mice lacking Adcy10 are infertile, as their spermatozoa are immotile. However, there is another report claiming that this isoform is indispensable for motility activation but does not have any direct effects on triggering hyperactivation [60]. By contrast, since the spermatozoon is a compartmented cell, it is difficult to diffuse the Adcy10-catalyzed cAMP from the middle piece to the head. Thus, this isoform is unlikely to be involved in the acrosome reaction and fusion with the oocyte plasma membrane. In fact, Adcy10-null spermatozoa have the normal capacity to undergo the acrosome reaction, indicating that Adcy10 is barely functional in the capacitation-associated changes leading to the acrosome reaction in the sperm head. The transmembrane isoforms including Adcy3 are present in the head and working as cAMP suppliers that stimulate the intracellular signal transduction regulating the capacitation-associated changes [61, 62].
Likewise, searches of the NCBI database imply the expression of mRNAs of transmembrane ADCYs#1-9 and ADCYs2, 6 and 9 in cattle and pigs, respectively. Moreover, both full-length and truncated forms of ADCY10 are also detectable in the testis from livestock [22, 23]. The truncated form is translated from mRNA lacking exon 11 that is derived by the alternative splicing in the testis and exclusively contained in mature spermatozoa. The molecular masses of the boar sperm truncated forms are 48 kDa and 70 kDa. The molecular mass of the former corresponds to that of the truncated form of rodent Adcy10 with catalytic domains in the N-terminal region [20, 21], whereas the latter may be another truncated form that is limited to boar spermatozoa. However, livestock sperm ADCY10 [22, 23] is distributed in the head as well as the connecting and principal pieces, unlike rodent spermatozoa (middle piece). This indicates that the livestock ADCY10 is potentially related to the functions of both the heads and flagella. Thus, there are differences in the regulatory system for cAMP synthesis in the sperm heads between livestock and rodents. In addition, soluble adenylyl cyclase is present in the head of sea urchin spermatozoa and participates in the cAMP signal transduction regulating the acrosome reaction [63].
My colleagues and I also pay attention to the localization of ADCY10 in the connecting and principal pieces of livestock spermatozoa [22, 23], because a variety of cAMP signaling molecules exist specifically in these segments [49, 64,65,66,67]. In other words, we hypothesized that livestock spermatozoa always maintain relatively higher levels of intracellular cAMP locally in the connecting and principal pieces by the action of ADCY10 and consequently produce constant stimulation of cAMP signal transduction in the flagella.
The main direct targets of cAMP are PKA and exchange protein directly activated by cAMP [EPAC, also known as “Rap guanine nucleotide exchange factor (RAPGEF)”] [68, 69]. PKA is a heterotetramer kinase that is composed of two cAMP-binding regulatory subunits and two inactive catalytic subunits [69]. Once the intracellular cAMP concentration increases, four molecules of cAMPs bind to one regulatory subunit dimmer to bring about release of catalytic subunits by a constructive change in regulatory subunits [70]. At this step, PKA becomes partially active and then fully active as the result of the phosphorylation at the Thr197 residue of the catalytic subunit to improve its affinity for ATP and the efficiency of phosphorylation to target proteins [71,72,73]. The amino acid motif of the activation loop is universal among different isoforms from various species, including humans (α, β and γ), mice (α and β) and pigs (α and β). The active PKA recognizes the substrate proteins containing amino acid motifs of Arg-X-X-Ser/Thr or Arg-X-Ser and transfers the phosphate group from ATP to serine or threonine residue [73]. Meanwhile, EPAC was discovered in 1998 as a novel cAMP-targeting factor [74, 75]. It is activated directly by cAMP for the function of the guanine-nucleotide exchange factors for small GTPases. The cAMP-EPAC-RAP1 signaling cascades are involved in the regulation of cellular adhesion [68, 76, 77], insulin secretion [68, 78] and cellular differentiation in somatic cells [68, 69].
These targets of cAMP have been found in mammalian spermatozoa. In the spermatozoa from several species, the distribution of PKA has been determined by immunological observation. In mouse spermatozoa, anti-PKA catalytic subunit antibody recognized flagellar antigens but not antigens of the heads [79]. However, Visconti et al. [80] reported that the regulatory and catalytic subunits of PKA were present in all regions. Anti-PKA regulatory subunit II antibody also reacted to the middle, principal and end pieces [81]. In bull spermatozoa, anti-PKA regulatory subunit I antibody produced intense staining of the acrosome and a light staining of connecting and middle pieces. In contrast, anti-PKA regulatory subunit II stained middle and end pieces intensively and principal piece less intensively [82]. In human spermatozoa, regulatory subunit II α was located on the axonemal microtubule wall of flagella, whereas a different isoform of broader specificity was present in the cytoplasm at the periphery of the coarse fibers and fibrous sheath. This subunit was also found in the mitochondria of the middle piece [83]. Moreover, a unique isoform of PKA catalytic subunit α-s was immunolocalized in the middle piece [84]. In boar spermatozoa, the anti-PKA catalytic subunit antibody stained prominently the principal and connecting pieces [85]. These suggest that PKA is localized mainly in the flagella of boar spermatozoa and that the localization of sperm PKA may vary among different species.
Although EPAC is less understood in mammalian spermatozoa compared with PKA, two isoforms are detectable in the mouse testis and are involved in the regulation of spermatogenesis [86, 87]. My colleagues and I [86] provided evidence for existence of cAMP-Epac signaling cascades in the heads of mouse spermatozoa. Branham et al. [88, 89] investigated roles of the cAMP-EPAC1-small G protein signaling cascades in the acrosome reaction of human spermatozoa. Briefly, they showed that Ca2+ influx for initiation of the acrosome reaction was fully induced by treatment with a cAMP analog, that the spermatozoa failed to undergo the acrosome reaction when EPAC was reacted with specific blocking antibodies and that the cell-permeable EPAC-selective cAMP analog induced the acrosome reaction. Kinukawa et al. [90] reported the cAMP-dependent activation of the Epac2-Rap2 signaling cascades regulating the conversion of microtubule sliding into flagellar bending in hamster epididymal spermatozoa. Moreover, Miro-Moran et al. [91] reported that an immunodetection signal of EPAC1 was observed in the marginal segment of the acrosome and middle piece of boar spermatozoa and that RAP1 and E-cadherin may be cAMP-EPAC1 signaling molecules. In addition, details on other elements of the intracellular cAMP signal transduction in mammalian spermatozoa are available in a previous review [92].
Acrosome Reaction
The mammalian spermatozoon is structurally composed of a head, neck (connecting piece) and flagellum. The head is divided into two parts, the acrosomal and postacrosomal regions, based on light-microscopic characteristics. The former region is further divided into three domains, the marginal, principal and equatorial segments. The marginal and principal segments are collectively termed the acrosomal cap [93]. The acrosome reaction is the exocytosis of the acrosomal contents following multiple partial fusions between the plasma and outer acrosomal membranes. It is apparently triggered by an increase in intracellular Ca2+, which is recruited from the extracellular space as well as the internal store (outer acrosomal membrane) through cation channels, including the voltage-operated Ca2+ channels (VOCCs), inositol triphosphate receptor (IP3R) and store-operated channels (SOCs). Recently, the group research of Darszon and Visconti indicates that acrosome reaction-inducible Ca2+ entry into mouse spermatozoa is mediated mainly via VOCCs, which are activated by membrane potential hyperpolarization during capacitation [94].
For the last several decades, it has been believed that a specific zona pellucida glycoprotein (ZP3 of mouse oocytes) has dual functions as a specific sperm-binding site (sperm receptor) and a physiological inducer of the acrosome reaction [95]. However, Gahlay et al. [96] claimed that another zona pellucida glycoprotein (ZP2), rather than ZP3, was pivotally involved in the sperm binding to the zona pellucida in mice. Jin et al. [97] observed using a video microscopic in vitro fertilization system that mouse spermatozoa underwent the acrosome reaction before contact with the zona pellucida and then fertilized oocytes. Moreover, spermatozoa with an intact acrosome barely initiated the reaction on the zona pellucida. These findings strongly require reexamination on the roles of the zona pellucida glycoprotein in the acrosome reaction. Alternatively, it should be noted that progesterone (included by the cumulus oophorus) can stimulate Ca2+ entry from the extracellular milieu [98], which activates the cAMP-EPAC-small G-protein (RAP1 and RAB3A) signaling cascades and then the SNARE-dependent mechanism, leading to the acrosome reaction in human spermatozoa [88, 89]. Effects of this steroid on the acrosome reaction have been reported to be mediated non-genomically by the unique receptors on the plasma membrane in various species [42, 99,100,101,102]. The involvement of the cAMP signal transduction in the acrosome reaction is also supported by research with boar spermatozoa showing that treatment with a cell-permeable cAMP analog can induce the acrosome reaction after the capacitation-associated changes in the sperm head [38, 41].
Whether release of acrosomal serine proteases during the acrosome reaction is important for sperm fertilization with oocytes had also been controversial. This was finally demonstrated by the observation that epididymal spermatozoa from mice lacking sperm ACR (acrosin) and PRSS21 (testisin/TESP5) failed to penetrate into the zona pellucida in vitro. However, this mutant mouse was surprisingly subfertile (but not infertile), because ejaculated spermatozoa of this mutant mouse were partially capable of fertilizing oocytes in vivo due to interaction with uterine fluid during capacitation [103].
The mouse sperm-specific protein IZUMO1 is essential for sperm-oocyte plasma membrane binding and fusion. During the acrosome reaction, this protein migrates to the equatorial segment of the sperm head, which initially binds to and fuses with the oocyte plasma membrane [104, 105, 106]. This fact indicates that the acrosome reaction has another function for migration and modification of fertilization-related proteins in spermatozoa. Thus, this reaction can act as a functional switch that converts spermatozoa into a fusion-competent state [28].
We also need to reconsider the concept that fully acrosome-reacted spermatozoa are unable to penetrate into the zona pellucida and consequently have lost their fertilizing ability, because mouse spermatozoa long after the acrosome reaction are still capable of penetrating into the zona pellucida and fertilizing other oocytes [107]. Thus, the roles of the acrosome reaction in sperm fertilization and characteristics of the acrosome-reacted spermatozoa should be reexamined in all mammalian species.
Progressive Motility and Hyperactivation
The flagellum of the mammalian spermatozoon is structurally divided into the middle, principal and end pieces. These pieces share a long axoneme in the central part that is composed of a central pair of microtubule doublets and nine outer microtubule doublets. However, the structures surrounding the axoneme are different among these pieces. In the middle piece, an outer dense fiber is localized outside of each outer microtubule doublet of the axoneme. These structures are surrounded by the mitochondrial sheath, which is positioned along the internal circumference of the plasma membrane [93, 108]. Although it has long been believed that oxidative phosphorylation in the mitochondrial sheath produces sufficient ATP to sustain sperm progressive motility under aerobic conditions, spermatozoa of many species can remain motile even under anaerobic conditions or when mitochondrial oxidative phosphorylation is pharmacologically inhibited. Moreover, it is uncertain whether sufficient ATP can effectively be diffused from the mitochondrial sheath to the distal flagellum. In spermatozoa from mice lacking a sperm-specific glycolytic enzyme, “glyceraldehyde 3-phosphate dehydrogenase-S”, moreover, the ATP level was greatly reduced to 10% of that in wild-type mouse spermatozoa, though mitochondrial oxygen consumption was barely affected. These indicate that cytoplasmic glycolysis rather than mitochondrial oxidative phosphorylation is mainly functional in the supply of sufficient ATP to the distal flagellum for the exhibition of sperm progressive motility. However, arguments that the balance between these ATP-supplying systems varies among species and that local glycolysis is not solely responsible for the supply of ATP to the distal flagellum are still put forward. Indeed, deficiency of oxygen in the seminal plasma results in a remarkable reduction in the motility of boar spermatozoa ejaculated and then stored at room temperature [108, 109, 110].
The spermatozoa ejaculated immediately into the female reproductive tract exhibit progressive motility resulting from the regular and fast beating of the principal piece. The sperm flagella are driven by the interaction between ATP and the axonemal motor protein “dynein”. In the principal piece, outer dense fibers are extended from the middle piece. The fibrous sheath replaces the mitochondrial sheath and forms longitudinal columns at positions of the outer dense fibers #3 and #8. These axoneme-surrounding structures possess scaffolding proteins, “A-kinase anchoring proteins 3 and 4”, which gather the intracellular signaling molecules regulating flagellar beating and movement patterns. The end piece consists of the axoneme and plasma membrane without the axonemal-surrounding structures. However, the roles of this piece in sperm motility remain unclear [108, 111, 112].
While mammalian spermatozoa are in the late/terminal phase(s) of capacitation, their movement patterns change from progressive motility to hyperactivation. Hyperactivation is characterized by intensive and asymmetric whiplash beating of the middle and principal pieces and enhances the ability of spermatozoa to detach from the wall of the oviduct, to move around in the labyrinthine lumen of the oviduct, to pass through mucous substances and to penetrate the extracellular matrix of oocytes [4, 113, 114, 115]. Apparently, initiation and continuance of hyperactivation is regulated by the increase in intracellular Ca2+ (from approximately 50 nM to 400 nM) in the flagellum [116]. As of now, it is considered that the increased Ca2+ is recruited from the internal store (redundant nuclear envelop of the neck) through IP3R [65, 117, 118, 119] and subsequently from the extracellular space supposedly through SOCs including the transient receptor potential (TRP) channel family [120, 121] as well as from the extracellular space through the cationic channel of sperm “CATSPER” [122, 123, 124, 125]. However, it is likely that these two distinct calcium signaling cascades regulate different flagellar beating patterns in mouse hyperactivated spermatozoa [126]. Specifically, mouse epididymal spermatozoa, which were incubated under capacitating conditions for 2 h or treated with the putative CATSPER activator (procaine or 4-aminopyridine) under non-capacitating conditions for several minutes, underwent an intracellular Ca2+ increase in the proximal principal piece and phosphorylation at the serine/threonine residues of many proteins and then exhibited high-amplitude, pro-hook flagellar beating (a deep bend in the same direction as the hook of the head) [126]. The validity of the putative CATSPER activator (procaine) for the induction of hyperactivation in uncapacitated spermatozoa was also confirmed in the livestock (bulls and stallions) [127, 128]. In contrast, mouse epididymal spermatozoa increased the intracellular Ca2+ in the base (connecting and proximal middle pieces) of flagella after treatment with a putative releaser (thimerosal) of Ca2+ from the internal store of the connecting piece under non-capacitation conditions and consequently exhibited high-amplitude anti-hook beating of the flagella (a deep bend in the direction opposite the hook of the head) [126]. Moreover, recent experiments using male Acr-EGFP mice revealed that most ejaculated spermatozoa after in vivo capacitation exhibited high-amplitude anti-hook beating of the flagella in the ampulla of the oviduct [129]. From these complicated results, it is postulated that hyperactivation of mouse spermatozoa may initially be triggered by the influx of extracellular Ca2+ through CATSPER and subsequently be modulated by the action of Ca2+ from the internal store through IP3R and/or extracellular Ca2+ through SOCs to reverse the direction of the flagellar bend (from pro-hook beating to anti-hook beating). Also, it can be stated that there are basic differences in hyperactivation between in vivo-capacitated and in vitro-capacitated spermatozoa, because the environment of the female reproductive tract is quite different from that provided by incubation in capacitation-supporting medium [129]. Moreover, we should consider the possibility that differences in functions between epididymal and ejaculated spermatozoa have influences on hyperactivation, since seminal plasma (secretions from male accessory genital glands) contains a variety of functional regulators for sperm motility, metabolism, membrane fluidity, capacitation status and so on [130]. Additionally, in a previous experiment [131], mouse hyperactivation-like epididymal spermatozoa, in which serine-/threonine-phosphorylated proteins with molecular masses of 170, 155, 140 and 42 kDa were increased by incubation with calyculin A for 1.5 h, showed high-amplitude anti-hook flagellar beating, as observed in in vivo-capacitated spermatozoa [126]. However, it is hard to distinguish the direction of flagellar beating in the hyperactivated spermatozoa of livestock because their sperm heads are bilaterally symmetrical. Meanwhile, controversial data have been obtained in other pharmacological experiments indicating that a CATSPER-mediated increase in intracellular Ca2+ was induced in an early phase of capacitation and that this Ca2+ increase activated the motility to promote penetration into cervical mucus or cumulus matrix but barely induced hyperactivation [132, 133]. Instead, the IP3R-meditated release of stored Ca2+ at the connecting piece was effective mainly in initiation and continuance of human sperm hyperactivation [133]. Thus, further research is necessary in order to determine the types of calcium channels that are requisite for the initiation and continuance of hyperactivation.
Roles of Intracellular cAMP Signal Transduction in Sperm Capacitation and Subsequent Hyperactivation
It is generally accepted that the mammalian sperm acrosome reaction and flagellar hyperactivation are observed in the late/terminal phase(s) of the capacitation. In particular, hyperactivation is correlated with the cAMP-dependent enhancement of the protein tyrosine phosphorylation state, which is a hallmark of capacitation [132, 134, 135]. Thus, I am thinking that it is necessary to investigate the relationship between cAMP signal transduction and calcium signaling cascades leading to hyperactivation for the purpose of understanding the molecular basis of capacitation.
Mouse spermatozoa
Roles of cAMP signal transduction in regulation of the ion channels have been proposed for mouse spermatozoa [119]. Briefly, intracellular alkalization is observed during passage through the female reproductive tract or incubation in a capacitation-supporting medium. It is regulated by the uptake of bicarbonate [17,18,19] and also promoted by a sperm-specific sodium/hydrogen exchanger (sNHE) in the principal piece of the flagella [136]. The sNHE contains a potential voltage sensor and a consensus cyclic nucleotide-binding motif, suggesting possible interaction with cAMP. Interestingly, sNHE-null male mice are infertile and have severely diminished sperm motility [136]. Subsequently, intracellular alkalization activates the potassium channels including SLO3, leading to membrane hyperpolarization of the flagella [137, 138]. Sperm SLO3 is stimulated by cAMP through PKA-dependent phosphorylation [119]. Alternatively, a Cl- channel, the cystic fibrosis transmembrane conductance regulator (CFTR), which is modulated by the cAMP-PKA signaling cascades and ATP levels, promotes membrane hyperpolarization by closing epithelial Na+ channels (ENaCs) in the middle piece [139,140,141]. Such capacitation-associated membrane hyperpolarization is necessary for entry of external Ca2+ via the voltage-dependent ion channels including CATSPER and contributes to the occurrence of hyperactivation [119]. Moreover, mouse spermatozoa are highly enhanced in the tyrosine phosphorylation state of flagellar proteins and coincidentally exhibit hyperactivation by simple incubation in a capacitation-supporting medium including the activators of cAMP signal transduction (bicarbonate [21] and Ca2+ [54]) without further addition of external stimulators (e.g., procaine, 4-aminopyridine, caffeine, progesterone, thimerosal and Ca2+ ionophore A23187) for the calcium signaling cascades [126, 129, 142]. These observations suggest that mouse spermatozoa may be spontaneously capable of switching on the calcium signaling cascades leading to hyperactivation when they accomplish sufficient activation of cAMP signal transduction during capacitation.
Boar spermatozoa
In boar spermatozoa, hyperactivation was barely induced by simple incubation in a capacitation-supporting medium (unpublished data). Similar results were obtained in bull spermatozoa incubated under capacitating conditions in vitro [127]. Moreover, a clear increase of the tyrosine phosphorylation state was detectable in only limited proteins of boar spermatozoa that were incubated in a capacitation-supporting medium [143,144,145]. These observations indicate that incubation under capacitating conditions in vitro cannot sufficiently activate the intracellular cAMP signal transduction leading to enhancement of the protein tyrosine phosphorylation state and the occurrence of hyperactivation in boar spermatozoa, unlike the case in mouse spermatozoa. This might be because full activation of sperm cAMP signal transduction requires much stronger stimulators in boars than mice.
Thus, in our laboratory, my colleagues and I attempted to induce both capacitation-associated changes and hyperactivation in boar spermatozoa in vitro by stronger stimulation of intracellular cAMP signal transduction and found that transition of motility from the progressive type to hyperactivation was highly induced in boar spermatozoa by incubation with a cell-permeable cAMP analog, “Sp-5,6-dichloro-1-β-D-ribofuranosylbenzimidazole-3´,5´-monophoshorothioate” (cBiMPS), for 180 min [49, 66, 67, 85]. During this incubation period, the capacitation state in the sperm head (as assessed by chlortetracycline staining) and tyrosine phosphorylation state in the flagellar proteins were enhanced coincidently with the transition of motility to hyperactivation [38, 64, 67, 85]. These findings demonstrate that our simulation system can mimic the capacitation-associated changes leading to hyperactivation in boar spermatozoa. To my knowledge, our system is the only method that can highly induce both capacitation-associated changes and hyperactivation in livestock spermatozoa.
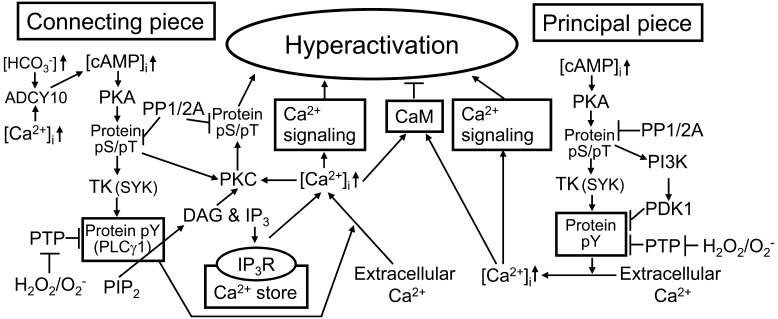
Our previous articles suggest that several cAMP signaling cascades regulating hyperactivation are activated in boar spermatozoa by our simulation system (Fig. 1). In brief, the treatment with cBiMPS (i.e., increase of intracellular cAMP) can induce protein serine/threonine phosphorylation rapidly by the activation of PKA and then induce protein tyrosine phosphorylation with a time lag of a few hours by activation of protein tyrosine kinases [e.g., spleen tyrosine kinase (SYK)] in the connecting and principal pieces. The possible functions of the cAMP-dependent protein tyrosine phosphorylation may include activation of phospholipase Cγ1 (PLCγ1) that is linked to the release of Ca2+ from the internal store in the connecting piece [65]. Moreover, other serine/threonine kinases including protein kinase C (PKC) of the connecting piece are activated by the actions of the cAMP-PKA signaling cascades and/or release of Ca2+ from the internal store [66]. On the other hand, it is likely that another cAMP-dependent signaling cascade suppresses tyrosine phosphorylation of flagellar proteins via the phosphatidylinositol-3 kinase (PI3K) and phosphoinositide-dependent protein kinase-1 (PDK1) in order to prevent the occurrence of precocious hyperactivation [85]. Recently, my colleagues and I [135] indicated that these capacitation-associated changes in protein tyrosine phosphorylation state in the connecting and principal pieces are necessary for critical actions of external Ca2+ to trigger hyperactivation.
Fig. 1.
Possible segment-specific cAMP signal transductions regulating transition of the flagellar movement pattern to hyperactivation in boar spermatozoa. ADCY10, adenylyl cyclase 10; cAMP, cyclic adenosine 3´,5´-monophosphate; PKA, protein kinase A (cAMP-dependent protein kinase); pS/pT, serine/threonine phosphorylation; PP, protein phosphatase; TK, tyrosine kinase; SYK, spleen tyrosine kinase; PTP, protein tyrosine phosphatase; pY, tyrosine phosphorylation; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, 1,2-diacylglycerol; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; PKC, protein kinase C; PI3K, phosphatidylinositol-3 kinase; PDK1, phosphoinositide-dependent protein kinase-1; CaM, calmodulin.
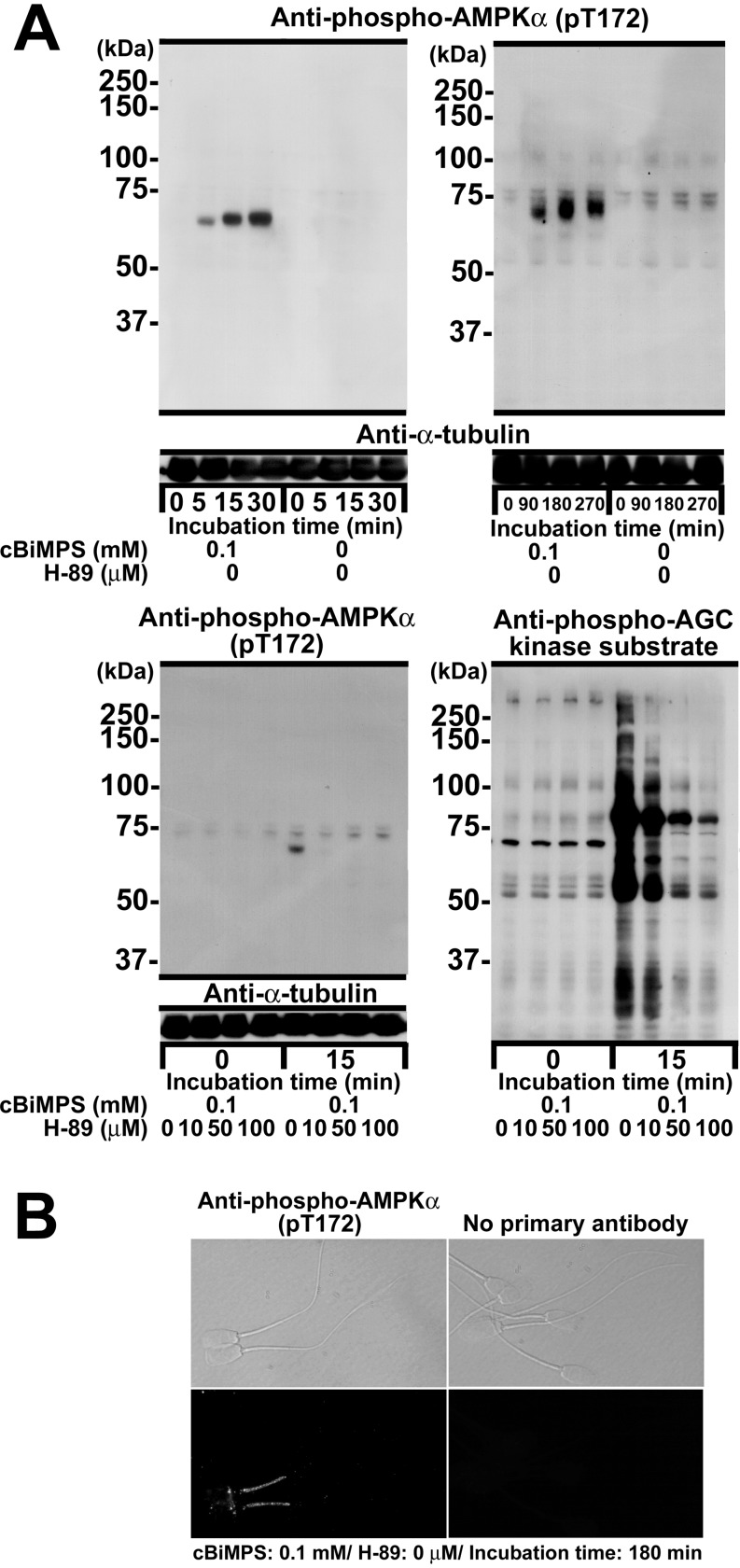
Unlike the case of mouse spermatozoa, the capacitation-associated protein phosphorylation at the serine/threonine and tyrosine residues is less intensive in the middle pieces of boar spermatozoa because of a deficiency of PKA and protein tyrosine kinase (SYK) in this segment [64, 85]. Thus, few investigations have been made into the cAMP-dependent changes in the protein phosphorylation state of the middle piece. In our preliminary experiment, however, my colleagues and I detected an increase in the active form of the AMP-activated protein kinase (AMPK) 2α catalytic subunit (phosphorylated at Thr172) mainly in the middle piece of boar spermatozoa during incubation with cBiMPS to induce hyperactivation (Fig. 2). Moreover, this increase of the active form was suppressed by addition of the PKA inhibitor H-89, indicating interaction with the cAMP-PKA signaling cascades (Fig. 2). Pharmacological inhibition of AMPK with compound C suppressed the occurrence of hyperactivation without deleterious effects on the motility rates and intensity of flagellar beating, but had almost no influence on the state of cAMP-dependently tyrosine-phosphorylated proteins and serine/threonine-phosphorylated PKA substrates in the connecting and principal pieces (data not shown). AMPK is a key regulatory kinase for cellular energy homeostasis, including carbohydrate metabolism. An increase in the intracellular AMP/ATP ratio leads to activation of AMPK by phosphorylation at Thr172 of the activation loop in the catalytic α subunit [146, 147]. Another research group [148] also demonstrated the presence of AMPKs in boar spermatozoa and indicated their possible roles in motility. As sperm movement is accompanied by the consumption of a large amount of ATPs by the action of flagellar dynein-ATPases, I hypothesize that carbohydrate metabolism-related signaling cascades may participate in the occurrence of sperm hyperactivation. In brief, the AMPK2α catalytic subunit of the middle piece is unlikely a PKA substrate, since PKA is barely detected in this piece [85]. One of the causal factors for the increase in the active form of the AMPK2α catalytic subunit may be an increase in the intracellular AMP/ATP ratio, which is due to rapid consumption of ATPs by the PKA-dependent dynein-ATPase. Considering the localization of cAMP-dependently tyrosine-phosphorylated proteins, this indicates the existence of AMPK-mediated signaling cascades in the middle piece that affect the occurrence of hyperactivation and are independent of capacitation-associated protein tyrosine phosphorylation.
Fig. 2.
Detection of AMP-activated protein kinase (AMPK) in boar spermatozoa. For the immunodetection of the AMPK2α catalytic subunit protein, washed spermatozoa were incubated with cBiMPS and an inhibitor for PKA H-89 at 38.5 C. In panel A (Western blotting: a representative of three replicates), aliquots of each sperm suspension (1 × 106 spermatozoa/lane) were recovered immediately before and after incubation, used for SDS-PAGE and transblotting to the membranes, treated with a diluted rabbit anti-phospho-AMPK α polyclonal antibody [Thr172, an active form, anti-phospho-AMPKα (pT172), 1:1,000-2,000] or with a diluted rabbit anti-phospho-AGC kinase substrate polyclonal antibody (anti-phospho-AGC kinase substrate, 1:5,000) and then treated with HRP-conjugated donkey anti-rabbit immunoglobulin polyclonal antibody (1:1,000–1:2,000). In addition, the tubulin was detected solely with HRP-conjugated mouse anti-α-tubulin monoclonal antibody (1:10,000, anti-α-tubulin) as loading controls. In panel B (indirect immunofluorescence: a representative of three replicates), aliquots of each sperm suspension (5 × 105 spermatozoa/preparation) were recovered immediately after incubation for 180 min and treated with paraformaldehyde and Triton X-100. The fixed and membrane-permeated spermatozoa were blocked with bovine serum albumin (BSA) in PBS (blocking buffer), treated overnight at 4 C with the anti-phospho-AMPKα antibody (1:50) or a blocking buffer without the primary antibody (no primary antibody) and then treated with fluorescein isothiocyanate (FITC)-conjugated swine anti-rabbit immunoglobulins (1:50). In each set of photographs, the upper photograph is from differential interference contrast microscopy, and the bottom photograph is from immunofluorescence microscopy.
Concluding Remarks
In conclusion, I would like to propose the suggestion that there are differences in the mechanism for the sperm capacitation between mice and boars. Moreover, I would also like to point out the existence of segment-specific cAMP signal transductions in boar spermatozoa. In the connecting and principal pieces, capacitation-associated protein tyrosine phosphorylation is related to modulation of the calcium signaling cascades leading to hyperactivation. In the middle piece, by contrast, the activation of AMPK (which is promoted by the indirect action of cAMP-PKA signaling cascades) is independent of capacitation-associated protein tyrosine phosphorylation and involved in the occurrence of hyperactivation. These segment-specific cAMP signal transductions must be orchestrated during capacitation for full development of flagellar movement into hyperactivation in boar spermatozoa.
Acknowledgments
I would like to express my gratitude to the Society for Reproduction and Development (SRD) for awarding me an SRD Outstanding Research Award in 2012. I am also very grateful to Dr T Miyano and Dr M Miyake for valuable suggestions and kind advice concerning my research and especially to Dr S Kato, Dr S Kanda, Dr H Masuda and Dr RH Hammerstedt (my former supervisors) for their warm-hearted guidance in the research on the mammalian sperm function. I am thankful to Dr M Fukushima, Dr M Sakase, Dr O Shidara, Dr S Ishikawa (General Technological Center of Hyogo Prefecture for Agriculture, Forest and Fishery) and Dr S Watanabe (NARO Institute of Livestock and Grassland Science) for their kind cooperation. Finally, I would really like to thank all the students in our laboratory very much for technical assistance and helpful suggestions. This work was supported in part by the Grants-in-Aid (23658225, 25450394) from Japan Society for the Promotion of Science to HH.
References
- 1.Bedford JM. Maturation, transport and fate of spermatozoa in the epididymis. In: Astwood EB, Greep RO (eds.), Handbook of Physiology, Section 7: Washington DC: American Physiological Society; 1975: 303–318.
- 2.Orgebin-Crist MC, Danzo BJ, Davies J. Endocrine control of the development and maintenance of sperm fertilizing ability in the epididymis. In: Astwood EB, Greep RO (eds.), Handbook of Physiology, Section 7. Washington DC: American Physiological Society; 1975: 319–338.
- 3.Hammerstedt RH, Parks JE. Changes in sperm surfaces associated with epididymal transit. J Reprod Fertil Suppl 1987; 34: 133–149 [PubMed] [Google Scholar]
- 4.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD (eds.), The Physiology of Reproduction. 2nd edition. New York: Raven Press; 1994: 189–317.
- 5.Lewis B, Aitken RJ. Impact of epididymal maturation on the tyrosine phosphorylation patterns exhibited by rat spermatozoa. Biol Reprod 2001; 64: 1545–1556 [DOI] [PubMed] [Google Scholar]
- 6.Hunter RH, Holtz W, Henfrey PJ. Epididymal function in the boar in relation to the fertilizing ability of spermatozoa. J Reprod Fertil 1976; 46: 463–466 [DOI] [PubMed] [Google Scholar]
- 7.Dacheux JL, Paquignon M, Combarnous Y. Head-to-head agglutination of ram and boar epididymal spermatozoa and evidence for an epididymal antagglutinin. J Reprod Fertil 1983; 67: 181–189 [DOI] [PubMed] [Google Scholar]
- 8.Harayama H, Kusunoki H, Kato S. Motility and penetrability into zona-free hamster eggs of boar spermatozoa collected from various regions of the epididymis. J Reprod Dev 1993; 39: 41–45 [Google Scholar]
- 9.Harayama H, Kusunoki H, Kato S. Capacity of rete testicular and cauda epididymal boar spermatozoa to undergo the acrosome reaction and subsequent fusion with egg plasma membrane. Mol Reprod Dev 1993; 35: 62–68 [DOI] [PubMed] [Google Scholar]
- 10.Hunter RHF, Holtz W, Herrmann H. Stabilizing role of epididymal plasma in relation to the capacitation time of boar spermatozoa. Anim Reprod Sci 1978; 1: 161–166 [Google Scholar]
- 11.Harayama H, Miyano T, Masuda H, Miyake M, Kato S. Detection of the 25-kDa anti-agglutinin in epididymal plasma and spermatozoa collected from various regions of boar epididymis. J Reprod Dev 1995; 41: 113–121 [Google Scholar]
- 12.Harayama H, Kato S, Hammerstedt RH. Electrophoretic characterization of boar epididymal antiagglutinin. Biol Reprod 1996; 55: 325–332 [DOI] [PubMed] [Google Scholar]
- 13.Harayama H, Liao PC, Gage DA, Miyake M, Kato S, Hammerstedt RH. Biochemical characterization of sialoprotein “anti-agglutinin” purified from boar epididymal and seminal plasma. Mol Reprod Dev 2000; 55: 96–103 [DOI] [PubMed] [Google Scholar]
- 14.Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem 1985; 260: 9699–9705 [PubMed] [Google Scholar]
- 15.Ekstedt E, Ridderstråle Y, Plöen L, Rodriguez-Martinez H. Histochemical localization of carbonic anhydrase in the testis and epididymis of the boar. Acta Anat (Basel) 1991; 141: 257–261 [DOI] [PubMed] [Google Scholar]
- 16.Parkkila S, Kaunisto K, Kellokumpu S, Rajaniemi H. A high activity carbonic anhydrase isoenzyme (CA II) is present in mammalian spermatozoa. Histochemistry 1991; 95: 477–482 [DOI] [PubMed] [Google Scholar]
- 17.Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. Involvement of a Na+/HCO-3 cotransporter in mouse sperm capacitation. J Biol Chem 2003; 278: 7001–7009 [DOI] [PubMed] [Google Scholar]
- 18.Parkkila S, Rajaniemi H, Kellokumpu S. Polarized expression of a band 3-related protein in mammalian sperm cells. Biol Reprod 1993; 49: 326–331 [DOI] [PubMed] [Google Scholar]
- 19.Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, Tsang LL, Chung YW, Höglund P, Chan HC, Shi QX. Cl- is required for HCO3- entry necessary for sperm capacitation in guinea pig: involvement of a Cl-/HCO3- exchanger (SLC26A3) and CFTR. Biol Reprod 2009; 80: 115–123 [DOI] [PubMed] [Google Scholar]
- 20.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA 1999; 96: 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000; 289: 625–628 [DOI] [PubMed] [Google Scholar]
- 22.Tate S, Nakamura K, Suzuki C, Noda T, Lee J, Harayama H. Evidence of the existence of adenylyl cyclase 10 (ADCY10) ortholog proteins in the heads and connecting pieces of boar spermatozoa. J Reprod Dev 2010; 56: 271–278 [DOI] [PubMed] [Google Scholar]
- 23.Noda T, Sakase M, Fukushima M, Harayama H. Novel approach for the detection of the vestiges of testicular mRNA splicing errors in mature spermatozoa of Japanese Black bulls. PLoS One 2013; 8: e57296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tash JS, Means AR. Regulation of protein phosphorylation and motility of sperm by cyclic adenosine monophosphate and calcium. Biol Reprod 1982; 26: 745–763 [DOI] [PubMed] [Google Scholar]
- 25.Tash JS, Means AR. Cyclic adenosine 3´,5´ monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod 1983; 28: 75–104 [DOI] [PubMed] [Google Scholar]
- 26.Tash JS, Bracho GE. Regulation of sperm motility: emerging evidence for a major role for protein phosphatases. J Androl 1994; 15: 505–509 [PubMed] [Google Scholar]
- 27.Tash JS, Kakar SS, Means AR. Flagellar motility requires the cAMP-dependent phosphorylation of a heat-stable NP-40-soluble 56 kd protein, axokinin. Cell 1984; 38: 551–559 [DOI] [PubMed] [Google Scholar]
- 28.Florman HM, Ducibella T. Fertilization in Mammals. In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM (eds.), Knobil and Neill’s Physiology of Reproduction. 3rd edition. Waltham: Academic Press; 2006: 55–112.
- 29.Visconti PE, Krapf D, de la Vega-Beltrán JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl 2011; 13: 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, Colenbrander B, Gadella BM. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci 2001; 114: 3543–3555 [DOI] [PubMed] [Google Scholar]
- 31.Gadella BM, Harrison RA. Capacitation induces cyclic adenosine 3´,5´-monophosphate-dependent, but apoptosis-unrelated, exposure of aminophospholipids at the apical head plasma membrane of boar sperm cells. Biol Reprod 2002; 67: 340–350 [DOI] [PubMed] [Google Scholar]
- 32.van Gestel RA, Helms JB, Brouwers JF, Gadella BM. Effects of methyl-β-cyclodextrin-mediated cholesterol depletion in porcine sperm compared to somatic cells. Mol Reprod Dev 2005; 72: 386–395 [DOI] [PubMed] [Google Scholar]
- 33.Gadella BM, Tsai PS, Boerke A, Brewis IA. Sperm head membrane reorganisation during capacitation. Int J Dev Biol 2008; 52: 473–480 [DOI] [PubMed] [Google Scholar]
- 34.Tsai PS, Garcia-Gil N, van Haeften T, Gadella BM. How pig sperm prepares to fertilize: stable acrosome docking to the plasma membrane. PLoS One 2010; 5: e11204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai PS, Brewis IA, van Maaren J, Gadella BM. Involvement of complexin 2 in docking, locking and unlocking of different SNARE complexes during sperm capacitation and induced acrosomal exocytosis. PLoS One 2012; 7: e32603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashworth PJ, Harrison RA, Miller NG, Plummer JM, Watson PF. Flow cytometric detection of bicarbonate-induced changes in lectin binding in boar and ram sperm populations. Mol Reprod Dev 1995; 40: 164–176 [DOI] [PubMed] [Google Scholar]
- 37.Harrison RA. Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol Reprod Dev 2004; 67: 337–352 [DOI] [PubMed] [Google Scholar]
- 38.Adachi J, Tate S, Miyake M, Harayama H. Effects of protein phosphatase inhibitor calyculin a on the postacrosomal protein serine/threonine phosphorylation state and acrosome reaction in boar spermatozoa incubated with a cAMP analog. J Reprod Dev 2008; 54: 171–176 [DOI] [PubMed] [Google Scholar]
- 39.Okamura N, Tanba M, Fukuda A, Sugita Y, Nagai T. Forskolin stimulates porcine sperm capacitation by increasing calcium uptake. FEBS Lett 1993; 316: 283–286 [DOI] [PubMed] [Google Scholar]
- 40.Harayama H, Okada K, Miyake M. Involvement of cytoplasmic free calcium in boar sperm: head-to-head agglutination induced by a cell-permeable cyclic adenosine monophosphate analog. J Androl 2003; 24: 91–99 [PubMed] [Google Scholar]
- 41.Harayama H, Sasaki K, Miyake M. A unique mechanism for cyclic adenosine 3´,5´-monophosphate-induced increase of 32-kDa tyrosine-phosphorylated protein in boar spermatozoa. Mol Reprod Dev 2004; 69: 194–204 [DOI] [PubMed] [Google Scholar]
- 42.Roldan ER, Vazquez JM. Bicarbonate/CO2 induces rapid activation of phospholipase A2 and renders boar spermatozoa capable of undergoing acrosomal exocytosis in response to progesterone. FEBS Lett 1996; 396: 227–232 [DOI] [PubMed] [Google Scholar]
- 43.Bernabò N, Berardinelli P, Mauro A, Russo V, Lucidi P, Mattioli M, Barboni B. The role of actin in capacitation-related signaling: an in silico and in vitro study. BMC Syst Biol 2011; 5: 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernabò N, Palestini P, Chiarini M, Maccarrone M, Mattioli M, Barboni B. Endocannabinoid-binding CB1 and TRPV1 receptors as modulators of sperm capacitation. Commun Integr Biol 2012; 5: 68–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harayama H, Miyake M, Shidara O, Iwamoto E, Kato S. Effects of calcium and bicarbonate on head-to-head agglutination in ejaculated boar spermatozoa. Reprod Fertil Dev 1998; 10: 445–450 [DOI] [PubMed] [Google Scholar]
- 46.Harayama H, Magargee SF, Kunze E, Shidara O, Iwamoto E, Arikawa S, Miyake M, Kato S, Hammerstedt RH. Changes in epididymal protein anti-agglutinin on ejaculated boar spermatozoa during capacitation in vitro. Reprod Fertil Dev 1999; 11: 193–199 [DOI] [PubMed] [Google Scholar]
- 47.Harayama H, Miyake M, Kato S. Role of cyclic adenosine 3´,5´-monophosphate and serum albumin in head-to-head agglutination of boar spermatozoa. Reprod Fertil Dev 2000; 12: 307–318 [DOI] [PubMed] [Google Scholar]
- 48.Harayama H, Kato S. Relationship between bicarbonate and cyclic nucleotide in the promoting effects on head-to-head agglutination in boar spermatozoa. Asian J Androl 2002; 4: 87–96 [PubMed] [Google Scholar]
- 49.Harayama H. Viability and protein phosphorylation patterns of boar spermatozoa agglutinated by treatment with a cell-permeable cyclic adenosine 3´,5´-monophosphate analog. J Androl 2003; 24: 831–842 [DOI] [PubMed] [Google Scholar]
- 50.Harrison RA. Sperm plasma membrane characteristics and boar semen fertility. J Reprod Fertil (Suppl) 1997; 52: 195–211 [PubMed] [Google Scholar]
- 51.Rall TW, Sutherland EW. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem 1958; 232: 1065–1076 [PubMed] [Google Scholar]
- 52.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 1958; 232: 1077–1091 [PubMed] [Google Scholar]
- 53.Kresge N, Simoni RD, Hill RL, Earl W. Sutherland’s discovery of cyclic adenine monophosphate and the second messenger system. J Biol Chem 2005; 280: e39 [Google Scholar]
- 54.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA 2003; 100: 10676–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA 2004; 101: 2993–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 2005; 9: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 2006; 296: 353–362 [DOI] [PubMed] [Google Scholar]
- 58.Chaloupka JA, Bullock SA, Iourgenko V, Levin LR, Buck J. Autoinhibitory regulation of soluble adenylyl cyclase. Mol Reprod Dev 2006; 73: 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlson AE, Hille B, Babcock DF. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol 2007; 312: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marquez B, Suarez SS. Soluble adenylyl cyclase is required for activation of sperm but does not have a direct effect on hyperactivation. Reprod Fertil Dev 2008; 20: 247–252 [DOI] [PubMed] [Google Scholar]
- 61.Baxendale RW, Fraser LR. Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa. Mol Reprod Dev 2003; 66: 181–189 [DOI] [PubMed] [Google Scholar]
- 62.Livera G, Xie F, Garcia MA, Jaiswal B, Chen J, Law E, Storm DR, Conti M. Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol Endocrinol 2005; 19: 1277–1290 [DOI] [PubMed] [Google Scholar]
- 63.Beltrán C, Vacquier VD, Moy G, Chen Y, Buck J, Levin LR, Darszon A. Particulate and soluble adenylyl cyclases participate in the sperm acrosome reaction. Biochem Biophys Res Commun 2007; 358: 1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harayama H, Muroga M, Miyake M. A cyclic adenosine 3´,5´-monophosphate-induced tyrosine phosphorylation of Syk protein tyrosine kinase in the flagella of boar spermatozoa. Mol Reprod Dev 2004; 69: 436–447 [DOI] [PubMed] [Google Scholar]
- 65.Harayama H, Murase T, Miyake M. A cyclic adenosine 3´,5´-monophosphate stimulates phospholipase Cγ1-calcium signaling via the activation of tyrosine kinase in boar spermatozoa. J Androl 2005; 26: 732–740 [DOI] [PubMed] [Google Scholar]
- 66.Harayama H, Miyake M. A cyclic adenosine 3´,5´-monophosphate-dependent protein kinase C activation is involved in the hyperactivation of boar spermatozoa. Mol Reprod Dev 2006; 73: 1169–1178 [DOI] [PubMed] [Google Scholar]
- 67.Harayama H, Nishijima K, Murase T, Sakase M, Fukushima M. Relationship of protein tyrosine phosphorylation state with tolerance to frozen storage and the potential to undergo cyclic AMP-dependent hyperactivation in the spermatozoa of Japanese Black bulls. Mol Reprod Dev 2010; 77: 910–921 [DOI] [PubMed] [Google Scholar]
- 68.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 2003; 4: 733–738 [DOI] [PubMed] [Google Scholar]
- 69.Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci 2008; 38: 578–588 [DOI] [PubMed] [Google Scholar]
- 70.Traugh JA, Ashby CD, Walsh DA. Criteria for the classification of protein kinases. Methods Enzymol 1974; 38: 290–299 [DOI] [PubMed] [Google Scholar]
- 71.Adams JA, McGlone ML, Gibson R, Taylor SS. Phosphorylation modulates catalytic function and regulation in the cAMP-dependent protein kinase. Biochemistry 1995; 34: 2447–2454 [DOI] [PubMed] [Google Scholar]
- 72.Moore MJ, Kanter JR, Jones KC, Taylor SS. Phosphorylation of the catalytic subunit of protein kinase A. Autophosphorylation versus phosphorylation by phosphoinositide-dependent kinase-1. J Biol Chem 2002; 277: 47878–47884 [DOI] [PubMed] [Google Scholar]
- 73.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 2010; 11: 9–22 [DOI] [PubMed] [Google Scholar]
- 74.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 1998; 282: 2275–2279 [DOI] [PubMed] [Google Scholar]
- 75.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998; 396: 474–477 [DOI] [PubMed] [Google Scholar]
- 76.Bos JL, de Bruyn K, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, Riedl J, de Rooij J, van Mansfeld F, Zwartkruis F. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans 2003; 31: 83–86 [DOI] [PubMed] [Google Scholar]
- 77.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 2005; 579: 4966–4972 [DOI] [PubMed] [Google Scholar]
- 78.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2´-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem 2003; 278: 8279–8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci 2006; 119: 3182–3192 [DOI] [PubMed] [Google Scholar]
- 80.Visconti PE, Johnson LR, Oyaski M, Fornés M, Moss SB, Gerton GL, Kopf GS. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol 1997; 192: 351–363 [DOI] [PubMed] [Google Scholar]
- 81.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA 2004; 101: 13483–13488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vijayaraghavan S, Olson GE, NagDas S, Winfrey VP, Carr DW. Subcellular localization of the regulatory subunits of cyclic adenosine 3´,5´-monophosphate-dependent protein kinase in bovine spermatozoa. Biol Reprod 1997; 57: 1517–1523 [DOI] [PubMed] [Google Scholar]
- 83.Pariset C, Weinman S. Differential localization of two isoforms of the regulatory subunit RIIα of cAMP-dependent protein kinase in human sperm: biochemical and cytochemical study. Mol Reprod Dev 1994; 39: 415–422 [DOI] [PubMed] [Google Scholar]
- 84.Reinton N, Orstavik S, Haugen TB, Jahnsen T, Taskén K, Skålhegg BS. A novel isoform of human cyclic 3´,5´-adenosine monophosphate-dependent protein kinase, c α-s, localizes to sperm midpiece. Biol Reprod 2000; 63: 607–611 [DOI] [PubMed] [Google Scholar]
- 85.Harayama H, Nakamura K. Changes of PKA and PDK1 in the principal piece of boar spermatozoa treated with a cell-permeable cAMP analog to induce flagellar hyperactivation. Mol Reprod Dev 2008; 75: 1396–1407 [DOI] [PubMed] [Google Scholar]
- 86.Amano R, Lee J, Goto N, Harayama H. Evidence for existence of cAMP-Epac signaling in the heads of mouse epididymal spermatozoa. J Reprod Dev 2007; 53: 127–133 [DOI] [PubMed] [Google Scholar]
- 87.Aivatiadou E, Ripolone M, Brunetti F, Berruti G. cAMP-Epac2-mediated activation of Rap1 in developing male germ cells: RA-RhoGAP as a possible direct down-stream effector. Mol Reprod Dev 2009; 76: 407–416 [DOI] [PubMed] [Google Scholar]
- 88.Branham MT, Mayorga LS, Tomes CN. Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J Biol Chem 2006; 281: 8656–8666 [DOI] [PubMed] [Google Scholar]
- 89.Branham MT, Bustos MA, De Blas GA, Rehmann H, Zarelli VE, Treviño CL, Darszon A, Mayorga LS, Tomes CN. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J Biol Chem 2009; 284: 24825–24839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinukawa M, Oda S, Shirakura Y, Okabe M, Ohmuro J, Baba SA, Nagata M, Aoki F. Roles of cAMP in regulating microtubule sliding and flagellar bending in demembranated hamster spermatozoa. FEBS Lett 2006; 580: 1515–1520 [DOI] [PubMed] [Google Scholar]
- 91.Miro-Moran A, Jardin I, Ortega-Ferrusola C, Salido GM, Peña FJ, Tapia JA, Aparicio IM. Identification and function of exchange proteins activated directly by cyclic AMP (Epac) in mammalian spermatozoa. PLoS One 2012; 7: e37713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Signorelli J, Diaz ES, Morales P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res 2012; 349: 765–782 [DOI] [PubMed] [Google Scholar]
- 93.Eddy EM. The Spermatozoon. In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM (eds.), Knobil and Neill’s Physiology of Reproduction. 3rd edition. Waltham: Academic Press; 2006: 3–54.
- 94.De La Vega-Beltran JL, Sánchez-Cárdenas C, Krapf D, Hernandez-González EO, Wertheimer E, Treviño CL, Visconti PE, Darszon A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J Biol Chem 2012; 287: 44384–44393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Litscher ES, Williams Z, Wassarman PM. Zona pellucida glycoprotein ZP3 and fertilization in mammals. Mol Reprod Dev 2009; 76: 933–941 [DOI] [PubMed] [Google Scholar]
- 96.Gahlay G, Gauthier L, Baibakov B, Epifano O, Dean J. Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science 2010; 329: 216–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA 2011; 108: 4892–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011; 471: 387–391 [DOI] [PubMed] [Google Scholar]
- 99.Thomas P, Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem J 1989; 264: 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blackmore PF, Lattanzio FA. Cell surface localization of a novel non-genomic progesterone receptor on the head of human sperm. Biochem Biophys Res Commun 1991; 181: 331–336 [DOI] [PubMed] [Google Scholar]
- 101.Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science 1994; 266: 1578–1581 [DOI] [PubMed] [Google Scholar]
- 102.Melendrez CS, Meizel S, Berger T. Comparison of the ability of progesterone and heat solubilized porcine zona pellucida to initiate the porcine sperm acrosome reaction in vitro. Mol Reprod Dev 1994; 39: 433–438 [DOI] [PubMed] [Google Scholar]
- 103.Kawano N, Kang W, Yamashita M, Koga Y, Yamazaki T, Hata T, Miyado K, Baba T. Mice lacking two sperm serine proteases, ACR and PRSS21, are subfertile, but the mutant sperm are infertile in vitro. Biol Reprod 2010; 83: 359–369 [DOI] [PubMed] [Google Scholar]
- 104.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434: 234–238 [DOI] [PubMed] [Google Scholar]
- 105.Fujihara Y, Murakami M, Inoue N, Satouh Y, Kaseda K, Ikawa M, Okabe M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J Cell Sci 2010; 123: 1531–1536 [DOI] [PubMed] [Google Scholar]
- 106.Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci 2012; 125: 4985–4990 [DOI] [PubMed] [Google Scholar]
- 107.Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci USA 2011; 108: 20008–20011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turner RM. Tales from the tail: what do we really know about sperm motility? J Androl 2003; 24: 790–803 [DOI] [PubMed] [Google Scholar]
- 109.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA 2004; 101: 16501–16506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update 2006; 12: 269–274 [DOI] [PubMed] [Google Scholar]
- 111.Miki K, Eddy EM. Identification of tethering domains for protein kinase A type Iα regulatory subunits on sperm fibrous sheath protein FSC1. J Biol Chem 1998; 273: 34384–34390 [DOI] [PubMed] [Google Scholar]
- 112.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech 2003; 61: 103–115 [DOI] [PubMed] [Google Scholar]
- 113.Katz DF, Yanagimachi R, Dresdner RD. Movement characteristics and power output of guinea-pig and hamster spermatozoa in relation to activation. J Reprod Fertil 1978; 52: 167–172 [DOI] [PubMed] [Google Scholar]
- 114.Suarez SS, Ho HC. Hyperactivation of mammalian sperm. Cell Mol Biol (Noisy-le-grand) 2003; 49: 351–356 [PubMed] [Google Scholar]
- 115.Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 2008; 52: 455–462 [DOI] [PubMed] [Google Scholar]
- 116.Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol 2002; 250: 208–217 [DOI] [PubMed] [Google Scholar]
- 117.Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol Reprod 2001; 65: 1606–1615 [DOI] [PubMed] [Google Scholar]
- 118.Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod 2003; 68: 1590–1596 [DOI] [PubMed] [Google Scholar]
- 119.Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev 2011; 91: 1305–1355 [DOI] [PubMed] [Google Scholar]
- 120.Costello S, Michelangeli F, Nash K, Lefievre L, Morris J, Machado-Oliveira G, Barratt C, Kirkman-Brown J, Publicover S. Ca2+-stores in sperm: their identities and functions. Reproduction 2009; 138: 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darszon A, Sánchez-Cárdenas C, Orta G, Sánchez-Tusie AA, Beltrán C, López-González I, Granados-González G, Treviño CL. Are TRP channels involved in sperm development and function? Cell Tissue Res 2012; 349: 749–764 [DOI] [PubMed] [Google Scholar]
- 122.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature 2001; 413: 603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA 2001; 98: 12527–12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci USA 2003; 100: 14864–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA 2007; 104: 1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chang H, Suarez SS. Two distinct Ca2+ signaling pathways modulate sperm flagellar beating patterns in mice. Biol Reprod 2011; 85: 296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marquez B, Suarez SS. Different signaling pathways in bovine sperm regulate capacitation and hyperactivation. Biol Reprod 2004; 70: 1626–1633 [DOI] [PubMed] [Google Scholar]
- 128.McPartlin LA, Suarez SS, Czaya CA, Hinrichs K, Bedford-Guaus SJ. Hyperactivation of stallion sperm is required for successful in vitro fertilization of equine oocytes. Biol Reprod 2009; 81: 199–206 [DOI] [PubMed] [Google Scholar]
- 129.Chang H, Suarez SS. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol Reprod 2012; 86: 140,1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Risbridger GP, Taylor RA. Physiology of the male accessory sex structures: the prostate gland, seminal vesicles, and bulbourethral glands. In: Neill JD, Plant TM, Pfaff DW, Challis JRG, de Kretser DM, Richards JS, Wassarman PM (eds.), Knobil and Neill’s Physiology of Reproduction. 3rd ed. Waltham: Academic Press; 2006: 1149–1172.
- 131.Goto N, Harayama H. Calyculin A-sensitive protein phosphatases are involved in maintenance of progressive movement in mouse spermatozoa in vitro by suppression of autophosphorylation of protein kinase A. J Reprod Dev 2009; 55: 327–334 [DOI] [PubMed] [Google Scholar]
- 132.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci USA 2009; 106: 667–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L, Ramalho-Santos J, Kirkman-Brown J, Michelangeli F, Publicover S, Barratt CL. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human sperm. J Biol Chem 2013; 288: 6248–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 1998; 59: 1–6 [DOI] [PubMed] [Google Scholar]
- 135.Harayama H, Noda T, Ishikawa S, Shidara O. Relationship between cyclic AMP-dependent protein tyrosine phosphorylation and extracellular calcium during hyperactivation of boar spermatozoa. Mol Reprod Dev 2012; 79: 727–739 [DOI] [PubMed] [Google Scholar]
- 136.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol 2003; 5: 1117–1122 [DOI] [PubMed] [Google Scholar]
- 137.Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 2010; 584: 1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci USA 2011; 108: 5879–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hernández-González EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, López-González I, Demarco I, Wertheimer E, Darszon A, Visconti PE. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem 2006; 281: 5623–5633 [DOI] [PubMed] [Google Scholar]
- 140.Hernández-González EO, Treviño CL, Castellano LE, de la Vega-Beltrán JL, Ocampo AY, Wertheimer E, Visconti PE, Darszon A. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem 2007; 282: 24397–24406 [DOI] [PubMed] [Google Scholar]
- 141.Figueiras-Fierro D, Acevedo JJ, Martínez-López P, Escoffier J, Sepúlveda FV, Balderas E, Orta G, Visconti PE, Darszon A. Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm. J Cell Physiol 2013; 228: 590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL. Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev 2012; 79: 4–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tardif S, Dubé C, Chevalier S, Bailey JL. Capacitation is associated with tyrosine phosphorylation and tyrosine kinase-like activity of pig sperm proteins. Biol Reprod 2001; 65: 784–792 [DOI] [PubMed] [Google Scholar]
- 144.Kaneto M, Harayama H, Miyake M, Kato S. Capacitation-like alterations in cooled boar spermatozoa: assessment by the chlortetracycline staining assay and immunodetection of tyrosine-phosphorylated sperm proteins. Anim Reprod Sci 2002; 73: 197–209 [DOI] [PubMed] [Google Scholar]
- 145.Bravo MM, Aparicio IM, Garcia-Herreros M, Gil MC, Peña FJ, Garcia-Marin LJ. Changes in tyrosine phosphorylation associated with true capacitation and capacitation-like state in boar spermatozoa. Mol Reprod Dev 2005; 71: 88–96 [DOI] [PubMed] [Google Scholar]
- 146.Hardie DG. AMP-activated protein kinase: a master switch in glucose and lipid metabolism. Rev Endocr Metab Disord 2004; 5: 119–125 [DOI] [PubMed] [Google Scholar]
- 147.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1: 15–25 [DOI] [PubMed] [Google Scholar]
- 148.Hurtado de Llera A, Martin-Hidalgo D, Gil MC, Garcia-Marin LJ, Bragado MJ. AMP-activated kinase AMPK is expressed in boar spermatozoa and regulates motility. PLoS One 2012; 7: e38840 [DOI] [PMC free article] [PubMed] [Google Scholar]