Abstract
Accumulating evidence suggests that the arcuate nucleus (ARC) kisspeptin/neurokinin B (NKB)/dynorphin (KNDy) neurons play a role in estrogen negative feedback action on pulsatile gonadotropin-releasing hormone (GnRH)/luteinizing hormone (LH) release. The present study aimed to determine if dynorphin (Dyn) is involved in estrogen negative feedback on pulsatile GnRH/LH release. The effect of the injection of nor-binaltorphimine (nor-BNI), a kappa-opioid receptor (KOR) antagonist, into the third cerebroventricle (3V) on LH pulses was determined in ovariectomized (OVX) adult female rats with/without replacement of negative feedback levels of estradiol (low E2). The mean LH concentrations and baseline levels of LH secretion in nor-BNI-injected, low E2-treated rats were significantly higher compared with vehicle-treated controls. On the other hand, the nor-BNI treatment failed to affect any LH pulse parameters in OVX rats without low E2 treatment. These results suggest that Dyn is involved in the estrogen negative feedback regulation of pulsatile GnRH/LH release. The low E2 treatment had no significant effect on the numbers of ARC Pdyn (Dyn gene)-,Kiss1- and Tac2 (NKB gene)-expressing cells. The treatment also did not affect mRNA levels of Pdyn and Oprk1 (KOR gene) in the ARC-median eminence region, but significantly increased the ARC kisspeptin immunoreactivity. These findings suggest that the negative feedback level of estrogen suppresses kisspeptin release from the ARC KNDy neurons through an unknown mechanism without affecting the Dyn and KOR expressions in the ARC. Taken together, the present result suggests that Dyn-KOR signaling is a part of estrogen negative feedback action on GnRH/LH pulses by reducing the kisspeptin release in female rats.
Keywords: Arcuate nucleus, GnRH, Kisspeptin, Neurokinin B
Animal reproduction is regulated by the hypothalamo-pituitary-gonadal axis. Two modes of gonadotropin-releasing hormone (GnRH) release, pulse and surge, are considered to regulate follicular maturation/steroidogenesis and ovulation, respectively, in female mammals [1,2,3,4]. The pulsatile GnRH/luteinizing hormone (LH) release is negatively regulated by circulating estrogen from the ovary [5] to fine-tune gonadotropin secretion for follicular maturation [6, 7]. The mechanism generating GnRH/LH pulses, called the GnRH pulse generator, has been suggested to be localized in the mediobasal hypothalamus (MBH) in rats and monkeys [8,9,10].
It has been well accepted that kisspeptin-GPR54 signaling plays a key role in controlling reproduction via stimulating GnRH release [2, 3, 11]. More specifically, a population of kisspeptin neurons located in the arcuate nucleus (ARC) has been considered to play an important role in GnRH/LH pulse generation in many mammalian species, including rats [12,13,14], mice [15], goats [16, 17], sheep [18, 19] and monkeys [20]. The ARC kisspeptin neurons are recently referred to as KNDy neurons [19] because they co-express neurokinin B (NKB) and dynorphin (Dyn) [15, 17, 21]. Wakabayashi et al. [17] showed that central infusion of a Dyn antagonist or NKB increases the GnRH/LH pulse generator activity in ovariectomized (OVX) goats. Their results suggest that Dyn and NKB play an inhibitory and stimulatory role, respectively, in controlling GnRH pulses. It is speculated that kisspeptin is released in a pulsatile manner from the ARC KNDy neurons to stimulate GnRH pulses by acting on GnRH neuronal terminals at the median eminence (ME). This notion could be adapted in many mammalian species, such as mice [15], rats [13, 22] and sheep [18, 19].
Dynorphin exerts its effects mainly through the kappa-opioid receptor (KOR), a G-protein-coupled receptor. Dynorphin-KOR signaling is considered to be involved in the suppression of GnRH/LH pulses, because central administration of nor-binaltorphimine (nor-BNI), a selective KOR antagonist, increases the amplitude and frequency of LH pulses during early pregnancy [23] and the mean LH level and LH pulse frequency during midpregnancy [24] in rats. Further, Goodman et al. [25] suggested that the central Dyn-KOR system mediates progesterone negative feedback on GnRH/LH pulses in ewes. LH pulses are strongly suppressed in prepubertal rats, in which both Kiss1 (kisspeptin gene) expression and kisspeptin immunoreactivity in the ARC are suppressed in the presence of a negative feedback level of estrogen [26]. Since estrogen receptor (ER) α is located in ARC KNDy neurons [27,28,29], it is possible that Dyn-KOR signaling mediates the estrogen negative feedback effect on GnRH/LH pulses in adult female rats.
The present study, therefore, aimed to determine if Dyn-KOR signaling mediates estrogen negative feedback on GnRH/LH release. We first examined the effects of central administration of a KOR antagonist on pulsatile LH release in OVX rats in the presence or absence of a negative feedback level of estradiol (E2) [30]. We also determined the effects of this negative feedback level of E2 on the gene expressions of Pdyn (Dyn gene), Oprk1 (KOR gene), Kiss1, and Tac2 (NKB gene) in the ARC-ME region to investigate if estrogen exerts its negative regulation of GnRH/LH pulses through the changes in these gene expressions. In addition, we examined kisspeptin immunoreactivity in the ARC in the presence or absence of a negative feedback level of E2 to investigate if the current E2 treatment affects the kisspeptin expression in this nucleus.
Materials and Methods
Animals
Adult female Wistar-Imamichi rats at 10–12 weeks of age (230–280 g BW) were used. They were maintained under a controlled environment (14 h light and 10 h darkness, lights on at 0500 h; 23 ± 3 C) with free access to food (CE2, Clea, Tokyo, Japan) and water. Vaginal smears were checked daily to determine estrous cyclicity, and females having at least two consecutive estrous cycles were used. Rats were bilaterally ovariectomized 2 weeks before the blood or brain sampling to serve as the OVX group. Some OVX rats immediately received subcutaneous Silastic implants (i.d., 1.57 mm; o.d., 3.18 mm; 25 mm in length; Dow Corning, Midland, MI, USA) filled with E2 (20 µg/ml peanut oil) for 1 week to serve as the OVX + low E2 group. The low E2 treatment was previously confirmed to produce a plasma E2 level of 35.8 pg/ml and to produce a negative feedback effect on LH pulses but not to induce LH surges [30]. All surgeries were performed under ketamine/xylazine anesthesia and aseptic conditions. All rats were injected with antibiotics (Mycillin Sl; Meiji Seika, Tokyo, Japan) after any surgery. All experiments were conducted in accordance with the guidelines of the Committee of Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University, Japan.
Brain surgery
Some OVX and OVX + low E2 rats were stereotaxically implanted with a stainless-steel guide cannula (22 G, Plastics One, Roanoke, VA, USA) for drug administration into the third cerebroventricle (3V) with its tip 0.8 mm posterior and 7.5 mm ventral to the bregma at the midline according to the rat brain atlas [31]. The rats were allowed a one-week recovery period prior to blood sampling.
Drug administration and blood sampling
To examine the effect of blockade of central KOR on pulsatile LH release, nor-BNI (Sigma-Aldrich, St. Louis, MO, USA), a selective KOR antagonist [23, 32, 33], was infused into the 3V at a dose of 20 µg/head. The dose of nor-BNI was chosen according to a previous study, in which central nor-BNI treatment increased LH pulse frequency and mean LH levels during midpregnancy in rats [24]. Nor-BNI (10 μg/µl) was dissolved in ultrapure water (UPW) and administered into the 3 V of OVX rats with/without low E2 treatment at a flow rate of 1 µl/min for 2 min using a microsyringe pump (Eicom, Kyoto, Japan) through an inner cannula (28 G, Plastics One), which was inserted into the guide cannula. The drug was administered just after the first blood sampling at 1300 h. Control rats were infused with an equivalent volume of UPW in the same manner. Blood samples (100 µl) were collected every 6 min for 3 h from free-moving conscious rats via a silicone cannula (i.d., 0.5 mm; o.d., 1.0 mm; Shin-Etsu Polymer, Tokyo, Japan) that was inserted into the right atrium through the jugular vein on the day before blood sampling. An equivalent volume of rat red blood cells taken from donor rats and prepared in heparinized saline was replaced through the same atrial cannula after each blood collection. Plasma was separated by centrifugation and stored at –20 C until LH assay. At the end of the experiment, the animals were anesthetized and infused with 3% brilliant blue dye solution at the same flow rate as drug administration to check if the drug was infused into the 3 V. The brain was removed, and cannula placement and blue dye staining in the brain were visually verified. All animals used in the present study were confirmed to have correct 3V cannula placement and drug administration.
Radioimmunoassay (RIA) for LH
Plasma LH concentrations were measured using a rat LH RIA kit provided by the National Hormone and Peptide Program. The concentrations were expressed in terms of NIDDK rat LH RP-3. The least detectable LH concentration was 0.16 ng/ml for 50 µl of plasma, and the intra- and interassay co-efficient of variations were 8.81% at 2.60 ng/ml and 7.68% at 2.76 ng/ml, respectively.
In situ hybridization (ISH) for Pdyn, Kiss1 and Tac2
To detect Pdyn, Kiss1 and Tac2 mRNA expressions in the brain, we performed non-radioactive free-floating single ISH with rat Pdyn-, Kiss1- or Tac2-specific digoxigenin (DIG)-labeled probes in coronal sections (50-µm thickness) of the hypothalamus taken from OVX rats with/without low E2 treatment as previously described [27]. DIG-labeled antisense cRNA probes for rat Pdyn (position 315-731; GenBank accession no. NM_019374), Kiss1 (postion 33-348; GenBank accession no. AY196983) and Tac2 (position 180-483; GenBank accession no. NM_019162) were synthesized by in vitro transcription from the cDNA clones. Overnight hybridization with DIG-labeled cRNA probes was executed at 60 C. Hybridized probes were detected using an alkaline phosphatase-conjugated anti-DIG Fab fragment (Roche Diagnostics, Mannheim, Germany) and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium chloride (Roche Diagnostics). Numbers of Pdyn-, Kiss1- and Tac2-expressing cells were counted under a light microscope, and the sum of the cell number in the ARC (11 sections) was obtained. The ARC area was identified according to the rat brain atlas [31].
Semiquantitative analysis of mRNA expressions of Pdyn and Oprk1 in the ARC-ME region by RT-PCR
The ARC-ME tissue of OVX rats with/without low E2 was dissected out from the brain according to rat brain atlas as previously described [31, 34]. Total RNA was extracted from the ARC-ME tissue using ISOGEN reagents (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. A total of 500 ng of RNA was used for reverse transcription using MultiScribe Reverse Transcriptase enzyme (Applied Biosystems, Foster City, CA, USA) to synthesize the cDNA. All primer sequences are described in Table 1. Pdyn and Oprk1 were analyzed by RT-PCR. β-Actin (Actb) was used as the internal control. The RT-PCR for the mRNAs for Pdyn, Oprk1 and Actb was performed using AmpliTaq Gold DNA Polymerase (Applied Biosystems) under the following conditions: 95 C for 5 min and 35 cycles of 94 C for 30 sec, 62 C for 1 min and 72 C for 1 min for Pdyn; 95 C for 5 min and 35 cycles of 94 C for 30 sec, 64 C for 1 min and 72 C for 1 min for Oprk1; and 95 C for 5 min and 30 cycles of 94 C for 30 sec, 60 C for 1 min and 72 C for 1 min for Actb. The final cycle was followed by a 10 min extension step at 72 C before reducing the temperature to 4 C for storage. The mRNA levels of Pdyn and Oprk1 were semiquantified using the ImageJ software (version 1.45s; http://rsb.info.nih.gov/ij/). The intensity was then expressed as a value relative to that of the Actb amplicon.
Table 1. Primer set sequences for RT-PCR used in this study.
| Gene | Forward primer (5´ to 3´) | Reverse primer (5´ to 3´) | Product size (bp) | GeneBank accession ID |
| Pdyn | GTTCCCTGTGTGCAGTGAGGAC | TAGCGTTTGGCCTGTTTTCTCA | 418 | NM_019374.3 |
| Oprk1 | GATGTCATTGAATGCTCCTTGC | CAGGATCATCAGGGTGTAGCAG | 138 | NM_017167.2 |
| Actb | TGTCACCAACTGGGACGATA | GGGGTGTTGAAGGTCTTCAAA | 165 | NM_031144.3 |
Immunohistochemistry
Ovariectomized or low E2-treated OVX rats were perfused with 4% paraformaldehyde at 1400 h, and their brains were quickly removed. Frozen coronal sections (50 µm) were obtained using a cryostat. Every sixth section through the ARC (6 sections, from 2.3 to 4.1 mm posterior to the bregma) from each rat was stained with mouse monoclonal anti-rat kisspeptin antibody (no. 254 outstretched in Takeda Pharmaceutical, Osaka, Japan), the cross-reactivity of which was described previously [26]. Brain tissue sections from each rat were incubated with the anti-rat kisspeptin antibody (1:50,000) for 24 h at room temperature, followed by incubation with Alexa Fluor 488-conjugated donkey antimouse IgG (1:800; Molecular Probes, Eugene, OR, USA). Then the sections were mounted with an antifade reagent (FluoroGuard; Bio-Rad, Hercules, CA, USA). Fluorescence images were obtained on an ApoTome microscope (ApoTome; Carl Zeiss, Jena, Germany). Six digital photomicrographs of each ARC per rat were processed by the ImageJ analysis software. The ARC area was outlined on the gray-scale image and processed for density measurement. Nonspecific background density points were eliminated using the same threshold for each rat.
Statistical analysis
LH pulses were identified using the PULSAR computer program [35] as previously described [36]. The statistical differences in LH pulse parameters were determined by the Student's t-test between nor-BNI-treated rats and vehicle-treated controls within OVX + low E2 and OVX groups. The statistical differences in the mRNA expressions of Pdyn and Oprk1 relative to Actb and kisspeptin immunoreactive areas between the OVX + low E2 and OVX groups were also determined by the Student's t-test.
Results
Effect of nor-BNI injection into 3V on pulsatile LH release in OVX rats with/without low E2
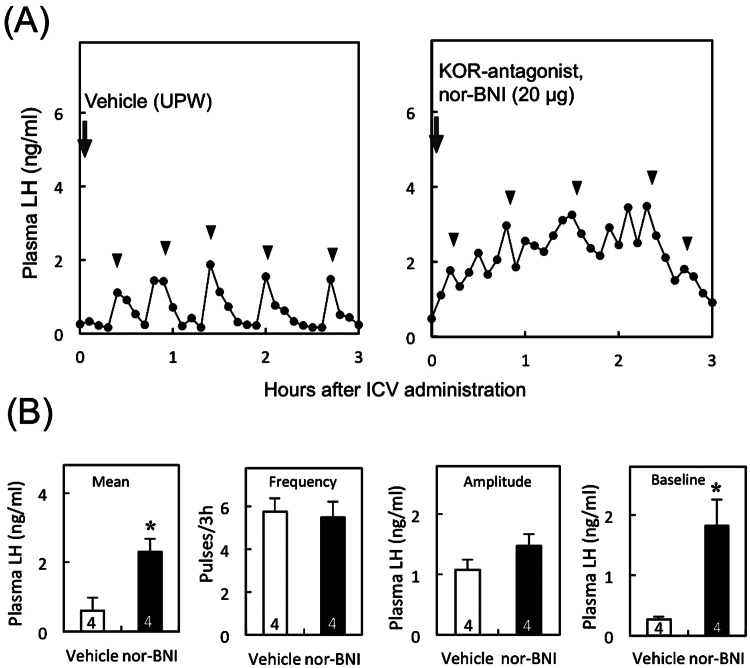
Figure 1A shows LH profiles of representative OVX rats with a negative feedback level of E2 that received a 3V injection of a KOR antagonist, nor-BNI, or vehicle. The mean LH level was significantly (P < 0.05, Student's t-test) higher in nor-BNI-injected animals compared with that in vehicle-treated controls. The mean baseline level of LH pulses was significantly (P < 0.05) higher in the animals treated with nor-BNI compared with that in controls (Fig. 1B), but the frequency and amplitude of LH pulses did not significantly differ between these two groups.
Fig. 1.
Effect of central administration of KOR antagonist on pulsatile LH release in OVX rats treated with a negative feedback level of E2 (low E2). Profiles of LH release in representative OVX + E2 rats treated with nor-BNI, a KOR antagonist, or vehicle (A). Nor-BNI or vehicle (UPW) was injected into the 3V immediately after the start of blood sampling (arrows). Blood samples were collected for 3 h at 6-min intervals. Arrowheads indicate the peaks of LH pulses identified by the PULSAR computer program. Mean plasma LH concentrations and frequency, amplitude and baseline levels of LH pulses in low E2-treated OVX rats (B). Values are means ± SEM. The numbers in each column represent the numbers of animals used in each group. * P < 0.05 vs. the vehicle-treated controls (Student's t-test).
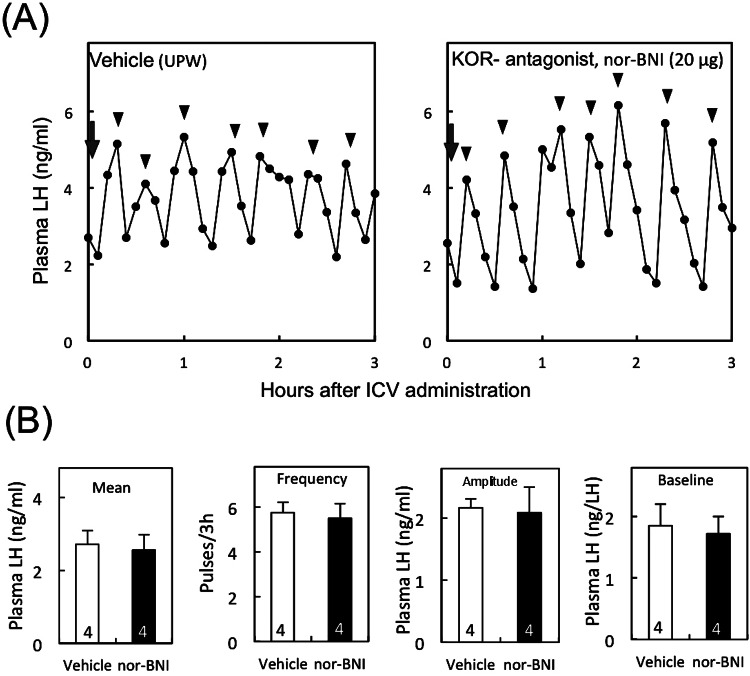
Figure 2A shows the representative profiles of LH release in OVX individuals treated with nor-BNI or vehicle. There were no significant differences in any LH pulse parameters between nor-BNI- and vehicle treated-OVX rats (Fig. 2B).
Fig. 2.
Effect of central administration of KOR antagonist on pulsatile LH release in OVX rats without E2 treatment. Profiles of LH release in representative OVX rats treated with nor-BNI or vehicle (arrows) (A). Arrowheads indicate the peaks of LH pulses. Mean plasma LH concentrations and frequency, amplitude and baseline levels of LH pulses in OVX rats (B). The numbers in each column represent the numbers of animals used in each group. See Fig. 1 for details.
Effects of low E2 treatment on numbers of Pdyn, Kiss1 and Tac2 mRNA-expressing cells in the ARC
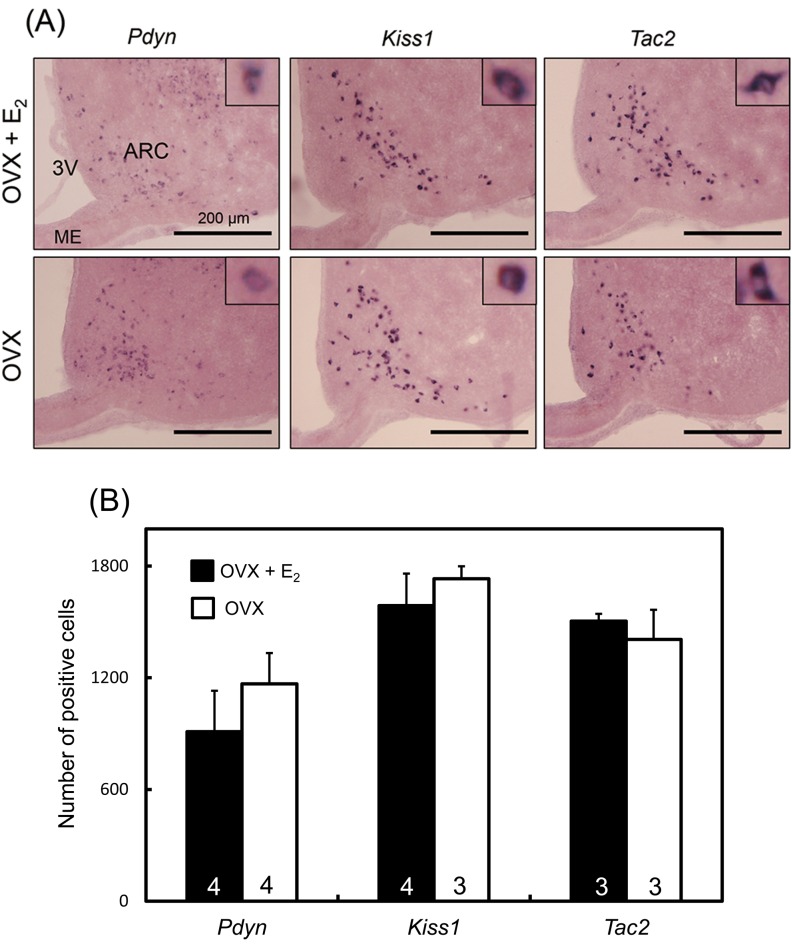
Figure 3A shows the Pdyn, Kiss1 and Tac2 mRNA expressions in the ARC of representative OVX rats with or without a negative feedback level of E2 treatment. We found that Pdyn, Kiss1 and Tac2 mRNA-expressing cells were abundantly located in the ARC in both OVX + low E2 and OVX rats. There were no significant differences in the number of Pdyn mRNA-expressing cells in the ARC between the OVX + low E2 and OVX groups (Fig. 3B). Similarly, there were no significant differences between OVX + low E2 and OVX rats in the number of Kiss1- and Tac2-expressing cells.
Fig. 3.
Pdyn, Kiss1 and Tac2 mRNA expressions in the ARC in OVX + low E2 and OVX rats determined by in situ hybridization. The upper and lower panels show the Pdyn, kiss1 and Tac2 mRNA expressions in representative rats in the OVX + low E2 and OVX groups, respectively (A). Insets show the sections at higher magnification. ARC, arcuate nucleus; ME, median eminence; 3V, third ventricle. The numbers of Pdyn, Kiss1 or Tac2 mRNA-positive cells in the ARC of OVX + low E2 and OVX groups (B). Values are means ± SEM. The numbers in each column represent the numbers of animals used in each group.
Effects of low E2 treatment on mRNA expressions of Pdyn and Oprk1 in the ARC-ME region
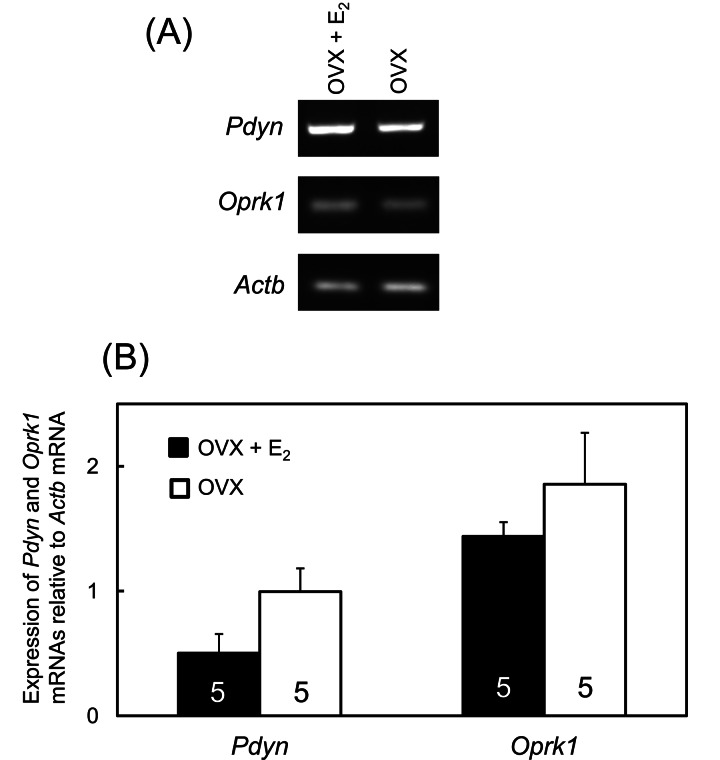
Representative images of mRNA expressions of Pdyn andOprk1 in the ARC-ME region of OVX + low E2 and OVX rats are shown in Fig. 4A. Semiquantitative measurements revealed no significant differences in Pdyn and Oprk1 mRNA expression levels between these two experimental groups (Fig. 4B).
Fig. 4.
Pdyn and Oprk1 mRNA expressions in the ARC-ME region of OVX + low E2 and OVX rats. Representative images of mRNA expressions of Pdyn, Oprk1 and Actb in the ARC-ME region of the OVX + low E2 and OVX groups (A). mRNA levels of Pdyn and Oprk1 were determined semiquantitatively by RT-PCR with the primers shown in Table 1 (B). The analysis was carried out using the ImageJ software of the National Institutes of Health. Values are indicated as the expression relative to Actb, as an internal control. Values are means ± SEM. The numbers in each column represent the numbers of animals used in each group.
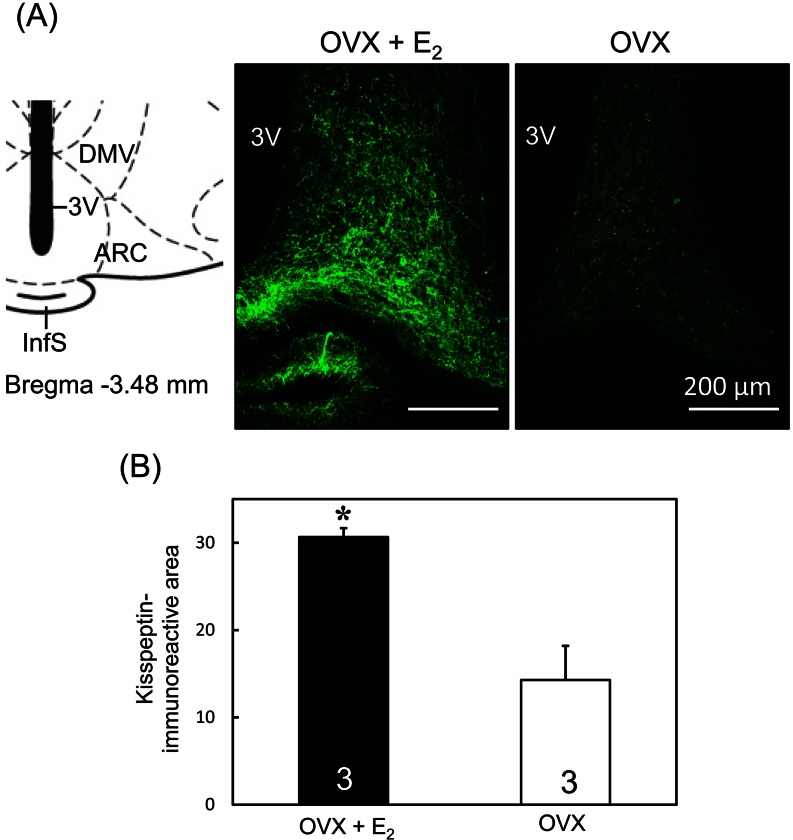
Effects of low E2 treatment on kisspeptin-immunoreactivity in the ARC
Kisspeptin-immunoreactive cell bodies and fibers were dense in the ARC of low E2 -treated OVX rats (Fig. 5A), whereas few kisspeptin immunoreactivities were observed in OVX rats (Fig. 5A). ImageJ analysis showed that the kisspeptin-immunoreactive area in the ARC was significantly (P < 0.05, Student's t-test) higher in the OVX + low E2 rats compared with the OVX rats (Fig. 5B).
Fig. 5.
Kisspeptin immunoreactivities in the ARC of OVX + low E2 and OVX rats. Schematic illustration and photomicrographs of coronal sections of rat brains including the ARC (A). Schematic drawing of the ARC according to Paxinos and Watson [31] (left). The value in the drawing indicates the distance (mm) to the bregma. Photomicrographs (right) show kisspeptin immunoreactivity (green) in representative animals in the ARC of OVX + low E2 and OVX groups. ARC, arcuate nucleus; DMV, ventral dorsomedial hypothalamic nucleus; InfS, infundibular stem; 3V, third ventricle. Scale bars: 200 µm. Semiquantitative analysis of kisspeptin immunoreactivity in the ARC of OVX + low E2 and OVX rats was determined using the ImageJ software (B). Values are means ± SEM. The numbers in each column represent the numbers of animals used in each group.
Discussion
The present study demonstrates that Dyn-KOR signaling is involved in the estrogen negative feedback action on GnRH/LH release in adult female rats, because central nor-BNI administration significantly increased LH release only in the presence of a negative feedback level of E2 in OVX rats. The Dyn-KOR signaling may be involved in the suppression of basal LH release but not in suppression of the GnRH/LH pulse generation system, because baseline levels of LH pulses were significantly increased but LH pulse frequency and amplitude were not affected by nor-BNI administration. Interestingly, the current low E2 treatment did not affect the number of Pdyn-expressing cells and gene expression levels of Pdyn and Oprk1 gene in the ARC. These results suggest that the negative feedback level of E2 alters Dyn-KOR tone in brain areas other than the ARC. The present E2 treatment increased kisspeptin immunoreactivity in the ARC KNDy neurons without affecting Kiss1 mRNA expressions, suggesting that the current E2 treatment inhibited the release of kisspeptin from the ARC kisspeptin neurons. ARC KNDy neurons are considered to play a role in GnRH pulse generation, and kisspeptin is a direct stimulator for GnRH release in several mammals [15, 17, 19, 22]. Thus, the present study suggests that Dyn-KOR signaling in other brain areas than the ARC KNDy neurons is partly involved in estrogen negative feedback actions on GnRH/LH pulses via inhibition of kisspeptin release from KNDy neurons.
Involvement of KOR in suppression of LH release is consistent with previous studies that demonstrated a stimulatory effect of KOR antagonists on GnRH/LH secretion [17, 23,24,25]. In E2-treated ewes, WIN 44,441-3, a KOR antagonist [37], increased LH pulse amplitude [38]. In addition, nor-BNI also reversed the inhibitory effect of progesterone on pulsatile LH secretion in the ewe by increasing the mean LH level and pulse frequency, suggesting that the Dyn-KOR signaling plays a major role in mediating progesterone negative feedback [25]. Central administration of nor-BNI increased LH pulse frequency and mean LH levels in pregnant rats, in which LH pulses are strongly suppressed [23, 24]. The LH pulse suppression during pregnancy could be more dependent on Dyn-KOR signaling than the suppression by estrogen negative feedback, because our results showed that nor-BNI increased the mean and baseline levels of LH pulses in low E2-treated rats without affecting LH pulse frequency and amplitude.
The present study showed that the nor-BNI treatment failed to alter LH pulses in OVX rats, suggesting that Dyn-KOR signaling is not activated in the absence of E2. On the other hand, central administration of nor-BNI increased the frequency of MUA volleys in OVX goats [17], suggesting that Dyn-KOR signaling plays a role in suppressing KNDy neuronal activity and then GnRH/LH pulses even in the absence of estrogen. The inconsistency between their results and ours may be due to the species difference in the role of Dyn-KOR in controlling GnRH release.
The present study demonstrated that the current E2 treatment did not affect the numbers of Pdyn-, Kiss1- and Tac2-expressing cells as well as Pdyn and Oprk1 expressions in the ARC-ME region in OVX rats. These results suggest that the ARC KNDy neurons may not be an estrogen action site that exerts a negative feedback effect on LH pulses via Dyn-KOR signaling. Dyn neurons are abundantly distributed in several regions in the rat brain, such as the hypothalamus, medulla-pons, midbrain and spinal cord [39]. In the rat hypothalamus, Dyn expressions are obvious in the paraventricular nucleus (PVN) [40, 41], MBH and preoptic area (POA) [42]. Therefore, it is possible that some brain areas other than the ARC could be estrogen action sites that mediate the negative feedback effect on LH pulses via Dyn-KOR signaling in rats. Further studies are required to address this issue in more detail. The current low E2 treatment increased ARC kisspeptin immunoreactivity without affecting Kiss1 gene expressions in the nucleus. In terms of Kiss1 gene expression, the present result is consistent with our previous reports, which showed that the negative feedback level of E2 had no suppressive effect on ARC Kiss1 gene expression. On the other hand, a number of studies showed that a positive feedback level of E2 suppresses both Kiss1 and kisspeptin expressions in the ARC of female rodents [15, 27,28,29]. The increased ARC kisspeptin immunoreactivity caused by the current low E2 treatment without an accompanying effect on Kiss1 expression implies that kisspeptin release from the ARC KNDy neurons was diminished by the low E2 treatment and that ARC kisspeptin was consequently increased.
Previous studies revealed that estrogen mainly exerts its feedback action to control GnRH/LH secretion through ERα, a receptor subtype in the brain [43,44,45]. This estrogen negative feedback action on LH secretion is considered to be mediated by nonclassical ERα action instead of classical ERα action [43, 45]. These studies suggest that estrogen response element (ERE)-independent ERα is sufficient to convey a major portion of estrogen negative feedback action on LH release. This nonclassical estrogen action involves interactions of ERα with DNA-bound transcription factors, such as specificity protein and activating protein 1 [46], whereas, in the classical genomic pathway, ERα binds to EREs in DNA to alter the transcription of genes [47]. Thus, it is conceivable that our negative feedback level of E2 acts through the nonclassical ERα pathway without affecting the gene expressions of Pdyn, Kiss1, Tac2 and Oprk1 in the ARC-ME and may suppress the kisspeptin release from KNDy neurons. It is also possible that a membrane receptor, such as GPR30 [48], is involved in the estrogen negative feedback regulation of LH secretion. This mechanism should be investigated in greater detail in future studies.
Interestingly, recent studies showed that central NKB receptors rather than KOR would be involved in the suppression of GnRH/LH pulses in rats [49, 50]. These studies showed that central administration of NK3 receptor agonist increases LH pulse intervals, while both Dyn agonist and antagonist had no effect on LH pulses in diestrous rats and OVX rats treated with a negative feedback level of E2. More recently, O'Byrne and colleagues showed that KOR in the ARC mediates LH pulse suppression induced by the activation of NKB receptors within the ARC in rats [50]. These findings suggest that roles of Dyn and NKB signaling in controlling GnRH/LH pulses are still controversial.
In conclusion, the present study suggests that Dyn-KOR signaling at least partly mediates estrogen negative feedback on GnRH/LH release by reducing kisspeptin release from the ARC KNDy neurons in female rats. The estrogen action sites that enhance the Dyn-KOR signaling would be in other brain regions than the ARC KNDy neurons. Further studies are warranted to address the precise mechanism involved in the estrogen negative feedback on GnRH/LH release via alteration of Dyn-KOR signaling.
Acknowledgment
We are grateful to the National Hormone and Peptide Program for the rat LH RIA kit. The RIA and LH pulse analyses were performed at the Nagoya University Radioisotope Center and Information Technology Center, respectively. This study was supported in part by a Grant-in-Aid from the Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development (to HT) and Grants-in-Aid for Scientific Research (No. 23380163 to HT, No. 23580402 to YU and No. 24380157 to KM) from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Rahe CH, Owens RE, Fleeger JL, Newton HJ, Harms PG. Pattern of plasma luteinizing hormone in the cyclic cow: dependence upon the period of the cycle. Endocrinology 1980; 107: 498–503. [DOI] [PubMed] [Google Scholar]
- 2.Ohkura S, Uenoyama Y, Yamada S, Homma T, Takase K, Inoue N, Maeda K, Tsukamura H. Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides 2009; 30: 49–56. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord 2007; 8: 21–29. [DOI] [PubMed] [Google Scholar]
- 4.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 2010; 1364: 103–115. [DOI] [PubMed] [Google Scholar]
- 5.Karsch FJ. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol 1987; 49: 365–382. [DOI] [PubMed] [Google Scholar]
- 6.Akema T, Tadokoro Y, Kimura F. Regional specificity in the effect of estrogen implantation within the forebrain on the frequency of pulsatile luteinizing hormone secretion in the ovariectomized rat. Neuroendocrinology 1984; 39: 517–523. [DOI] [PubMed] [Google Scholar]
- 7.Evans NP, Dahl GE, Glover BH, Karsch FJ. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 1994; 134: 1806–1811. [DOI] [PubMed] [Google Scholar]
- 8.Hálasz B, Pupp L. Hormone secretion of the anterior pituitary gland after physical interruption of all nervous pathways to the hypophysiotrophic area. Endocrinology 1965; 77: 553–562. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara M, Hiruma H, Kimura F. Interactions between the noradrenergic and opioid peptidergic systems in controlling the electrical activity of luteinizing hormone-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology 1991; 54: 321–326. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology 1984; 39: 256–260. [DOI] [PubMed] [Google Scholar]
- 11.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev 2009; 30: 713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology 2008; 149: 1004–1008. [DOI] [PubMed] [Google Scholar]
- 13.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 2012; 153: 2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 2011; 300: E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman RL, Holaskova I, Nestor CC, Connors JM, Billings HJ, Valent M, Lehman MN, Hileman SM. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology 2011; 152: 3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010; 151: 3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010; 151: 4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007; 148: 5752–5760. [DOI] [PubMed] [Google Scholar]
- 22.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 2010; 1364: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo RV. Kappa-opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J Neuroendocrinol 1990; 2: 685–691. [DOI] [PubMed] [Google Scholar]
- 24.Zhen S, Gallo RV. The effect of blockade of kappa-opioid receptors in the medial basal hypothalamus and medial preoptic area on luteinizing hormone release during midpregnancy in the rat. Endocrinology 1992; 131: 1650–1656. [DOI] [PubMed] [Google Scholar]
- 25.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 2004; 145: 2959–2967. [DOI] [PubMed] [Google Scholar]
- 26.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 2009; 21: 527–537. [DOI] [PubMed] [Google Scholar]
- 27.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436. [DOI] [PubMed] [Google Scholar]
- 29.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686–3692. [DOI] [PubMed] [Google Scholar]
- 30.Cagampang FR, Maeda KI, Tsukamura H, Ohkura S, Ota K. Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J Endocrinol 1991; 129: 321–328. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Steriotaxic Coordinates, Sixth edition. San Diego: Academic Press: 2007. [Google Scholar]
- 32.Portoghese AS, Lipkowski AW, Takemori AE. Bimorphinans as highly selective, potent kappa opioid receptor antagonists. J Med Chem 1987; 30: 238–239. [DOI] [PubMed] [Google Scholar]
- 33.Tortella FC, Echevarria E, Lipkowski AW, Takemori AE, Portoghese PS, Holaday JW. Selective kappa antagonist properties of nor-binaltorphimine in the rat MES seizure model. Life Sci 1989; 44: 661–665. [DOI] [PubMed] [Google Scholar]
- 34.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 2009; 81: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 35.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]
- 36.Maeda KI, Tsukamura H, Uchida E, Ohkura N, Ohkura S, Yokoyama A. Changes in the pulsatile secretion of LH after the removal of and subsequent resuckling by pups in ovariectomized lactating rats. J Endocrinol 1989; 121: 277–283. [DOI] [PubMed] [Google Scholar]
- 37.Wood PL, Pilapil C, Thakur M, Richard JW. WIN 44,441: A stereo-specific and long-acting narotic antagonist. Pharm Res 1984; 1: 46–48. [DOI] [PubMed] [Google Scholar]
- 38.Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod 1988; 39: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein A, Ghazarossian VE. Immunoreactive dynorphin in pituitary and brain. Proc Natl Acad Sci USA 1980; 77: 6207–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol 2001; 433: 222–238. [DOI] [PubMed] [Google Scholar]
- 41.Lightman SL, Young WS., 3rdVasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol 1987; 394: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol 1986; 249: 293–336. [DOI] [PubMed] [Google Scholar]
- 43.Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 2007; 104: 8173–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 1998; 19: 302–330. [DOI] [PubMed] [Google Scholar]
- 45.McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol 2008; 290: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol 2008; 41: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod 1992; 46: 163–167. [DOI] [PubMed] [Google Scholar]
- 48.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 2009; 23: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, Millar RP, Lightman SL, O'Byrne KT. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One 2012; 7: e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O'Byrne KT. Suppression of the GnRH pulse generator by neurokinin B involves a kappa-opioid receptor-dependent mechanism. Endocrinology 2012; 153: 4894–4904. [DOI] [PubMed] [Google Scholar]