Abstract
Prostaglandin F2α (PGF2α) induces luteolysis in cows and causes infiltration of immune cells, which resembles inflammatory immune response. Since the general immune response is mediated by the lymphatic system, we hypothesized that luteolysis is associated with generation of an immune response that involves lymphatic vessels in the bovine corpus luteum (CL). The CL was obtained from Holstein cows at the mid-luteal phase (days 10–12, ovulation = day 0) by ovariectomy at various time points after PGF2α injection. Lymphatic endothelial cell (LyEC) marker, endothelial hyaluronan receptor 1 (LYVE1), levels decreased significantly 12 h after PGF2α injection. Podoplanin, another LyEC marker, decreased from 15 min after PGF2α injection. PGF2α also diminished mRNA expression of lymphangiogenic factors, such as vascular endothelial growth factor (VEGF) C, VEGFD and VEGF receptor 3 (VEGFR3). During PGF2α-induced luteolysis, the levels of mRNA expression of tumor necrosis factor α (TNFα; the major pro-inflammatory cytokine) and chemokine (C-X-C motif) ligand 1 (neutrophil chemokine) were increased. On the other hand, chemokine (C-C motif) ligand 21, which regulates outflow of immune cells from tissues via the lymphatic vessels during an immune response, was decreased. This study demonstrated that the lymphatic network in the CL is disrupted during luteolysis and suggests that during luteolysis, immune cells can induce a local immune response in the CL without using the lymphatic vessels.
Keywords: Corpus luteum, Cow, Luteolysis, Lymphatic vessel, Tumor necrosis factor α
The corpus luteum (CL) is a complex ovarian organ consisting of vascular endothelial, steroidogenic and immune cells. The luteolytic cascade of the bovine CL is primed by the pulsatile release of uterine prostaglandin F2α (PGF2α). The decrease in progesterone concentrations is closely followed by a structural degeneration of vasculature and apoptosis of steroidogenic cells [1, 2]. In the CL, the expression of vascular endothelial growth factor (VEGF) A, the most common angiogenic factor, was shown to be downregulated by PGF2α injection [3, 4], suggesting that PGF2α inhibits the angiogenic process in the regressing CL. Additionally, endothelin-1 (EDN1) and angiotensin (Ang) II, strong vasoconstrictive factors, were shown to be associated with the process of luteal regression in ruminants [5,6,7,8]. Furthermore, PGF2α upregulated the expression of EDN1 and Ang II in vivo and in vitro [5,6,7,8], resulting in intensive vasoconstriction and disruption of oxygen and nutrient supply during luteolysis.
The immune response also plays an essential role in luteolysis [9,10,11]. Leukocytes such as T lymphocytes, macrophages and neutrophils infiltrate the CL and produce different cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, interferon (IFN)-γ and monocyte chemoattractant protein 1 (MCP1) during CL regression [10, 12,13,14,15,16,17,18,19,20,21, 22]. TNFα is secreted by activated macrophages and has been implicated in neutrophil and monocyte recruitment to inflammatory sites [23,24,25,26,27]. Spontaneous or PGF2α-induced luteolysis are associated with a significant rise in intraluteal TNFα as shown in previous studies by using a CL microdialysis system [28]. Moreover, TNFα induces apoptotic death of steroidogenic and endothelial cells in vitro [11, 29, 30]. In short, the luteolytic phenomenon is an inflammatory-like immune response characterized by a massive recruitment of leukocytes and high production of cytokines.
The cardiovascular circulatory system consists of a tree-like hierarchy of vessels formed from a primitive vascular network. The lymphatic system is a distinct type of vascular system present in most organs of the body. Lymphatic endothelial cells (LyECs) form the vessels that drain the interstitial fluid from the tissues back into venous circulation after passage through the lymph node network [30, 31]. The lymphatic system also plays a crucial role in an immune response to infectious agents. LyECs act as a gatekeeper that controls immune cells, such as dendritic cells and macrophages, during migration from tissues to lymph nodes. Lymphatic drainage is essential for the recirculation of lymphocytes, allowing access of professional antigen-presenting cells to lymph nodes [32, 33]. During inflammation and infection, the number of dendritic cells reaching the lymph nodes drastically increases [34,35,36]. We recently reported the expression of lymphatic vessel markers, such as lymphatic endothelial hyaluronan receptor 1 (LYVE1) and podoplanin, in the bovine CL [37], suggesting that luteolysis involves the immune response by increasing the number of immune cells that traverse the lymphatic vessels into the CL.
This study aimed to evaluate the lymphatic system, including lymphatic vessel-related factors and cytokines in the CL, at different time points following PGF2α-induced luteolysis.
Materials and Methods
CL collection was conducted at the Clinic for Cattle of the University of Veterinary Medicine Hannover, Germany. The experimental procedures complied with the guidelines of the Ethics Committee on Animal Rights Protection of Oldenburg, Germany, in accordance with the German legislation on animal rights and welfare. The protocol was approved by the committee on the Ethics of Animal Experiments of the University of Veterinary Medicine Hannover (permit number: 33.9-42502-04-07/1275).
PGF2α-induced luteolysis
For collecting CLs during luteolysis, 29 normal cyclic German Holstein cows were used in this study. The day of estrus was designated Day 0. Cows (n = 4–5 for each time point) at the mid-luteal phase (days, 10–12) were injected with PGF2α via the intramuscular route (0 min; 0.5 mg of cloprostenol, 2.0 ml EstrumateTM, Essex Tierarznei, Munich, Germany), and ovaries were collected by ovariectomy [3] through the vagina before PGF2α injection (0 min), and at 15 min, 30 min, 2 h and 12 h after injection.
Processing of the corpus luteum
The CL was enucleated from the ovary and dissected, free of connective tissues, as described previously [38]. The CL tissue samples were then minced, immediately placed into a 1.5-ml microcentrifuge tube with or without 0.4 ml of TRIzol reagent (Invitrogen, Karlsruhe, Germany) and stored at –80 C until analysis.
RNA extraction, cDNA synthesis and reverse-transcription quantitative PCR
Total RNA was extracted from the CL following the protocol of Chomczynski and Sacchi [39] using TRIzol reagent, treated with DNase using a commercial kit (Promega, Madison, WI, USA) and frozen at –20 C in THE RNA Storage Solution (Ambion, Austin, TX, USA). The cDNA was synthesized as previously described [40]. The levels of mRNA expression of LYVE1, podoplanin, VEGFR3, VEGFC, VEGFD, TNFα, CXCL1, CCL21 and β-actin were quantified by reverse-transcription quantitative polymerase chain reaction (RT-qPCR) as previously described [40]. RT-qPCR reactions were performed in duplicate in a final volume of 10 μl containing 5 μl of QuantiTectTM SYBR Green PCR Buffer (QIAGEN GmbH, Hilden, Germany), 2.8 μl of H2O (Sigma, St. Louis, MO, USA), 0.1 μl of 50 μM forward and reverse primers (Table 1 lists primer sequences and accession numbers) and 2 μl of cDNA template or water (as a non-template negative control). RT-qPCR conditions were 10 min at 95 C, followed by 40 cycles of 95 C for 15 sec, 56 C for 30 sec and 72 C for 30 sec using a LightCycler (Roche Diagnostics, Mannheim, Germany). The PCR products were resolved by electrophoresis, and the target bands were cut out and purified using a DNA purification kit (SUPRECTM-01, Takara Bio, Otsu, Japan). The mRNA expression levels in the CL were normalized using β-actin as an internal standard. Each PCR amplification product was sequenced using an Applied Biosystems 3730 × l DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
Table 1. Primer sequences for the investigated genes.
| Gene | Primer sequence | Accession No. | Product size (bp) | |
| LYVE1 | FWD | AGG TTG AAG AAG CAC GGA AA | NM_205815 | 231 |
| REV | AGG GAT CAT CGG TGG TGA TA | |||
| Podoplanin | FWD | TGG CTA CGG AGC TTT TTC AT | ENSBTAT | 291 |
| REV | CAC ACC CAG GGT TGT TTT CT | 0000002341 | ||
| VEGFR3 | FWD | TGA GGA TAA AGG CAG CAT GGA | AF030379 | 66 |
| REV | CCC AGA AAA AGA CAG CGA TGA | |||
| VEGFC | FWD | CTC AAG GCC CCA AAC CAG T | NM_174488 | 71 |
| REV | CAT CCA GCT TAG ACA TGC ATC G | |||
| VEGFD | FWD | GGA GAA TGC CTT TTG AAC CA | NM_001101043 | 272 |
| REV | CCA GTC CTC GAA GTG TGT GA | XM_590821 | ||
| TNFα | FWD | TAA CAA GCC GGT AGC CCA CG | K_00622 | 221 |
| REV | GCA AGG GCT CTT GAT GGC AGA | |||
| CXCL1 | FWD | CTA TTT TTG GGG AGA GGG TAT TCC | U66096 | 94 |
| REV | CGT GAC CTA TCT GTT TGC TTG AAAC C | |||
| CCL21 | FWD | AGT TGC GCT ATG CCA GCT AT | NM_001038076.2 | 184 |
| REV | TTC CCT TCT TGC CAG ACT TG | |||
| β-actin | FWD | CCA AGG CCA ACC GTG AGA AGA T | K00622 | 256 |
| REV | CCA CGT TCC GTG AGG ATC TTC A | |||
Western blotting
The CL tissue samples were homogenized in lysis buffer containing 25 mM Tris-HCl (pH 7.4), 0.3 M sucrose, 2 mM Na2EDTA and cOmplete Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany), and then filtered with a 70-µm filter (Cell Strainer, REF 352350, BD Falcon, Franklin Lakes, NJ, USA). The proteins were dissolved in sample buffer (0.5 M Tris-HCl [pH 6.8], glycerol, 10% SDS and 0.5% bromophenol blue) and steamed for 5 min. The entire samples were subjected to electrophoresis on 10% SDS-PAGE gels for 50 min at 200 V. The proteins were transferred to PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA) for 2 h at 60 V. The membranes were blocked with 4% Block Ace Powder (DS Pharma Biomedical, Osaka, Japan) in TBS with 0.5% Tween-20 (Sigma) for 1 h at room temperature. The membranes were next incubated with a rabbit anti-mouse-LYVE1 polyclonal antibody (1:500 dilution, Abcam, Cambridge, UK) and a mouse anti-β-actin monoclonal clone AC-15 antibody (1:10,000 dilution, Sigma) at 4 C overnight. The membranes were then washed 3 times in TBS with 0.5% Tween-20, incubated with HRP-conjugated anti-rabbit (1:10,000 dilution, Rockland Immunochemicals, Gilbertsville, PA, USA) or anti-mouse (1:10,000 dilution, GE Healthcare, Buckinghamshire, UK) IgG antibodies for 1 h at room temperature, and washed 3 times with TBS with 0.5% Tween-20. The signals were detected using an ECL Western Blotting Detection System (GE Healthcare). The optical densities of the immunospecific bands were quantified using an NIH Image computer-assisted analysis system.
Statistical analysis
All data are presented as means ± standard error of the mean (SEM). The statistical significance of differences was assessed by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison. A P value less than 0.05 was considered significant.
Results
mRNA expression of LYVE1 and podoplanin and LYVE1 protein expression in the bovine CL during PGF2α-induced luteolysis
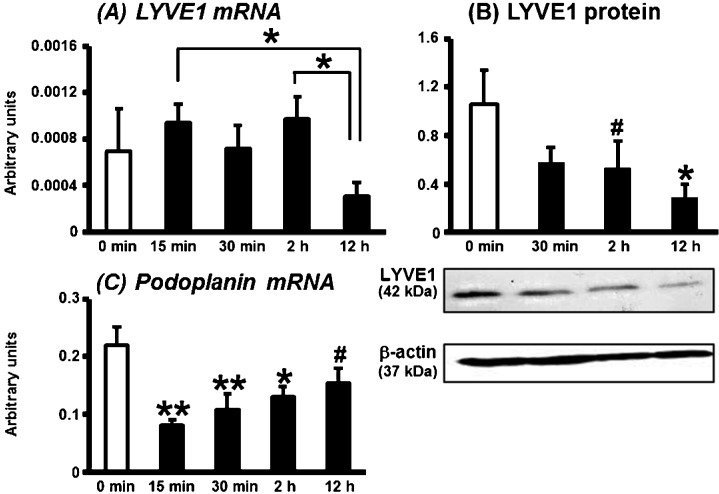
Figure 1 shows the mRNA expression of LYVE1 and podoplanin (markers of LyECs) and LYVE1 protein expression in the bovine CL during PGF2α-induced luteolysis (15 min, 30 min, 2 h and 12 h after PGF2α injection). The level of LYVE1 mRNA expression did not change from 0 min to 2 h, but decreased at 12 h compared with those at 15 min and 2 h after PGF2α injection (Fig. 1A; P<0.05). Lowered expression of LYVE1 protein was found for the first 2 h (P<0.1), and the expression was decreased 12 h after PGF2α injection (Fig. 1B; P<0.05). Lowered Podoplanin mRNA expression was found at 15 min, but the expression tended to recover after that up until 12 h after PGF2α injection (Fig. 1C; P<0.01, P<0.05 or P<0.1).
Fig. 1.
mRNA expression of LYVE1 and podoplanin and LYVE1 protein in the bovine CL during PGF2α-induced luteolysis. LYVE1 mRNA expression decreased in the CL at 12 h as compared with 15 min and 2 h after PGF2α injection (A). LYVE1 protein expression also decreased at 12 h compared with that at 0 min after PGF2α injection (B). Podoplanin mRNA expression was decreased in the CL at all points after PGF2α injection (C). All values are shown as means ± standard error of the mean (SEM; n = 4–5 in each time). *,** Significant difference (P<0.05 or P<0.01 compared with control) as determined by Bonferroni's multiple comparison test. # Tendency for a difference (P<0.1) as determined by Bonferroni's multiple comparison test.
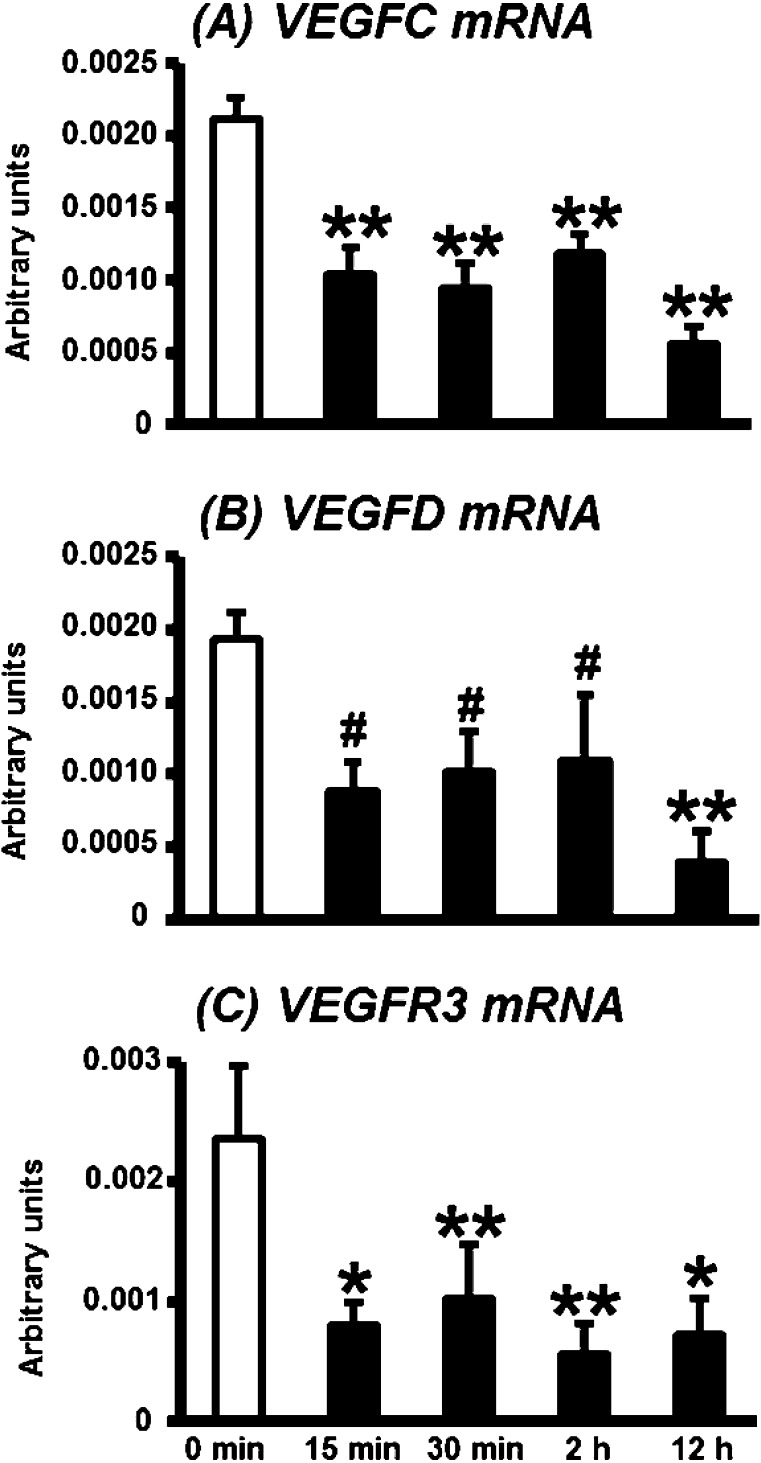
mRNA expression of lymphangiogenic factors in the bovine CL during PGF2α-induced luteolysis
Changes in the transcripts of lymphangiogenic factors (VEGFC, VEGFD and VEGFR3) in the CL during PGF2α-induced luteolysis are shown in Fig. 2. The levels of VEGFC and VEGFR3 mRNA expression were continuously suppressed after PGF2α injection (Figs. 2A and C; P<0.01 or 0.05). VEGFD mRNA expression decreased at 15 min to 2 h as compared with 0 min after PGF2α injection (P<0.1) and decreased significantly 12 h after PGF2α injection (Fig. 2B; P<0.05).
Fig. 2.
mRNA expression of VEGFC, VEGFD and VEGFR3 in the bovine CL during PGF2α-induced luteolysis. mRNA expressions of VEGFC (A), VEGFD (B) and VEGFR3 (C) started to decrease at 15 min after PGF2α injection and then remained at, then kept low levels. All values are shown as means ± standard error of the mean (SEM; n = 4–5 in each time). *,**Significant difference (P<0.05 or P<0.01 compared with control) as determined by Bonferroni's multiple comparison test. #Tendency for a difference (P<0.1) as determined by Bonferroni's multiple comparison test.
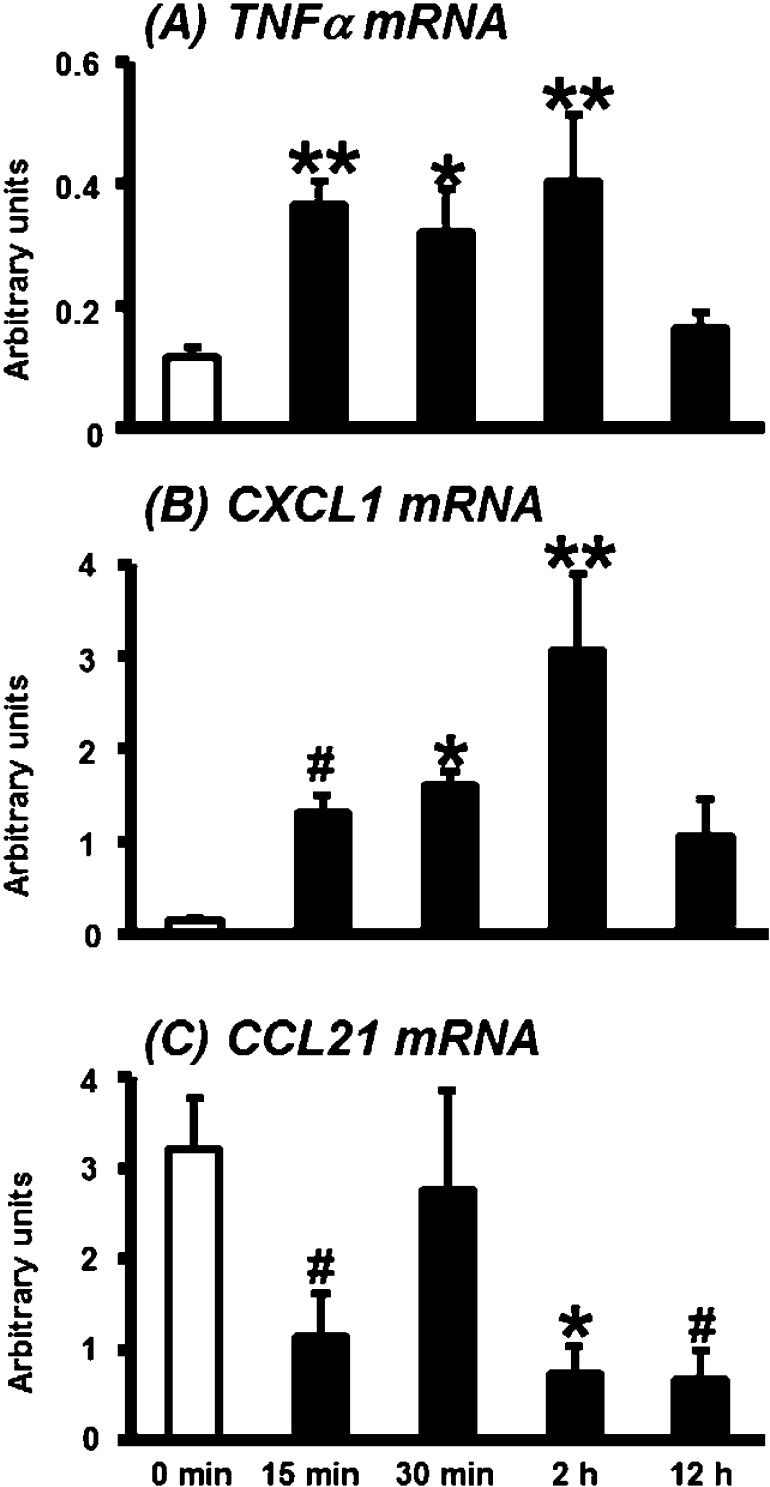
mRNA expression of TNFα, CXCL1 and CCL21 in the bovine CL during PGF2α-induced luteolysis
TNFα mRNA expression increased significantly in the CL at 15 and 30 min and 2 h as compared with 0 min after PGF2α injection (Fig. 3A; P<0.01 or 0.05). Chemokine (C-X-C motif) ligand 1 (CXCL1) enhances the recruitment of neutrophils and acts as a mediator of inflammation during the early wound healing process [41, 42]. CXCL1 mRNA expression also increased at 15 min (P<0.1) and was higher at 30 min and 2 h (P<0.01 or 0.05) as compared with 0 min after PGF2α injection (Fig. 3B).
Fig. 3.
mRNA expression of TNFα, CXCL1 and CCL21 in the bovine CL during PGF2α-induced luteolysis. mRNA expression of TNFα (A) and CXCL1 (B) increased in the CL after PGF2α injection, whereas CCL21 mRNA expression decreased (C, mean ± standard error of the mean [SEM; n = 4–5 in each time]). *,** Significant difference (P<0.05 or P<0.01 compared with control) as determined by Bonferroni's multiple comparison test. #Tendency for a difference (P<0.1) as determined by Bonferroni's multiple comparison test.
Chemokine (C-C motif) ligand 21 (CCL21) is involved in modulation of inflammatory responses and may play a role in the migration of leukocytes from peripheral tissues through afferent lymphatic vessels. CCL21 mRNA expression decreased at 15 min, 2 h and 12 h after PGF2α injection compared with the expression level at 0 min (Fig. 3C; P<0.1 or P<0.05).
Discussion
It is well known that cell death of luteal endothelial cells is induced during luteolysis, which is called structural luteolysis. The treatment with PGF2α resulted in a downregulation of fibroblast growth factor (FGF)-2 mRNA expression and mRNA and protein expression of VEGFA, which are potent angiogenic factors in the CL [3, 4]. In the CL, PGF2α decreased angiopoietin (ANPT) 1 mRNA expression [43] and stimulated a high level of angiopoietin ANPT 2 in relation to ANPT1, inducing the destabilization of blood vessels [3]. The presence of VEGFA may also define the fate of destabilized blood vessels [44], and thus a deficiency in VEGFA may result in the disruptive destabilization of blood vessels after PGF2α injection. The lymphatic vascular system has a role in the body's circulation system together with blood vessels, but there have been no studies about the changes of the lymphatic network in the CL during luteolysis. This study showed for the first time that lymphatic vessel markers, such as LYVE1 and podoplanin, and lymphangiogenic factors, such as VEGFC, VEGFD and VEGFR3, were downregulated in luteolysis. Interestingly, VEGFA and FGF2 have potent lymphangiogenic activity [45, 46]. Additionally, ANPT-1 resulted in lymphatic endothelial cell proliferation, lymphatic vessel enlargement, sprouting and branching in vivo [47] and promoted survival and proliferation of LyECs in vitro [48]. Thus, these findings suggest that luteolytic PGF2α downregulates the production of vascular-related factors, resulting in destruction of the vascular system through angiolysis and lymphangiolysis in the CL during luteolysis. Berisha et al. [49] showed that the number of VEGFR-3-immunostained large luteal cells significantly decreased in the bovine CL during regression (day>18). Immunohistochemical observation of lymphatic vessels markers in the CL after luteolysis is required to clarify how the luteal lymphatic structures disintegrate during PGF2α-induced luteolysis.
During luteolysis, leukocytes, especially neutrophils, macrophages and T lymphocytes, significantly increase in number in the CL [10, 14, 21, 22, 50]. Pro-inflammatory cytokines such as TNFα, IL-1β and IFNγ and chemokines such as MCP1 and IL-8 are associated with luteal regression [3, 13, 14, 51, 52]. These findings suggest that the luteolytic phenomenon is an inflammatory-like immune response. Accordingly, we hypothesized that various immune cells promote an immune response involving the lymphatic vessels during luteolysis in the CL. The immune cells that enter sites of inflammation, such as neutrophils, dendritic cells and macrophages, migrate from tissues and travel to lymph nodes through peripheral afferent lymphatic vessels [33, 34, 36, 53,54,55]. In the lymph nodes, dendritic cells present antigens to T cells, and in the case of immune response, this leads to the clonal expansion and differentiation of antigen-specific T cells. These T cells recirculate from the lymph nodes to inflammatory peripheral tissues through the blood vessels, resulting in an effective immune response through the lymphatic vessels and lymph nodes, which is called lymphocyte homing. In this process, the homeostatic chemokine CCL21 plays an important role of regulating outflow of immune cells from tissue. With regard to the exit of leukocytes from peripheral tissues through afferent lymphatic vessels, the expression of homeostatic chemokine CCL21 by dermal afferent lymphatic vessels is essential in guiding naïve T cells, dendritic cells and neutrophils [32, 56, 57]. In the CL during luteolysis, CCL21 mRNA expression was decreased. Additionally, the decrease in the expression of lymphatic vessel-related factors suggests the loss of lymphatic vessels in the CL during luteolysis. Thus, luteolysis may be a local, not systemic, inflammatory-immune response that does not utilize lymphatic vessels and lymph nodes.
In summary, expression of lymphatic cell markers and lymphangiogenic factors dramatically decreased in the CL during luteolysis, suggesting that the lymphatic network is disrupted in the CL during luteolysis, as well as the vascular structure.
Acknowledgment
This study was supported by a Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (JSPS) and the Global COE Program, Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Juengel JL, Garverick HA, Johnson AL, Youngquist RS, Smith MF. Apoptosis during luteal regression in cattle. Endocrinology 1993; 132: 249–254 [DOI] [PubMed] [Google Scholar]
- 2.Pate JL. Cellular components involved in luteolysis. J Anim Sci 1994; 72: 1884–1890 [DOI] [PubMed] [Google Scholar]
- 3.Arvisais E, Hou X, Wyatt TA, Shirasuna K, Bollwein H, Miyamoto A, Hansen TR, Rueda BR, Davis JS. Prostaglandin F2alpha represses IGF-I-stimulated IRS1/phosphatidylinositol-3-kinase/AKT signaling in the corpus luteum: role of ERK and P70 ribosomal S6 kinase. Mol Endocrinol 2010; 24: 632–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berisha B, Meyer HH, Schams D. Effect of prostaglandin F2 alpha on local luteotropic and angiogenic factors during induced functional luteolysis in the bovine corpus luteum. Biol Reprod 2010; 82: 940–947 [DOI] [PubMed] [Google Scholar]
- 5.Girsh E, Milvae RA, Wang W, Meidan R. Effect of endothelin-1 on bovine luteal cell function: role in prostaglandin F2alpha-induced antisteroidogenic action. Endocrinology 1996; 137: 1306–1312 [DOI] [PubMed] [Google Scholar]
- 6.Hinckley ST, Milvae RA. Endothelin-1 mediates prostaglandin F(2alpha)-induced luteal regression in the ewe. Biol Reprod 2001; 64: 1619–1623 [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Miyamoto A. Angiotensin II interacts with prostaglandin F2alpha and endothelin-1 as a local luteolytic factor in the bovine corpus luteum in vitro. Biol Reprod 1999; 60: 1104–1109 [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto A, Kobayashi S, Arata S, Ohtani M, Fukui Y, Schams D. Prostaglandin F2 alpha promotes the inhibitory action of endothelin-1 on the bovine luteal function in vitro. J Endocrinol 1997; 152: R7–R11 [DOI] [PubMed] [Google Scholar]
- 9.Cannon MJ, Pate JL. The role of major histocompatibility complex molecules in luteal function. Reprod Biol Endocrinol 2003; 1: 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pate JL, Landis Keyes P. Immune cells in the corpus luteum: friends or foes? Reproduction 2001; 122: 665–676 [DOI] [PubMed] [Google Scholar]
- 11.Friedman A, Weiss S, Levy N, Meidan R. Role of tumor necrosis factor alpha and its type I receptor in luteal regression: induction of programmed cell death in bovine corpus luteum-derived endothelial cells. Biol Reprod 2000; 63: 1905–1912 [DOI] [PubMed] [Google Scholar]
- 12.Pate JL. Involvement of immune cells in regulation of ovarian function. J Reprod Fertil Suppl 1995; 49: 365–377 [PubMed] [Google Scholar]
- 13.Brännström M, Friden B. Immune regulation of corpus luteum function. Semin Reprod Endocrinol 1997; 15: 363–370 [DOI] [PubMed] [Google Scholar]
- 14.Penny LA, Armstrong D, Bramley TA, Webb R, Collins RA, Watson ED. Immune cells and cytokine production in the bovine corpus luteum throughout the oestrous cycle and after induced luteolysis. J Reprod Fertil 1999; 115: 87–96 [DOI] [PubMed] [Google Scholar]
- 15.Bowen JM, Towns R, Warren JS, Landis Keyes P. Luteal regression in the normally cycling rat: apoptosis, monocyte chemoattractant protein-1, and inflammatory cell involvement. Biol Reprod 1999; 60: 740–746 [DOI] [PubMed] [Google Scholar]
- 16.Bukulmez O, Arici A. Leukocytes in ovarian function. Hum Reprod Update 2000; 6: 1–15 [DOI] [PubMed] [Google Scholar]
- 17.Townson DH, O'Connor CL, Pru JK. Expression of monocyte chemoattractant protein-1 and distribution of immune cell populations in the bovine corpus luteum throughout the estrous cycle. Biol Reprod 2002; 66: 361–366 [DOI] [PubMed] [Google Scholar]
- 18.Neuvians TP, Schams D, Berisha B, Pfaffl MW. Involvement of pro-inflammatory cytokines, mediators of inflammation, and basic fibroblast growth factor in prostaglandin F2alpha-induced luteolysis in bovine corpus luteum. Biol Reprod 2004; 70: 473–480 [DOI] [PubMed] [Google Scholar]
- 19.Townson DH, Liptak AR. Chemokines in the corpus luteum: implications of leukocyte chemotaxis. Reprod Biol Endocrinol 2003; 1: 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuda K, Sakumoto R. Multiple roles of TNF super family members in corpus luteum function. Reprod Biol Endocrinol 2003; 1: 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirasuna K, Jiemtaweeboon S, Raddatz S, Nitta A, Schuberth HJ, Bollwein H, Shimizu T, Miyamoto A. Rapid accumulation of polymorphonuclear neutrophils in the corpus luteum during prostaglandin F(2alpha)-induced luteolysis in the cow. PLoS One 2012; 7: e29054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer M, Reibiger I, Spanel-Borowski K. Leucocyte proliferation in the bovine corpus luteum. Reproduction 2001; 121: 297–305 [PubMed] [Google Scholar]
- 23.Bombini G, Canetti C, Rocha FA, Cunha FQ. Tumour necrosis factor-alpha mediates neutrophil migration to the knee synovial cavity during immune inflammation. Eur J Pharmacol 2004; 496: 197–204 [DOI] [PubMed] [Google Scholar]
- 24.Canetti C, Silva JS, Ferreira SH, Cunha FQ. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Br J Pharmacol 2001; 134: 1619–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol 2003; 171: 1009–1015 [DOI] [PubMed] [Google Scholar]
- 26.Gosset P, Tonnel AB, Joseph M, Prin L, Mallart A, Charon J, Capron A. Secretion of a chemotactic factor for neutrophils and eosinophils by alveolar macrophages from asthmatic patients. J Allergy Clin Immunol 1984; 74: 827–834 [DOI] [PubMed] [Google Scholar]
- 27.Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vitro: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol 1994; 10: 148–153 [DOI] [PubMed] [Google Scholar]
- 28.Cabrera M, Shaw MA, Sharples C, Williams H, Castes M, Convit J, Blackwell JM. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med 1995; 182: 1259–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petroff MG, Petroff BK, Pate JL. Mechanisms of cytokine-induced death of cultured bovine luteal cells. Reproduction 2001; 121: 753–760 [PubMed] [Google Scholar]
- 30.Shaw DW, Britt JH. Concentrations of tumor necrosis factor alpha and progesterone within the bovine corpus luteum sampled by continuous-flow microdialysis during luteolysis in vivo. Biol Reprod 1995; 53: 847–854 [DOI] [PubMed] [Google Scholar]
- 31.Johnson LA, Jackson DG. Cell trafic and the lymphatic endothelium. Ann NY Acad Sci 2008; 1131: 119–133 [DOI] [PubMed] [Google Scholar]
- 32.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands : balancing immunity and tolerance. Nat Rev Immunol 2008; 8: 362–371 [DOI] [PubMed] [Google Scholar]
- 33.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med 1990; 171: 801–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 2005; 5: 617–628 [DOI] [PubMed] [Google Scholar]
- 35.Martin-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. J Exp Med 2003; 198: 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity 2008; 29: 325–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitta A, Shirasuna K, Haneda S, Matsui M, Shimizu T, Matsuyama S, Kimura K, Bollwein H, Miyamoto A. Possible involvement of IFNT in lymphangiogenesis in the corpus luteum during the maternal recognition period in the cow. Reproduction 2011; 142: 879–892 [DOI] [PubMed] [Google Scholar]
- 38.Shirasuna K, Watanabe S, Asahi T, Wijayagunawardane MP, Sasahara K, Jiang C, Matsui M, Sasaki M, Shimizu T, Davis JS, Miyamoto A. Prostaglandin F2alpha increases endothelial nitric oxide synthase in the periphery of the bovine corpus luteum: the possible regulation of blood flow at an early stage of luteolysis. Reproduction 2008; 135: 527–539 [DOI] [PubMed] [Google Scholar]
- 39.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–159 [DOI] [PubMed] [Google Scholar]
- 40.Watanabe S, Shirasuna K, Matsui M, Yamamoto D, Berisha B, Schams D, Miyamoto A. Effect of intraluteal injection of endothelin type A receptor antagonist on PGF2alpha-induced luteolysis in the cow. J Reprod Dev 2006; 52: 551–559 [DOI] [PubMed] [Google Scholar]
- 41.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998; 153: 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol 2005; 175: 5396–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka J, Acosta TJ, Berisha B, Tetsuka M, Matsui M, Kobayashi S, Schams D, Miyamoto A. Relative changes in mRNA expression of angiopoietins and receptors tie in bovine corpus luteum during estrous cycle and prostaglandin F2a-induced luteolysis: a possible mechanism for the initiation of luteal regression. J Reprod Dev 2004; 50: 619–626 [DOI] [PubMed] [Google Scholar]
- 44.Hanahan D. Signaling vascular morphogenesis and maintenance. Science 1997; 277: 48–50 [DOI] [PubMed] [Google Scholar]
- 45.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D' Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004; 113: 1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. PNAS 2002; 99: 8868–8873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 2005; 105: 4642–4648 [DOI] [PubMed] [Google Scholar]
- 48.Nguyen VPKH, Chen SH, Trinh J, Kim H, Coomber BL, Dumont DJ. Differential response of lymphatic, venous and arterial endothelial cells to angiopoietin-1 and angiopoietin-2. BMC Cell Biol 2007; 8: 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berisha B, Schilffarth S, Kenngott R, Sinowatz F, Meyer HHD, Shams D. Expression of lymphangiogenic vascular endothelial growth factor family members in bovine corpus luteum. Anat Histol Embryol 2012. (In press). 10.111/ahe.12016. . [DOI] [PubMed] [Google Scholar]
- 50.Murdoch WJ, Steadman LE, Belden EL. Immunoregulation of luteolysis. Med Hypotheses 1988; 27: 197–199 [DOI] [PubMed] [Google Scholar]
- 51.Bukovský A, Caudle MR, Keenan JA, Wimalasena J, Upadhyaya NB, Van Meter SE. Is corpus luteum regression an immune-mediated event? Localization of immune system components and luteinizing hormone receptor in human corpora lutea. Biol Reprod 1995; 53: 1373–1384 [DOI] [PubMed] [Google Scholar]
- 52.Cavicchio VA, Pru JK, Davis BS, Davis JS, Rueda BR, Townson DH. Secretion of monocyte chemoattractant protein-1 by endothelial cells of the bovine corpus luteum: regulation by cytokines but not prostaglandin F2alpha. Endocrinology 2002; 143: 3582–3589 [DOI] [PubMed] [Google Scholar]
- 53.Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, Moron VG, Pistoresi-Palencia MC. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood 2006; 108: 3094–3102 [DOI] [PubMed] [Google Scholar]
- 54.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 2005; 106: 1843–1850 [DOI] [PubMed] [Google Scholar]
- 55.Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science 1985; 230: 1277–1280 [DOI] [PubMed] [Google Scholar]
- 56.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, Magistrelli G, Masternak K, Chevailler A, Delneste Y, Jeannin P. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 2011; 117: 1196–1204 [DOI] [PubMed] [Google Scholar]
- 57.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol 1999; 162: 2472–2475 [PubMed] [Google Scholar]