Abstract
Luteinizing hormone (LH) regulates several ovarian functions. However, the luteoprotective mechanisms of LH involved in the maintenance of bovine corpus luteum (CL) function are not well understood. Since prostaglandin F2α (PGF), PGE2 and progesterone (P4) are well documented as antiapoptotic factors in the bovine CL, we hypothesized that LH protects the CL by stimulating the local production and action of PGF, PGE2 and P4. Cultured bovine luteal cells obtained at the mid-luteal stage (days 8–12 of the estrous cycle) were treated with LH (10 ng/ml), onapristone (OP: a specific P4 receptor antagonist, 100 μM) and indomethacin [INDO; a cyclooxygenase (COX) inhibitor, 100 μM] for 24 h. LH with and without OP significantly increased the mRNA and protein expressions of COX-2, PGF synthase and carbonyl reductase (P<0.05) but not the mRNA and protein expressions of COX-1 and PGE synthase in bovine luteal cells. In addition, these treatments significantly increased PGF and P4 production (P<0.05) but not PGE2 production. Luteal cell viability was significantly increased by LH alone (P<0.05), but LH-increased cell viability was reduced by LH in combination with INDO as well as OP (P<0.05). The overall results suggest that LH prevents luteal cell death by stimulating luteal PGF and P4 production and supports CL function during the luteal phase in cattle.
Keywords: Corpus luteum, Luteinizing hormone, Progesterone, Prostaglandin E2, Prostaglandin F2α
The corpus luteum (CL) is a transient endocrine organ that forms in the ovary of mammals after ovulation. If pregnancy does not occur, the CL regresses, allowing a new cycle to begin [1]. Luteal regression induced by endometrial prostaglandin F2α (PGF) is characterized by a reduction in progesterone (P4) production (functional luteolysis) and by tissue degeneration via apoptosis (structural luteolysis) [2, 3]. The major event that causes the structural regression of the CL is luteal cell death [4]. P4 is indispensable for the establishment and maintenance of pregnancy and is also known to suppress apoptosis in bovine luteal cells [5]. Since luteinizing hormone (LH) stimulates P4 production via a variety of signaling molecules in bovine luteal cells, such as cyclic AMP (cAMP), lipoxygenase and phospholipid-specific phospholipase-C [6,7,8], LH may play luteoprotective roles in the bovine CL during the active luteal phase.
PGs regulate CL function in many species. Although the uterine prostanoid PGF induces luteolysis in cattle [3], it neither reduces P4 secretion nor induces apoptosis in cultured bovine luteal cells [9,10,11]. Furthermore, PGF stimulates P4 production as well as PGE2 by cultured luteal cells [9, 10]. PGF and PGE2 produced by luteal cells also suppress apoptosis via stimulation of P4 in bovine luteal cells [11]. However, the modulators of intraluteal PG synthesis are still not well understood. The first step of PG synthesis is the release of arachidonic acid from the phospholipid of cell membranes by phospholipase A2 [12, 13]. Thereafter, arachidonic acid is metabolized to PGH2 by cyclooxygenase (COX)-1 and COX-2 [14, 15]. PGH2 is converted to PGF by PGF synthase (PGFS) or to PGE2 by PGE2 synthase (PGES) [16], and PGE2 is converted to PGF by carbonyl reductase (CBR1), which has the same activity as 9-ketoprostaglandin reductase [17]. LH has been shown to stimulate PGF production by cultured bovine luteal cells [18]. Moreover, human chorionic gonadotropin, whose molecular structure is similar to that of LH, stimulates COX-2 expression in bovine luteal cells [19]. Thus, we hypothesized that LH modulates PG production in the bovine CL by stimulating the expressions of COX-2 and/or other PG synthases and that these actions help to maintain CL function.
In the present study, to determine whether LH promotes cell viability by regulating P4 and intra-luteal PG production, we examined the effects of LH on 1) the mRNA and protein expressions of COX-1, COX-2, PGFS, PGES and CBR1; 2) the production of PGF, PGE2 and P4; and 3) cell viability using a luteal cell culture system.
Materials and Methods
Collection of bovine CLs
Ovaries with CLs from Holstein cows were collected at a local abattoir within 10–20 min after exsanguination. Luteal stages were classified as early, developing, mid, late or regressed by macroscopic observation of the ovary and uterus [20]. For cell culture experiments, the ovaries with mid-CLs (days 8–12 of the estrous cycle) were submerged in ice-cold physiological saline and transported to the laboratory.
Cell isolation
Luteal cells were obtained as described previously [21]. Briefly, mid-CL tissue from five cows was enzymatically dissociated, and the resulting cell suspensions were centrifuged (5 min at 50 × g) three times to separate the luteal cells (pellet) from endothelial cells and other types of luteal nonsteroidogenic cells (supernatant). The dissociated luteal cells were suspended in a culture medium, Dulbecco's Modified Eagle's Medium/Nutrient Mixture F-12 Ham (D/F, 1:1 [v/v]; no. D8900; Sigma-Aldrich, St. Louis, MO, USA) containing 5% calf serum (no. 16170–078; Life Technologies, Grand Island, NY, USA) and 20 μg/ml gentamicin (no. 15750–060; Life Technologies), under 5% CO2 in air. Cell viability was greater than 80%, as assessed by trypan blue exclusion. The cells in the cell suspension after centrifugation consisted of about 70% small luteal cells, 20% large luteal cells, 10% endothelial cells or fibrocytes and no erythrocytes.
Cell culture
The dispersed luteal cells were seeded at 2.0 × 105 viable cells in 1 ml in 24-well cluster dishes (no. 662160; Greiner Bio-One, Frickenhausen, Germany) for evaluating mRNA expression and PG and P4 production, at 1.2 × 106 viable cells in 6 ml in an 25-cm2 culture flask (no. 690160; Greiner Bio-One) for testing protein expression or at 2.0 × 104 viable cells in 0.1 ml in 96-well cluster dishes (no. 3860–096; Iwaki; Asahi Techno Glass, Chiba, Japan) for the cell viability experiment and were cultured in a humidified atmosphere of 5% CO2 in air at 38 C in an N2-O2-CO2-regulated incubator (no. BNP-110; ESPEC, Osaka, Japan). After 24 h of culture, the medium was replaced with fresh medium containing 0.1% BSA (no. 15408; Roche Diagnostics, Mannheim, Germany), 5 ng/ml sodium selenite (no. S5261; Sigma-Aldrich), 5 μg/ml transferrin (no. T3400; Sigma-Aldrich), 10 ng/ml LH (USDA-bLH-B6), 100 μM OP (a specific P4 receptor antagonist; no. ZK98299; Schering AG, Berlin, Germany) and 100 μM indomethacin (INDO; a COX inhibitor: no. 17378; Sigma-Aldrich). The doses of LH, OP and INDO were selected based on previous reports [5, 8, 11].
RNA isolation and cDNA synthesis
Total RNA was extracted from cultured luteal cells using TRIzol Reagent according to the manufacturer's directions (no. 15596–026; Invitrogen, Carlsbad, CA, USA). Total RNA (1 μg) was reverse transcribed using a ThermoScript RT-PCR System (no. 11146-016; Invitrogen).
Quantitative PCR (real-time PCR)
Gene expression was determined by real-time PCR using the MyiQ Optical Module (no. 170-9744; Bio-Rad, Tokyo, Japan) and iQ SYBR Green Supermix (no. 170-8880; Bio-Rad) starting with 2 ng of reverse-transcribed total RNA as described previously [22]. Briefly, GAPDH expression was used as an internal control. For quantification of the mRNA expression levels, the primer length (20 bp) and GC contents of each primer (50–60%) were synthesized (Table 1) and were chosen using an online software package [23]. PCR was performed under the following conditions: 95 C for 3 min, followed by 45 cycles of 94 C for 15 sec, 55 C for 20 sec and 72 C for 15 sec. Use of the iQ SYBR Green Supermix at elevated temperatures resulted in reliable and sensitive quantification of the RT-PCR products with high linearity (Pearson correlation coefficient r > 0.99). The expression of each gene was evaluated on the basis of the GAPDH expression in the individual samples.
Table 1. Primers for real-time PCR.
| Gene | Forward and reverse primers | Accession No. | Product |
| COX-1 | F5'-CTGGGAGTCCTTCTCCAATG-3' R5'-GCAACTGCTTCTTCCCTTTG-3' |
AF004943 | 101 bp |
| COX-2 | F5'-TGTGAAAGGGAGGAAAGAGC-3' R5'-GGCAAAGAATGCAAACATCA-3' |
AF004944 | 115 bp |
| PGFS | F5'-TTCCCTTCAACCAGAGTTGG-3' R5'-TCCCTGGCTTCAGAGACACT-3' |
J03570 | 113 bp |
| PGES | F5'-AGGACGCTCAGAGACATGGA-3' R5'-TTCGGTCCGAGGAAAGAGTA-3' |
AY032727 | 142 bp |
| CBR1 | F5'-AAAACCGCAAGGCAGAGTGGTG-3' R5'-CTCCATATGCGGTATCGGGCCA-3' |
NM0010345131 | 200 bp |
| GAPDH | F5'-CACCCTCAAGATTGTCAGCA-3' R5'-GGTCATAAGTCCCTCCACGA-3' |
BC102589 | 103 bp |
Protein analysis
Each protein in the cultured bovine luteal cells was detected by Western blotting analysis. The cultured cells were lysed in 30 μl lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 10% glycerol [no. G7757; Sigma-Aldrich], Complete [no. 11 697 498 001; Roche Diagnostics, Basel, Switzerland], pH 7.4). Protein concentrations in the lysates were determined by the method of Osnes et al. [24] using BSA as a standard. The proteins samples (50 μg protein) were then solubilized in SDS gel-loading buffer (50 mM Tris-HCl, 2% SDS [no. 31607–94; Nacalai Tesque, Kyoto, Japan], 10% glycerol, 1% β-mercaptoethanol [no. 137-06862; Wako Pure Chemical Industries, Osaka, Japan], pH 6.8), heated at 95 C for 10 min and subjected to electrophoresis on a 10% SDS-PAGE for 80 min at 200 V.
The separated proteins were electrophoretically transblotted to a 0.2-μm nitrocellulose membrane (no. LC2000; Invitrogen) at 250 mA for 3 h in transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% methanol, pH 8.3). The membrane was washed in TBS (25 mM Tris-HCl, 137 mM NaCl, pH 7.5) and incubated in blocking buffer (5% nonfat dry milk in TBS-T) for 1 h at room temperature. After blocking buffer incubation, the membrane was washed three times in TBS-T (0.1% Tween 20 in TBS) for 10 min at room temperature, and then the membrane was cut into two or three pieces. The membranes were then incubated separately with a primary antibody in blocking buffer specific to each protein (Table 2) overnight at 4 C. After primary antibody incubation, the membranes were washed one time for 10 min in blocking buffer at room temperature, and washed two times for 10 min in TBS-T. After washing, the membranes were incubated with secondary antibody in TBS-T (anti-rabbit Ig, HRP-linked whole antibody produced in donkey [Amersham Biosciences, San Francisco, CA, USA; no. NA934; 1: 4000] for COX-1, COX-2, PGFS and PGES protein; anti-goat, HRP-linked whole antibody produced in donkey [Santa Cruz Biotechnology, CA, USA; no, sc-2020; 1:4000] for CBR1 protein; anti-mouse, HRP-linked whole antibody produced in sheep [Amersham Biosciences Corp.; no. NA931; 1: 40000] for beta-actin protein) for 1 h at room temperature and washed three times in TBS for 10 min at room temperature. The signal was detected using an ECL Western Blotting Detection System (no. RPN2109; Amersham Biosciences).
Table 2. Primary antibodies for Western blotting.
| Protein | Manufacturing company | Molecular weight | Dilution rate |
| COX-1 | Cayman Chemical Campany, Ann Arbor, MI, USA; no. 160109 | 70 kDa | 1:200 |
| COX-2 | Alpha Diagnostic Intl., San Antonio, TX, USA; no. 70209A | 72 kDa | 1:200 |
| PGFS | Received from Dr. K Watanabe | 32 kDa | 1:500 |
| PGES | Cayman Chemical; no. 160150 | 23 kDa | 1:500 |
| CBR1 | Abcam plc., Cambridge, UK; no. ab4148 | 30 kDa | 1:200 |
| Beta-actin | Sigma-Aldrich; no. A2228 | 42 kDa | 1:4000 |
The intensity of the immunological reaction in the cells was estimated by measuring the optical density in the defined area by computerized densitometry using NIH Image (National Institutes of Health, Bethesda, MD, USA).
PG and P4 determination
The conditioned medium was collected in 1.5 ml tubes containing 5 μl of a stabilizer solution (0.3 M EDTA, 1% (w/v) acid acetyl salicylic, pH 7.3). The concentrations of PGF and PGE2 in the culture medium were determined by EIA [25]. The PGF standard curve ranged from 0.016 to 4 ng/ml, and the ED50 of the assay was 0.25 ng/ml. The intra- and interassay coefficients of variation were on average 2.8 and 7.7%, respectively. The PGE2 standard curve ranged from 0.039 to 10 ng/ ml, and the ED50 of the assay was 0.625 ng/ml. The intra- and interassay coefficients of variation were on average 11.3 and 13.3%, respectively.
The concentrations of P4 in the culture medium were determined by EIA [25]. The P4 standard curve ranged from 0.391 to 100 ng/ml, and the ED50 of the assay was 0.09 ng/ml. The intra- and interassay coefficients of variation were on average 5.3 and 7.9%, respectively.
Cell viability test
The cell viability was determined using a Dojindo Cell Counting Kit including WST-1 (no. 345-06463; Dojindo, Kumamoto, Japan) as described previously [11]. Briefly, WST-1, a derivative of MTT (3-[4,5-dimethyl-2 thiazolyl]-2,5-diphenyl-2H-tetrazolium/Br), is a yellow tetrazolium salt that is reduced to formazan by live cells containing active mitochondria. For the viability assay, the culture medium was replaced with 100 μl D/F medium without phenol red, and 10 μl assay solution (0.3% WST-1, 0.2 mM 1-methoxy-5-methylphenazinium methylsulfate [1-methoxy PMS] in PBS, pH 7.4) was added to each well. The cells were then incubated for 4 h at 38 C. The absorbance (A) was read at 450 nm using a microplate reader (model 450; Bio-Rad). Cell viability (%) was calculated as cell viability (%)= 100 × (Atest /Acontrol), where Acontrol was the mean A of nontreated wells, and Atest was the mean A of LH-, OP- and INDO-treated wells. The mean A of wells in the absence of the cells was subtracted from the mean A of all experimental wells.
Statistical analysis
All experimental data are shown as the mean ± SEM. The data for P4, PGF and PGE2 production, and cell viability is shown as a percentage of the control. The statistical significance of differences was assessed by analysis of variance (ANOVA) followed by a Fisher's protected least-significant difference procedure (PLSD) as a multiple comparison test.
Results
Effects of LH on PG production and expressions of COX-1, COX-2, PGFS, PGES and CBR1
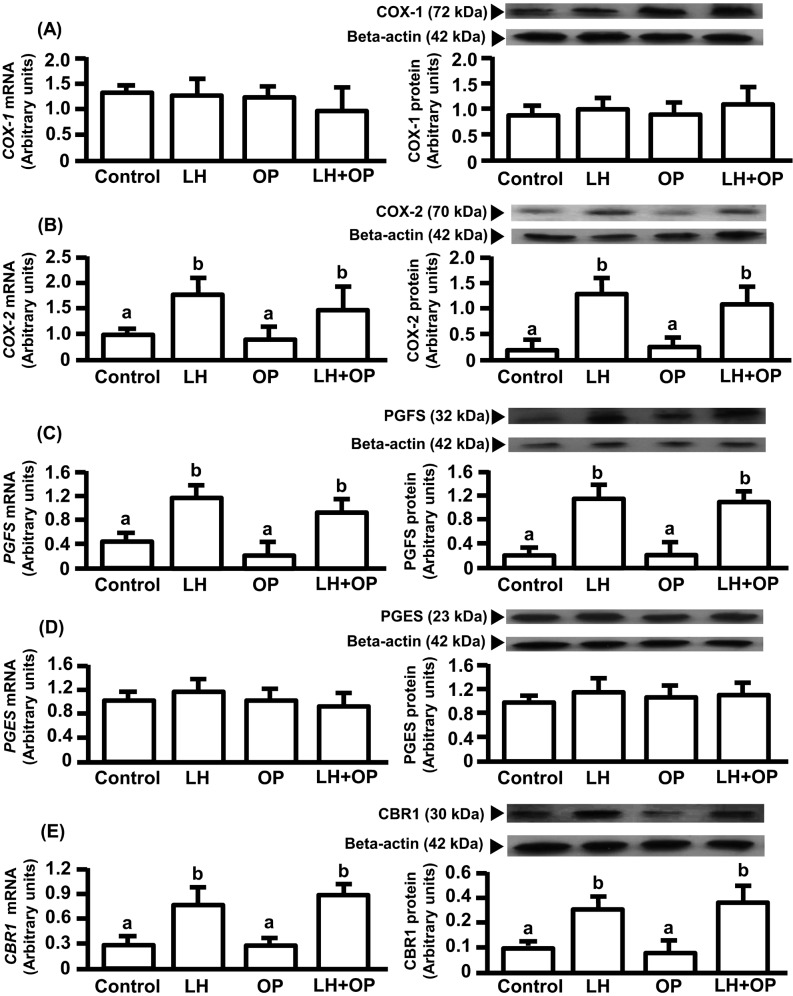
LH alone and in combination with OP significantly increased the mRNA and protein expressions of COX-2, PGFS and CBR1 (Fig. 1B, C, E; P<0.05) but did not affect the mRNA and protein expressions of COX-1 and PGES (Fig. 1A, D).
Fig. 1.
Effects of LH and/or OP on COX-1(A), COX-2(B), PGFS(C), PGES(D) and CBR1(E) expressions. The cells were treated with LH (10 ng/ml) alone or in combination with OP (100 μM) for 24 h. mRNA data are the mean ± SEM of five separate experiments each performed in duplicate and are expressed as levels relative to GAPDH levels. Protein data are the mean ± SEM of five experiments each performed with separate cell preparations and are expressed as levels relative to beta-actin levels. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by a Fisher's PLSD as a multiple comparison test.
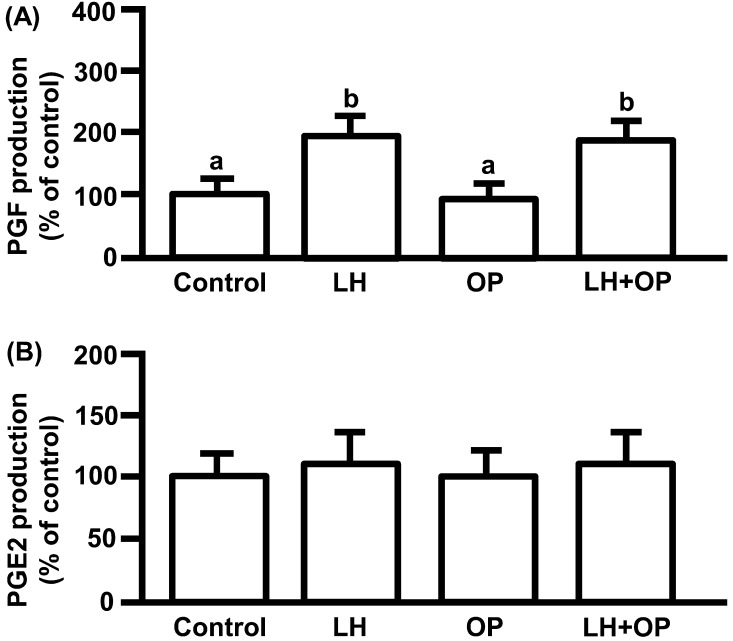
LH significantly increased PGF production (Fig. 2A; P<0.05) but did not affect PGE2 production (Fig. 2B). OP did not affect basal and LH-stimulated PGF production, and basal PGE2 production (Fig. 2A, B).
Fig. 2.
Effects of LH and/or OP on PGF (A) and PGE2 (B) production. The cells were treated with LH (10 ng/ml) alone or in combination with OP (100 μM) for 24 h. All values are expressed as a percentage of control and represent means ± SEM of five separate experiments each performed in duplicate. The mean PGF concentration in the controls was 169 ± 9.6 pg/ml. The mean PGE2 concentration in the controls was 13.7 ± 1.9 ng/ml. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by a Fisher's PLSD as a multiple comparison test.
Effects of LH on cell viability and P4 production
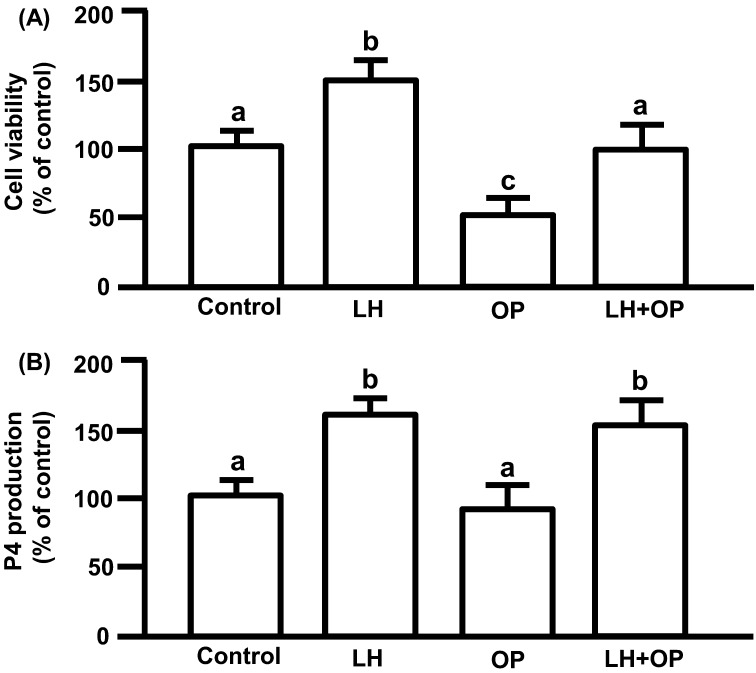
LH significantly increased P4 production (Fig. 3A; P<0.05). OP did not affect basal and LH-stimulated P4 production (Fig. 3A).
Fig. 3.
(A) Effects of LH and/or OP on P4 production. (B) Effects of LH and/or OP on cell viability. The cells were treated with LH (10 ng/ml) alone or in combination with OP (100 μM) for 24 h. P4 production is expressed as a percentage of the control and represents the mean ± SEM of five separate experiments each performed in duplicate. Cell viability is expressed as a percentage of control and represents the mean ± SEM of five separate experiments each performed in triplicate. The mean P4 concentration in the control was 698.7 ± 42.8 ng/ml. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by Fisher's PLSD as a multiple comparison test.
LH significantly increased cell viability (Fig. 3B; P<0.05). OP significantly reduced the viability of basal (control) and LH-induced cells (Fig. 3B; P<0.05). Luteal cell viability when treated with LH in combination with OP was higher than the viability of cells treated with OP alone (Fig. 3B; P<0.05).
Effects of LH and PG on cell viability and P4 production
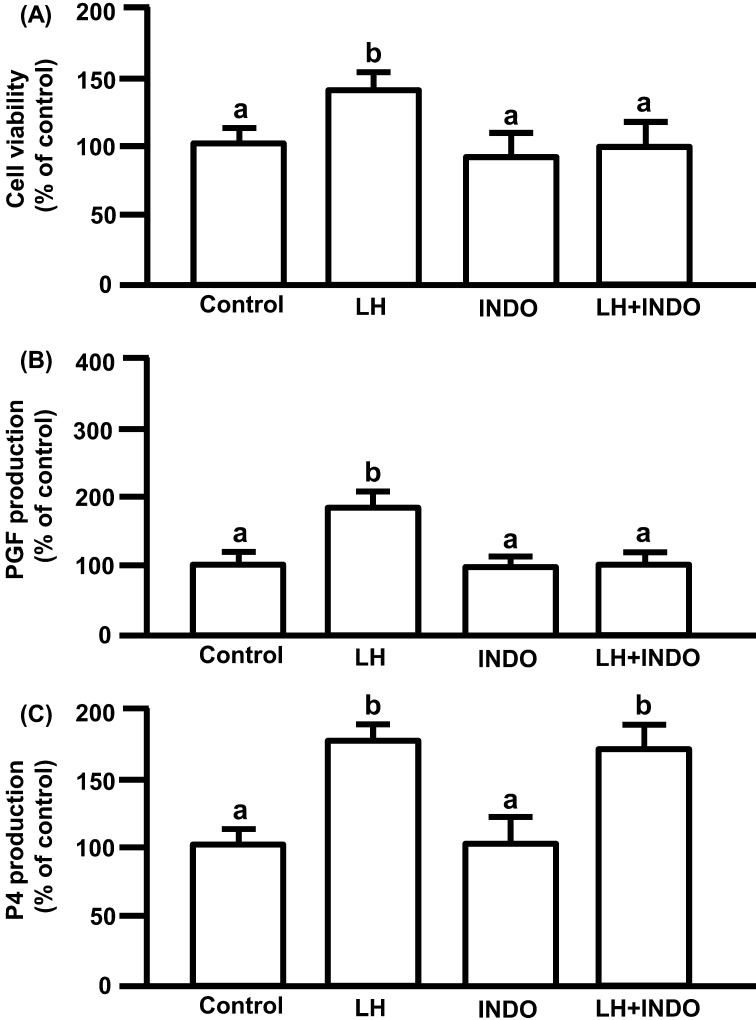
LH significantly increased cell viability. INDO, a COX inhibitor, did not affect cell viability, but significantly decreased LH-induced cell viability to the control level. The viability of cells treated with INDO in combination with LH was not significantly different from the viability of cells treated with INDO alone (Fig. 4A; P<0.05).
Fig. 4.
(A) Effects of LH and/or INDO on cell viability. (B) Effects of LH and/or INDO on PGF production. (C) Effects of LH and/or INDO on P4 production. The cells were treated with LH (10 ng/ml) alone or in combination with INDO (100 μM) for 24 h. Cell viability is expressed as a percentage of the control and represents the mean ± SEM of five separate experiments each performed in triplicate. PGF and P4 production are expressed as a percentage of control and represents the mean ± SEM of five separate experiments each performed in duplicate. The mean PGF concentration in the control was 173 ± 5.7 pg/ml. The mean P4 concentration in the control was 688.3 ± 42.9 ng/ml. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by Fisher's PLSD as a multiple comparison test.
LH significantly increased PGF production. INDO did not affect basal PGF production, but significantly decreased LH-increased PGF production to the control level (Fig. 4B; P<0.05).
LH significantly increased P4 production. INDO did not affect basal and LH-stimulated P4 production (Fig. 4C; P<0.05).
Discussion
LH is an important regulator of ovarian function. The main role of LH in bovine luteal cells is to stimulate P4 secretion, which suppresses apoptosis of these cells [5]. LH also strongly stimulates P4 production by cultured bovine luteal cells [26]. Thus, although LH seems to play a luteoprotective role by stimulating P4 production, the other luteoprotective roles of LH in bovine luteal cells have not been well understood. In fact, LH increased the viability of luteal cells in vitro in the present study. Although we previously demonstrated that PGF and PGE2 as well as P4 play anti-apoptotic roles in the bovine CL [5, 11], it is not known whether LH increases cell viability by regulating survival factors, such as P4 and PGs. At first, to confirm the luteoprotective action of LH is mediated by P4 in the present study, onapristone (OP: a specific P4 receptor antagonist) was used to inhibit the action of P4 on cell viability. OP decreased LH-induced cell viability, indicating that one of the means of CL protection by LH is mediated by P4. On the other hand, LH rescued the decrease in cell viability caused by OP, suggesting that a mechanism other than P4 stimulation is induced by LH to rescue cell viability. cAMP, which acts as a primary second messenger of LH action, has important roles in many biological processes through cAMP-dependent kinase (PKA) and/or in a PKA-independent manner [27,28,29]. In addition, cAMP analogues were found to act as anti-apoptotic agents not only in bovine luteal cells but also in non-steroidogenic cells [30, 31]. These findings support our suggestion that LH increases luteal cell viability by a mechanism other than P4 stimulation.
In many cell types including luteal cells, apoptosis is mediated by death receptors, such as FAS [32,33,34], and by many intracellular regulators, such as caspases (CASPs) [35, 36]. FAS is a receptor of the tumor necrosis factor α superfamily and is activated by binding to FAS ligand, leading to receptor aggregation and apoptotic signal transmission [32,33,34]. FAS induces cleavage of CASP3, thereby initiating the execution phase of apoptosis [32]. Both PGF and PGE2 produced by the CL increase the survivability of bovine luteal cells by suppressing FAS expression and CASP-mediated apoptosis [11]. In addition, since PGF stimulates P4 production in cultured bovine luteal cells [10], luteal PGF is thought to be a luteoprotective factor [11]. On the other hand, both intramuscular PGF injection [37] and endogenous uterine PGF [38] induce luteolysis. Moreover, luteal PGF stimulated by exogenous or uterine PGF has been demonstrated to promote luteolysis in the cow [39]. Therefore, there is still some controversy about whether PGF has different roles in the luteal and uterine origins. In the present study, the viability of luteal cells treated with LH was much higher than that of untreated cells, while the increase in viability caused by LH was decreased by indomethacin (INDO, a COX inhibitor). In addition, INDO decreased LH-increased PGF production, but did not affect P4 production in bovine luteal cells. The above findings suggest that the luteoprotective actions of LH are also mediated by PG production. Interestingly, although LH in combination with OP stimulated PGF production and LH in combination with INDO stimulated P4 production, both of these combinations decreased luteal cell viability in the present study. These results suggest that luteoprotective roles of LH require both P4 and PGF actions. Further studies are needed to clarify the exact mechanisms of luteoprotective actions of LH.
In the present study, LH stimulated the expressions of COX-2 and PGFS but not the expressions of COX-1 and PGES. In fact, LH stimulated PGF production but not PGE2 production. Furthermore, LH stimulated the CBR1 enzyme that converts PGE2 to PGF. This could be the reason why the PGE2 concentration in the medium was not increased, although COX-2 expression was stimulated by LH. In addition, since PGF stimulates P4 secretion in bovine luteal cells [10, 11], LH may stimulate P4 secretion not only directly but also by stimulating PGF. However, INDO did not affect LH-increased P4 production in the present study. Since LH has been demonstrated to increase P4 production by a variety of signaling molecules other than PGF [6,7,8], the increased level of P4 production could be mainly induced by LH rather than by LH-stimulated PGF.
In summary, LH stimulated P4 and PGF production but not PGE2 production. LH increased luteal cell viability, and this luteoprotective action of LH was inhibited by OP as well as by INDO. The overall findings suggest that LH protects CL function by stimulating the production of P4 and PGF in cows.
Acknowledgment
We are grateful to Dr Albert F Parlow, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), for bovine LH (USDA-bLH-B6); Dr K Chwalisz of Schering AG, Berlin, Germany, for a P4 receptor antagonist, OP; and Dr K Watanabe, University of East Asia, Yamaguchi, Japan, for the PGFS antibody. This research was supported by a Grant-in-Aid for Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development (REP-1002) from the Ministry of Agriculture, Forestry and Fisheries of Japan and a Grant-in-Aid for Scientific Research (No.24380155) from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev 2007; 28: 117–149 [DOI] [PubMed] [Google Scholar]
- 2.Knickerbocker JJ, Wiltbank MC, Niswender GD. Mechanisms of luteolysis in domestic livestock. Domest Anim Endocrinol 1988; 5: 91–107 [DOI] [PubMed] [Google Scholar]
- 3.McCracken JA, Custer EE, Lamsa JC. Luteolysis: a nuroendocrinemediated event. Physiol Rev 1999; 79: 263–323 [DOI] [PubMed] [Google Scholar]
- 4.Juengel JL, Garverick HA, Johnson AL, Youngquist RS, Smith MF. Apoptosis during luteal regression in cattle. Endocrinology 1993; 132: 249–254 [DOI] [PubMed] [Google Scholar]
- 5.Okuda K, Korzekwa A, Shibaya M, Murakami S, Nishimura R, Tsubouchi M, Woclawek-Potocka I, Skarzynski DJ. Progesterone is a suppressor of apoptosis in bovine luteal cells. Biol Reprod 2004; 71: 2065–2071 [DOI] [PubMed] [Google Scholar]
- 6.Williams MT, Glark MR, Ling WY, LeMaire WJ, Caron MG, Marsh JM. The role of cyclic AMP in the action of luteinizing hormone on steroidogenesis in the corpus luteum. Ady Cyclic Nucleotide Res 1978; 9: 573–582 [PubMed] [Google Scholar]
- 7.Taniguchi H, Uenoyama Y, Miyamoto Y, Okuda K. The lipoxygenase pathways are involved in LH-stimulated progesterone production in bovine corpus luteum. Prostaglandins Other Lipid Mediat 2002; 67: 49–60 [DOI] [PubMed] [Google Scholar]
- 8.Nishimura R, Shibaya M, Skarzynski DJ, Okuda K. Progesterone stimulation by LH involves the phospholipase-C pathway in bovine luteal cells. J Reprod Dev 2004; 50: 257–261 [DOI] [PubMed] [Google Scholar]
- 9.Milvae RA, Hansel W. Prostacyclin, prostaglandin F2α and progesterone production by bovine luteal cells during the estrous cycle. Biol Reprod 1983; 29: 1063–1068 [DOI] [PubMed] [Google Scholar]
- 10.Okuda K, Uenoyama Y, Lee KW, Sakumoto R, Skarzynski DJ. Progesterone stimulation by prostaglandin F2α involves the protein kinase C pathway in cultured bovine luteal cells. J Reprod Dev 1998; 44: 79–84 [Google Scholar]
- 11.Bowolaksono A, Nishimura R, Hojo T, Sakumoto R, Acosta TJ, Okuda K. Anti-apoptotic roles of prostaglandin E2 and F2α in bovine luteal steroidogenic cells. Biol Reprod 2008; 79: 310–317 [DOI] [PubMed] [Google Scholar]
- 12.Flint APF, Leat WM, Sheldrick EL, Stewart HJ. Stimulation of phosphoinositide hydrolysis by oxytocin and the mechanism by which oxytocin controls prostaglandin synthesis in the ovine endometrium. Biochem J 1986; 237: 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooqui AA, Ong WY, Horrocks LA, Farooqui T. Braincytosolic phospholipase A2: localization, role, and involvement in neurological diseases. Neuroscientist 2000; 6: 169–180 [Google Scholar]
- 14.Olofsson J, Leung PC. Auto/paracrine role of prostaglandins in corpus luteum function. Mol Cell Endocrinol 1994; 100: 87–91 [DOI] [PubMed] [Google Scholar]
- 15.Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 1996; 62: 167–215 [DOI] [PubMed] [Google Scholar]
- 16.Arosh JA, Banu SK, Chapdelaine P, Madore E, Sirois J, Fortier MA. Prostaglandin biosynthesis, transport, and signaling in corpus luteum: a basis for autoregulation of luteal function. Endocrinology 2004; 145: 2551–2560 [DOI] [PubMed] [Google Scholar]
- 17.Waclawik A, Ziecik AJ. Differential expression of prostaglandin (PG) synthesis enzymes in conceptus during peri-implantation period and endometrial expression of carbonyl reductase/PG 9-ketoreductase in the pig. J Endocrinol 2007; 194: 499–510 [DOI] [PubMed] [Google Scholar]
- 18.Grazul AT, Kirsch JD, Slanger WD, Marchello MJ, Redmer DA. Prostaglandin F2α, oxytocin and progesterone secretion by bovine luteal cells at several stages of luteal development: effects of oxytocin, luteinizing hormone, prostaglandin F2α and estradiol-17β. Prostaglandins 1989; 38: 307–318 [DOI] [PubMed] [Google Scholar]
- 19.Klipper E, Levit A, Mastich Y, Berisha B, Schams D, Meidan R. Induction of endothelin-2 expression by luteinizing hormone and hypoxia: possible role in bovine corpus luteum formation. Endocrinology 2010; 151: 1914–1922 [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto Y, Skarzynski DJ, Okuda K. Is tumor necrosis factor α a trigger for the initiation of endometrial prostaglandin F2α release at luteolysis in cattle? Biol Reprod 2000; 62: 1109–1115 [DOI] [PubMed] [Google Scholar]
- 21.Okuda K, Miyamoto A, Sauerwein H, Schweigert FJ, Schams D. Evidence for oxytocin receptors in cultured bovine luteal cells. Biol Reprod 1992; 46: 1001–1006 [DOI] [PubMed] [Google Scholar]
- 22.Sakumoto R, Komatsu T, Kasuya E, Saito T, Okuda K. Expression of mRNAs for interleukin-4, interleukin-6 and their receptors in porcine corpus luteum during the estrous cycle. Domest Anim Endocrinol 2006; 31: 246–257 [DOI] [PubMed] [Google Scholar]
- 23.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000; 132: 365–386 [DOI] [PubMed] [Google Scholar]
- 24.Osnes T, Sandstad O, Skar V, Osnes M, Kierulf P. Total protein in common duct bile measured by acetonitrile precipitation and a micro bicinchoninic acid (BCA) method. Scand J Clin Lab Invest 1993; 53: 757–763 [DOI] [PubMed] [Google Scholar]
- 25.Okuda K, Uenoyama Y, Fujita Y, Iga K, Sakamoto K, Kimura T. Functional oxytocin receptors in bovine granulosa cells. Biol Reprod 1997; 56: 625–631 [DOI] [PubMed] [Google Scholar]
- 26.Niswender GD. Molecular control of luteal secretion of progesterone. Reproduction 2002; 123: 333–339 [DOI] [PubMed] [Google Scholar]
- 27.Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 2002; 12: 258–266 [DOI] [PubMed] [Google Scholar]
- 28.Kobsar A, Heeg S, Krohne K, Opitz A, Walter U, Bock M, Gambaryan S, Eigenthaler M. Cyclic nucleotide-regulated proliferation and differentiation vary in human hematopoietic progenitor cells derived from healthy persons, tumor patients, and chronic myelocytic leukemia patients. Stem Cells Dev 2008; 17: 81–91 [DOI] [PubMed] [Google Scholar]
- 29.Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol 2010; 159: 265–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatsukawa Y, Bowolaksono A, Nishimura R, Komiyama J, Acosta TJ, Okuda K. Possible roles of intracellular cyclic AMP, protein kinase C and calcium ion in the apoptotic signaling pathway in bovine luteal cells. J Reprod Dev 2006; 52: 517–522 [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharjee R, Xiang W, Wang Y, Zhang X, Billiar TR. cAMP prevents TNF-induced apoptosis through inhibiting DISC complex formation in rat hepatocytes. Biochem Biophys Res Commun 2012; 423: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 1998; 17: 1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pru JK, Tilly JL. Programmed cell death in the ovary: insights and future prospects using genetic technologies. Mol Endocrinol 2001; 15: 845–853 [DOI] [PubMed] [Google Scholar]
- 34.Okuda K, Sakumoto R. Multiple roles of TNF super family members in corpus luteum function. Reprod Biol Endocrinol 2003; 1: 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilly JL. Apoptosis and ovarian function. Rev Reprod 1996; 1: 162–172 [DOI] [PubMed] [Google Scholar]
- 36.Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770–776 [DOI] [PubMed] [Google Scholar]
- 37.Wolfenson D, Thatcher WW, Drost M, Caton D, Foster DB, LeBlanc MM. Characteristics of prostaglandin F measurements in the ovarian circulation during the oestrous cycle and early pregnancy in the cow. J Reprod Fertil 1985; 75: 491–499 [DOI] [PubMed] [Google Scholar]
- 38.Schallenberger E, Schams D, Bullermann B, Walters DL. Pulsatile secretion of gonadotrophins, ovarian steroids and ovarian oxytocin during prostaglandin-induced regression of the corpus luteum in the cow. J Reprod Fertil 1984; 71: 493–501 [DOI] [PubMed] [Google Scholar]
- 39.Hayashi K, Acosta TJ, Berisha B, Kobayashi S, Ohtani M, Schams D, Miyamoto A. Changes in prostaglandin secretion by the regressing bovine corpus luteum. Prostaglandins Other Lipid Mediat 2003; 70: 339–349 [DOI] [PubMed] [Google Scholar]