Abstract
Luteoprotective mechanisms of luteinizing hormone (LH) involved in the maintenance of bovine corpus luteum (CL) function have not been completely clarified. Since antioxidant enzymes are well documented as antiapoptotic factors in the CL of many mammals, we hypothesized that the luteoprotective action of LH is mediated by stimulating the local production and action of antioxidant enzymes. To test the above hypothesis, in the present study, we examined the mechanisms involved in the luteoprotective actions of LH. Cultured bovine luteal cells obtained from the CL at the mid-luteal stage (days 8–12 of the estrous cycle) were treated with LH (10 ng/ml), onapristone (OP; a specific progesterone receptor antagonist, 100 μM) and diethyldithiocarbamate [DETC; an inhibitor of superoxide dismutase (SOD), 100 μM] for 24 h. LH in combination with or without OP significantly increased the mRNA and protein expressions of manganese SOD (Mn-SOD) and catalase (CATA) and SOD activity. While LH alone significantly increased the mRNA and protein expressions of SOD containing copper and zinc (Cu,Zn-SOD), OP in combination with or without LH significantly decreased the mRNA and protein expressions of Cu,Zn-SOD. In addition, Cu,Zn-SOD, Mn-SOD and CATA mRNA expressions were higher at the mid luteal phase than the other luteal phases. LH in combination with DETC significantly decreased LH-increased cell viability. The overall results suggest that LH increases cell viability by LH-increased antioxidant enzymes, resulting in maintenance of CL function during the luteal phase in cattle.
Keywords: Catalase, Luteinizing hormone, Progesterone, Reactive oxygen species, Superoxide dismutase
The corpus luteum (CL) is a transient endocrine organ that forms in the ovary of mammals after ovulation. The primary product of the CL, progesterone (P4), is required for the establishment and maintenance of pregnancy [1]. Luteinizing hormone (LH) is an important regulator of the synthesis and secretion of P4 in the CL of most mammals, including cows [2]. P4 production is initiated at the mitochondria and includes the uptake of cholesterol into the mitochondria mediated by the steroid acute regulatory protein (StAR) and its conversion into pregnenolone by cytochrome P450 side-chain cleavage (P450scc). Pregnenolone is further processed into P4 by 3β-hydroxysteroid dehydrogenase at the endoplasmic reticulum [1]. LH stimulates P4 production in part by activating P450scc [3].
It has been demonstrated that reactive oxygen species (ROS), such as the superoxide radicals and hydrogen peroxide (H2O2), are generated by activating P450scc [4, 5]. Therefore, LH, which activates P450scc, may increase not only P4 production but also ROS production. In fact, LH stimulates ROS production in rat theca cells [3]. ROS have some detrimental effects on cells including DNA and protein damage and lipid peroxidation, which primarily affect the membrane structure and the function of cells [4]. ROS, including nitric oxide, seem to play a big role in CL regression by inducing apoptosis in bovine luteal cells [6]. Since LH stimulates P4 synthesis and is necessary for establishing and maintaining pregnancy [1], we assume that ROS produced in the process of P4 production by the stimulation of LH should be metabolized by LH action.
ROS are metabolized to H2O2 and O2 by superoxide dismutase (SOD), and H2O2 is catabolized to H2O and O2 by catalase (CATA) [7]. There are two types of SOD: SOD containing copper and zinc (Cu,Zn-SOD), which is located in the cytosol, and manganese-SOD (Mn-SOD), which is located in the mitochondria [7]. It has been demonstrated that human chorionic gonadotropin, whose molecular structure is similar to that of LH, stimulates Cu,Zn-SOD activity and mRNA expression in the human CL [8]. Hence, we hypothesized that LH prevents the detrimental effects of ROS on luteal cells by increasing antioxidant enzyme production.
In the present study, to determine whether LH plays roles in regulating bovine CL function by increasing cell viability and influencing SOD and CATA expressions, we examined the effects of LH on 1) SOD activity; 2) the mRNA and protein expressions of Cu,Zn-SOD, Mn-SOD and CATA; and 3) the effect of LH and SOD on cell viability in bovine luteal cells. In addition, we examined 4) the changes in the mRNA and protein expressions of Cu,Zn-SOD, Mn-SOD and CATA in the bovine CL during the luteal phase.
Materials and Methods
Collection of bovine CLs
Ovaries with CLs from Holstein cows were collected at a local abattoir within 10–20 min after exsanguination. Luteal stages were classified as early (days 2–3), developing (days 5–6), mid (days 8–12), late (days 15–17) or regressed (days 19–21) by macroscopic observation of the ovary and uterus [9]. For experiments involving tissue extraction and cell culture, the ovaries were submerged in ice-cold physiological saline and transported to the laboratory.
Cell isolation
Luteal cells were obtained as described previously [10]. Briefly, mid-CL tissue from four cows was enzymatically dissociated, and the resulting cell suspensions were centrifuged (5 min at 50 × g) three times to separate the luteal cells (pellet) from endothelial cells and other types of luteal nonsteroidogenic cells (supernatant). The dissociated luteal cells were suspended in a culture medium, Dulbecco's Modified Eagle's Medium/Nutrient Mixture F-12 Ham (D/F, 1:1 [v/v]; no. D8900; Sigma-Aldrich, St. Louis, MO, USA) containing 5% calf serum (no. 16170–078; Life Technologies, Grand Island, NY, USA) and 20 μg/ml gentamicin (no. 15750–060; Life Technologies), under 5% CO2 in air. Cell viability was greater than 80%, as assessed by trypan blue exclusion. The cells in the cell suspension after centrifugation consisted of about 70% small luteal cells, 20% large luteal cells, 10% endothelial cells or fibrocytes and no erythrocytes.
Cell culture
The dispersed luteal cells were seeded at 2.0×105 viable cells in 1 ml in 24-well cluster dishes (no. 662160; Greiner Bio-One, Frickenhausen, Germany) for evaluating mRNA expression and P4 production, at 1.2×106 viable cells in 6 ml in an 25-cm2 culture flask (no. 690160; Greiner Bio-One) for testing protein expression, at 4.0×106 viable cells in 20 ml in an 75-cm2 culture flask (no. 658175; Greiner Bio-One) for SOD activity assay or at 2.0×104 viable cells in 0.1 ml in 96-well cluster dishes (no. 3860–096; Iwaki; Asahi Techno Glass, Chiba, Japan) for cell viability experiments and were cultured in a humidified atmosphere of 5% CO2 in air at 38 C in an N2-O2-CO2-regulated incubator (no. BNP-110; ESPEC, Osaka, Japan). After 24 h of culture, the medium was replaced with fresh medium containing 0.1% BSA (no. 15408; Roche Diagnostics, Mannheim, Germany), 5 ng/ml sodium selenite (no. S5261; Sigma-Aldrich), 5 μg/ml transferrin (no. T3400; Sigma-Aldrich), 10 ng/ml LH (USDA-bLH-B6), 100 μM onapristone (OP; a specific P4 receptor antagonist; no. ZK98299; Schering AG, Berlin, Germany) and 100 μM diethyldithiocarbamate (DETC; an inhibitor of SOD; no. D3132; Sigma-Aldrich). OP has been demonstrated to be one of the most specific P4 receptor antagonists [11]. The doses of LH, OP and DETC were selected based on previous reports [12,13,14].
RNA isolation and cDNA synthesis
Total RNA was extracted from cultured luteal cells using TRIzol Reagent according to the manufacturer's directions (no. 15596–026; Invitrogen, Carlsbad, CA, USA). Total RNA (1 μg) was reverse transcribed using a ThermoScript RT-PCR System (no. 11146-016; Invitrogen).
Quantitative PCR (real-time PCR)
Gene expression was determined by real-time PCR using a MyiQ Optics Module (no. 170-9744; Bio-Rad, Tokyo, Japan) and iQ SYBR Green Supermix (no. 170-8880; Bio-Rad) starting with 2 ng of reverse-transcribed total RNA as described previously [15]. Briefly, GAPDH expression was used as an internal control. For quantification of the mRNA expression levels, the primer length (20 bp) and GC contents of each primer (50–60%) were synthesized (Table 1) and were chosen using an online software package [16]. PCR was performed under the following conditions: 95 C for 3 min, followed by 45 cycles of 94 C for 15 sec, 55 C for 20 sec and 72 C for 15 sec. Use of the iQ SYBR Green Supermix at elevated temperatures resulted in reliable and sensitive quantification of the RT-PCR products with high linearity (Pearson correlation coefficient r > 0.99). The expression of each gene was evaluated on the basis of the GAPDH expression in the individual samples.
Table 1. Primers for real-time PCR.
| Gene | Forward and reverse primers | Accession No. | Product |
| Cu,Zn-SOD | F5'-AAGGCCGTCTGCGTGCTGAA-3' R5'-CAGGTCTCCAACATGCCTCT-3' |
M81129 | 240 bp |
| Mn-SOD | F5'-ATTGCTGGAAGCCATCAAAC-3' R5'-ACATTCCAAATGGCCTTCAG-3' |
NM201527 | 273 bp |
| CATA | F5'-CTGGGACCCAACTATCTCCA-3′ R5'-GATGCTCGGGAGCACTAAAG-3′ |
NM001035386 | 148 bp |
| GAPDH | F5'-CACCCTCAAGATTGTCAGCA-3' R5'-GGTCATAAGTCCCTCCACGA-3' |
BC102589 | 103 bp |
Protein analysis
The cultured cells were scraped and placed in ice-cold homogenization buffer for protein analysis (25 mM Tris-HCl, 300 mM sucrose, 2 mM EDTA, Complete [protease inhibitor cocktail; no. 1697498; Roche], pH 7.4), frozen in liquid nitrogen and then stored at –80 C until protein analysis by Western blotting. CL tissues were homogenized on ice in the homogenization buffer by a tissue homogenizer (Physcotron; Niti-on, Chiba, Japan; NS-50), followed by filtration with a metal wire mesh (150 μm). For protein analysis, nuclei were isolated from the tissue homogenates by centrifugation at 600 × g for 30 min. Mitochondria for Cu,Zn-SOD and CATA protein analyses and cytosol for Mn-SOD protein analyses were isolated from the resultant supernatant by centrifugation at 8000 × g for 30 min.
Each protein was detected by Western blotting analysis. Proteins were lysed in 30 μl lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 10% glycerol [no. G7757; Sigma-Aldrich], Complete [no. 11 697 498 001; Roche Diagnostics], pH 7.4). Protein concentrations in the lysates were determined by the method of Osnes et al. [17] using BSA as a standard. The protein samples (50 μg protein) were then solubilized in SDS gel-loading buffer (50 mM Tris-HCl, 2% SDS [no. 31607–94; Nacalai Tesque, Kyoto, Japan], 10% glycerol, 1% β-mercaptoethanol [no. 137-06862; Wako Pure Chemical Industries, Osaka, Japan], pH 6.8), heated at 95 C for 10 min and subjected to electrophoresis on a 10% SDS-PAGE for 80 min at 200 V.
The separated proteins were electrophoretically transblotted to a 0.2-μm nitrocellulose membrane (no. LC2000; Invitrogen) at 250 mA for 3 h in transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% methanol, pH 8.3). The membrane was washed in TBS (25 mM Tris-HCl, 137 mM NaCl, pH 7.5) and incubated in blocking buffer (5% nonfat dry milk in TBS-T) for 1 h at room temperature. After blocking buffer incubation, the membrane was washed three times in TBS-T (0.1% Tween 20 in TBS) for 10 min at room temperature, and then the membrane was cut into two or three pieces. The membranes were then incubated separately with a primary antibody in blocking buffer specific to each protein (Cu,Zn-SOD antibody [23 kDa; 1:500; no. sc-8637; Santa Cruz Biotechnology, CA, USA], Mn-SOD antibody [25 kDa; 1:500; no. sc-18503; Santa Cruz Biotechnology], CATA antibody [65 kDa; 1:10000; no. 200-4151; Rockland Immunochemicals, Gilbertsville, PA, USA], beta-actin antibody [42 kDa; 1:4000; no. A2228; Sigma-Aldrich]) overnight at 4 C. After primary antibody incubation, the membranes were washed one time for 10 min in blocking buffer at room temperature and washed two times for 10 min in TBS-T. After washing, the membranes were incubated with secondary antibody in TBS-T (anti-goat, HRP-linked whole antibody produced in donkey [Santa Cruz Biotechnology; no, sc-2020; 1:4000] for Cu,Zn-SOD and Mn-SOD protein; anti-rabbit Ig, HRP-linked whole antibody produced donkey [Amersham Biosciences, San Francisco, USA; no. NA934; 1: 4000] for CATA protein; anti-mouse, HRP-linked whole antibody produced in sheep [Amersham Biosciences; no. NA931; 1: 40000] for beta-actin) for 1 h at room temperature and washed three times in TBS for 10 min at room temperature. The signal was detected using an ECL Western Blotting Detection System (no. RPN2109; Amersham Biosciences).
The intensity of the immunological reaction in the cells was estimated by measuring the optical density in the defined area by computerized densitometry using NIH Image (National Institutes of Health, Bethesda, MD, USA).
P4 determination
The concentrations of P4 in the culture medium were determined by EIA [18]. The P4 standard curve ranged from 0.391 to 100 ng/ml, and the ED50 of the assay was 3.5 ng/ml. The intra- and interassay coefficients of variation were on average 5.3% and 7.9%, respectively.
SOD activity
SOD activity was measured with an SOD activity assay kit (no. S311; Dojindo Laboratories, Kumamoto, Japan). SOD activity in the luteal cells was evaluated as described previously [19]. SOD activity was calculated using a concurrent SOD standard curve, and expressed as a percentage of the control (raw SOD activity was expressed as units of activity per mg of cellular protein).
Cell viability test
The cell viability was determined using a Dojindo Cell Counting Kit including WST-1 (no. 345-06463; Dojindo, Kumamoto, Japan) as described previously [13]. Briefly, WST-1, a derivative of MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium/Br), is a yellow tetrazolium salt that is reduced to formazan by live cells containing active mitochondria. For the viability assay, the culture medium was replaced with 100 μl D/F medium without phenol red, and 10 μl assay solution (0.3% WST-1, 0.2 mM 1-methoxy-5-methylphenazinium methylsulfate [1-methoxy PMS] in PBS, pH 7.4) was added to each well. The cells were then incubated for 4 h at 38 C. The absorbance (A) was read at 450 nm using a microplate reader (model 450; Bio-Rad). Cell viability (%) was calculated as cell viability (%)= 100 × (Atest/Acontrol), where Acontrol was the mean A of nontreated wells, and Atest was the mean A of LH- and DETC-treated wells. The mean A of wells in the absence of the cells was subtracted from the mean A of all experimental wells.
Statistical analysis
All experimental data are shown as the mean ± SEM. The data for P4, SOD activity and cell viability are expressed as percentages of the control values. The statistical significance of differences was assessed by analysis of variance (ANOVA) followed by a Fisher's protected least-significant difference procedure (PLSD) as a multiple comparison test.
Results
Effects of LH on antioxidant enzymes expressions, SOD activity and P4 production
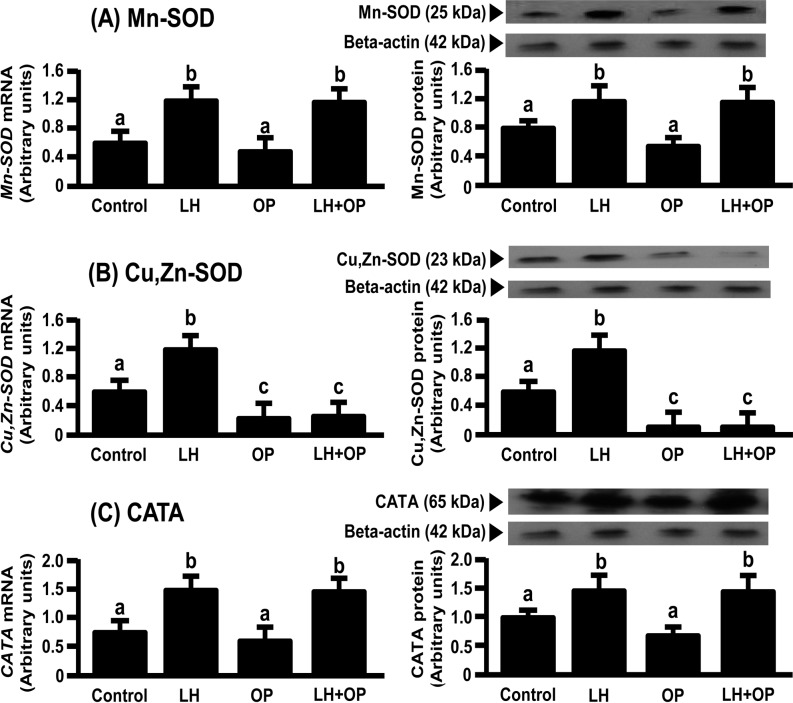
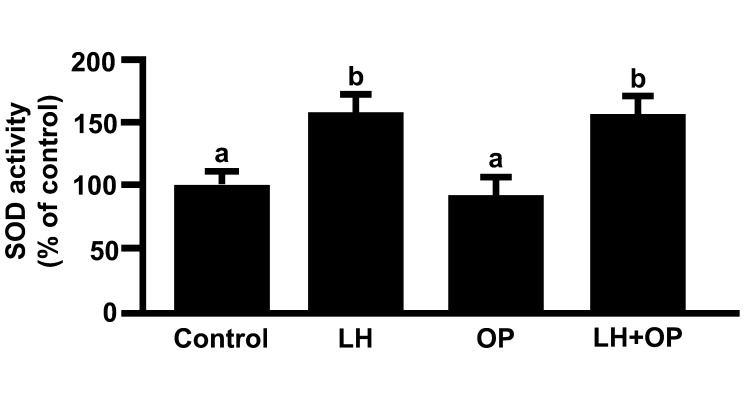
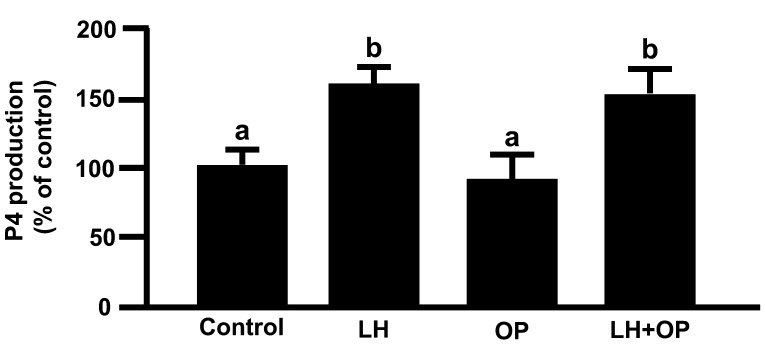
LH and LH in combination with OP significantly increased the mRNA and protein expressions of Mn-SOD and CATA (Figs. 1A and 1C; P<0.05), SOD activity (Fig. 2; P<0.05) and P4 production (Fig. 3; P<0.05). Although LH significantly increased the mRNA and protein expression of Cu,Zn-SOD, OP and OP in combination with LH significantly decreased the protein expression of Cu,Zn-SOD (Fig. 1B; P<0.05).
Fig. 1.
Effects of LH and/or OP on the amounts of (A) Mn-SOD, (B) Cu,Zn-SOD and (C) CATA expressions. The cells were treated with LH (10 ng/ml) alone or in combination with OP (100 μM) for 24 h. mRNA data are the mean ± SEM of four separate experiments each performed in duplicate and are expressed relative to the amount of GAPDH mRNA. Protein data are the mean ± SEM of four experiments each performed with separate cell preparations and are expressed relative to the amount of beta-actin protein. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by a Fisher's PLSD as a multiple comparison test.
Fig. 2.
Effects of LH and/or OP on SOD activity. The cells were treated with LH (10 ng/ml) alone or in combination with OP (100 μM) for 24 h. All values are expressed as a percentage of the control and represent the mean ± SEM of four separate experiments each performed in duplicate. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by Fisher's PLSD as a multiple comparison test.
Fig. 3.
Effects of LH and/or OP on P4 production. The cells were treated with LH (10 ng/ml) alone or in combination with OP (100 μM) for 24 h. All values are expressed as a percentage of the control and represent the mean ± SEM of four separate experiments each performed in duplicate. The mean P4 concentration in the control was 709.7 ± 32.8 ng/ml. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by Fisher's PLSD as a multiple comparison test.
Effects of LH and SOD on cell viability
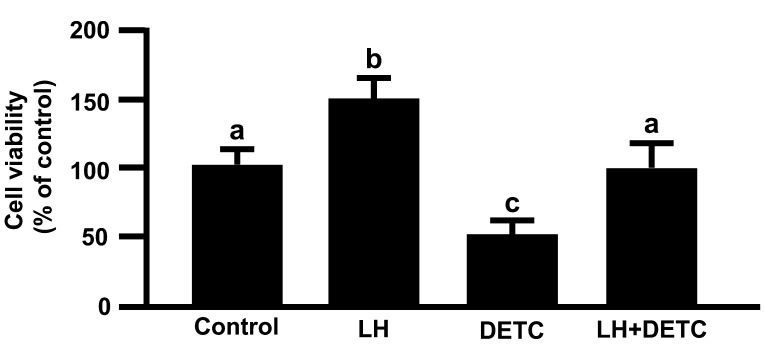
LH significantly increased cell viability, but DETC significantly decreased LH-stimulated cell viability (Fig. 4; P<0.05), indicating that LH increased the cell viability of cultured luteal cells via SOD.
Fig. 4.
Effecsts of LH and/or DETC on cell viability. The cells were treated with LH (10 ng/ml) alone or in combination with DETC (100 μM) for 24 h. All values are expressed as a percentage of the control and represent the mean ± SEM of four separate experiments each performed in triplicate. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by Fisher PLSD as a multiple comparison test.
mRNA and protein expressions of antioxidant enzymes
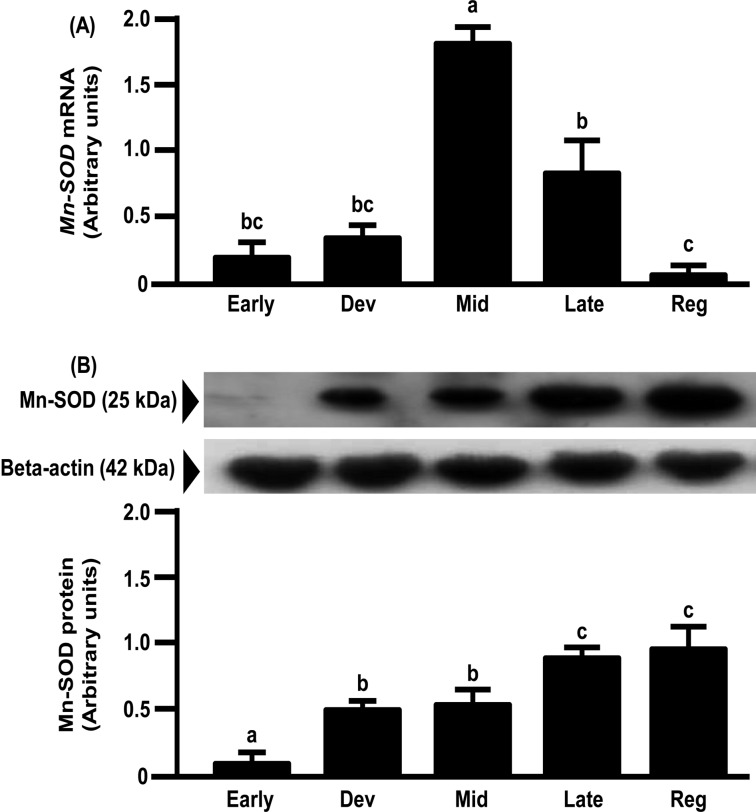
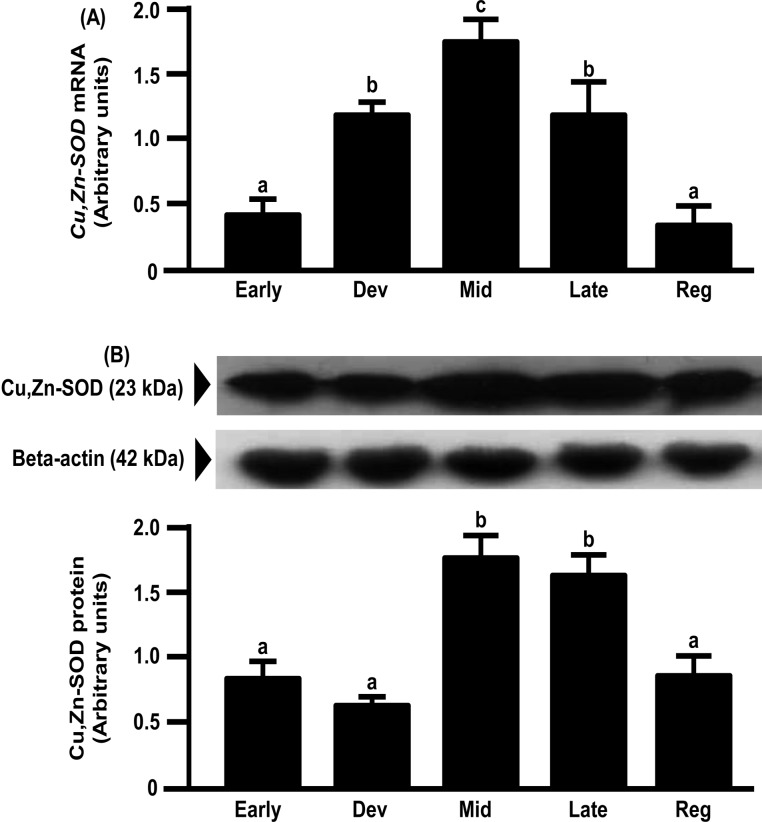
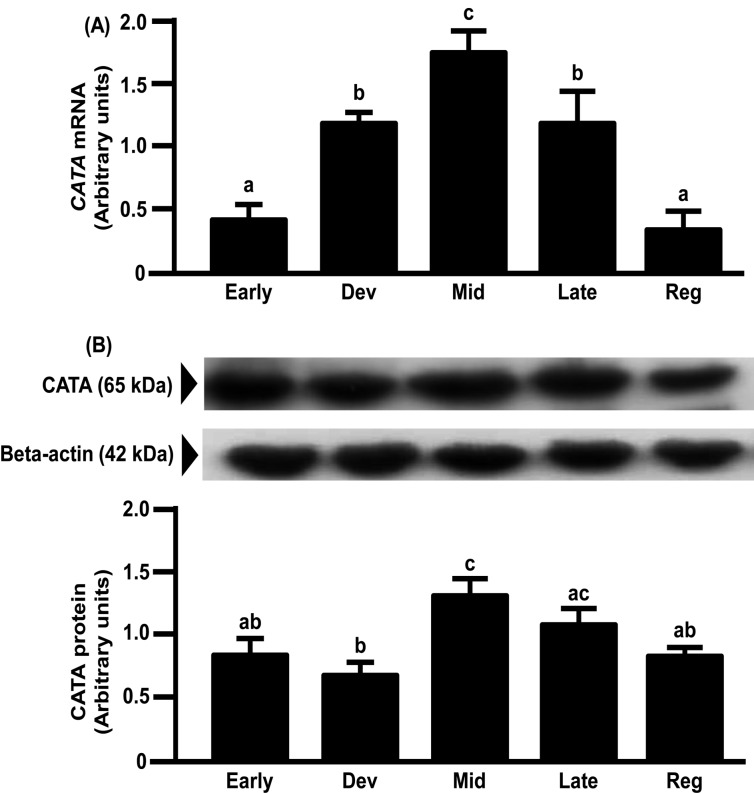
mRNA expression of Mn-SOD in the bovine CL increased from the early to mid-luteal phases and gradually decreased toward the regression phase (Fig. 5A). However, the protein expression of Mn-SOD gradually increased toward the regression luteal phase and was the highest in the regression luteal phase (Fig. 5B). mRNA and protein expressions of Cu,Zn-SOD and CATA in the bovine CL increased from the early to mid-luteal phases and gradually decreased toward the regression phase (Figs. 6 and 7).
Fig. 5.
Changes in relative amounts of Mn-SOD mRNA (A) and protein (B) in the bovine CL throughout the estrous cycle. mRNA data are the mean ± SEM of four separate experiments each performed in duplicate and are expressed relative to the amount of GAPDH mRNA. Protein data are the mean ± SEM of four experiments each performed with separate cell preparations and are expressed relative to the amount of beta-actin protein. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by a Fisher PLSD as a multiple comparison test (Early, days 2–3; Developing [Dev], days 5–6; Mid, days 8–12; Late, days 15–17; Regressed luteal stages [Reg], days 19–21).
Fig. 6.
Changes in relative amounts of Cu,Zn-SOD mRNA (A) and protein (B) in the bovine CL throughout the estrous cycle. mRNA data are the mean ± SEM of four separate experiments each performed in duplicate and are expressed relative to the amount of GAPDH mRNA. Protein data are the mean ± SEM of four experiments each performed with separate cell preparations and are expressed relative to the amount of beta-actin protein. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by a Fisher PLSD as a multiple comparison test (Early, days 2–3; Developing [Dev], days 5–6; Mid, days 8–12; Late, days 15–17; Regressed luteal stages [Reg], days 19–21).
Fig. 7.
Changes in relative amounts of CATA mRNA (A) and protein (B) in the bovine CL throughout the estrous cycle. mRNA data are the mean ± SEM of four separate experiments each performed in duplicate and are expressed relative to the amount of GAPDH mRNA. Protein data are the mean ± SEM of four experiments each performed with separate cell preparations and are expressed relative to the amount of beta-actin protein. Different letters indicate significant differences (P<0.05), as determined by ANOVA followed by a Fisher PLSD as a multiple comparison test (Early, days 2–3; Developing [Dev], days 5–6; Mid, days 8–12; Late, days 15–17; Regressed luteal stages [Reg], days 19–21).
Discussion
In most mammals, P4 is indispensable for the establishment and maintenance of pregnancy [1]. P4 works to develop tissue and blood vessels in the endometrium to assist in implantation [20] and also downregulates prostaglandin F2α in the bovine endometrium [21], which supports survival and development of the conceptus [22]. Furthermore, P4 suppresses apoptosis in bovine luteal cells [13]. A large amplitude of LH pulses is observed throughout the luteal phase, and it is thought that these pulses stimulate P4 production to maintain CL function in cattle [23]. LH stimulates P4 production in part by activating P450scc in rat preovulatory granulosa cells, which results in ROS production [3]. ROS have been shown to induce apoptosis in bovine luteal cells [24]. Therefore, pulsatile secretion of LH seems not only to stimulate P4 but also to induce luteolytic ROS production throughout luteal phase. Consequently, ROS production in the CL may be highest at the mid luteal phase, when P4 production is highest in the bovine CL [4]. CL function may be maintained at the mid luteal phase because LH stimulates antioxidant enzyme production. In the present study, we demonstrated that LH significantly increased the mRNA and protein expressions of Mn-SOD, Cu,Zn-SOD and CATA. In contrast, OP, a specific P4 receptor antagonist, decreased the mRNA and protein expressions of Cu,Zn-SOD, which suggests that LH stimulates the mRNA and protein expressions of Cu,Zn-SOD by mediating P4 action. On the other hand, since LH in combination with OP increased the mRNA and protein expressions of Mn-SOD and CATA and SOD activity, LH may stimulate the mRNA and protein expressions of Mn-SOD and CATA and SOD activity not by mediating P4 action. Cyclic AMP (cAMP), which acts as a primary second messenger of LH action, has been demonstrated to increase Mn-SOD expressions but not Cu,Zn-SOD expressions in human endometrial stromal cells [25]. In addition, since P4 stimulates Cu,Zn-SOD expressions in human endometrial stromal cells [26], LH could regulate the expressions of SODs by cAMP or P4 in bovine luteal cells. In any case, it could be assumed that ROS induced possibly by LH is metabolized by LH-induced antioxidant enzymes as well as LH-induced activities of the SODs to protect CL function. In fact, LH increased the cell viability of cultured luteal cells via SOD (Fig. 4). In addition, since LH in combination with DETC, a SOD activity inhibitor, increased the cell viability as compared with DETC alone, LH probably increases the cell viability by inducing one or more luteoprotective factors other than antioxidant enzymes.
In the present study, Cu,Zn-SOD, Mn-SOD and CATA mRNA expressions increased from the early to the mid luteal phases and gradually decreased towards the regression phase. Cu,Zn-SOD and CATA protein expressions were also highest at the mid luteal phase and gradually decreased towards the regression phase. The above findings imply that antioxidant enzymes are involved in maintaining CL function, because these changes were similar to the pattern of P4 production in the CL [4]. The pulsatile secretion of LH seems not only to stimulate P4 but also the expressions of antioxidant enzymes to metabolize ROS produced during P4 synthesis. However, Mn-SOD protein expression gradually increased towards the luteal regression phase and was highest in this phase. The pattern of the protein expressions of SODs during the luteal phase in the bovine CL was consistent with the pattern in the human CL [8]. However, Mn-SOD mRNA expression was not consistent with Mn-SOD protein expression in the bovine CL, suggesting that the latter is regulated at the translation level or during posttranslational processing. These results also suggest that Cu,Zn-SOD and Mn-SOD have different roles in luteal function. This suggestion is consistent with the findings that Cu,Zn-SOD plays important roles in the maintenance of cell function and that Mn-SOD is necessary for cell survival [8, 26, 27]. Further studies are needed to clarify the different roles of Cu,Zn-SOD and Mn-SOD in the bovine CL.
In conclusion, the overall results of the present study demonstrate that LH enhances the viability of luteal cells by stimulating the intraluteal expressions of Cu,Zn-SOD, Mn-SOD and CATA and by stimulating SOD activity in order to maintain luteal function in cows.
Acknowledgment
We are grateful to Dr Albert F Parlow, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), for bovine LH (USDA-bLH-B6) and Dr K Chwalisz of Schering AG, Berlin, Germany, for a P4 receptor antagonist (OP). This research was supported by a Grant-in-Aid for Research Program on Innovative Technologies for Animal Breeding, Reproduction, and Vaccine Development (REP-1002) from the Ministry of Agriculture, Forestry and Fisheries of Japan and a Grant-in-Aid for Scientific Research (No.24380155) from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 2000; 80: 1–29 [DOI] [PubMed] [Google Scholar]
- 2.Imakawa K, Day ML, Zalesky DD, Garcia-Winder M, Kittok RJ, Kinder JE. Regulation of pulsatile LH secretion by ovarian steroids in the heifer. J Anim Sci 1986; 63: 162–168 [DOI] [PubMed] [Google Scholar]
- 3.Yacobi K, Tsafriri A, Gross A. Luteinizing hormone-induced caspase activation in rat preovulatory follicles is coupled to mitochondrial steroidogenesis. Endocrinology 2007; 148: 1717–1726 [DOI] [PubMed] [Google Scholar]
- 4.Rapoport R, Sklan D, Wolfenson D, Shaham-Albalancy A, Hanukoglu I. Antioxidant capacity is correlated with steroidogenic status of the corpus luteum during the bovine estrous cycle. Biochim Biophys Acta 1998; 1380: 133–140 [DOI] [PubMed] [Google Scholar]
- 5.Sugino N. Role of reactive oxygen species in the corpus luteum. Anim Sci J 2006; 77: 556–565 [Google Scholar]
- 6.Korzekwa AJ, Okuda K, Woclawek-Potocka I, Murakami S, Skarzynski DJ. Nitric oxide induces apoptosis in bovine luteal cells. J Reprod Dev 2006; 52: 353–361 [DOI] [PubMed] [Google Scholar]
- 7.Takiguchi S, Sugino N, Kashida S, Yamagata Y, Nakamura Y, Kato H. Rescue of the corpus luteum and an increase in luteal superoxide dismutase expression induced by placental luteotropins in the rat: action of testosterone without conversion to estrogen. Biol Reprod 2000; 62: 398–403 [DOI] [PubMed] [Google Scholar]
- 8.Sugino N, Takiguchi S, Kashida S, Karube A, Nakamura Y, Kato H. Superoxide dismutase expression in the human corpus luteum during the menstrual cycle and in early pregnancy. Mol Hum Reprod 2000; 6: 19–25 [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto Y, Skarzynski DJ, Okuda K. Is tumor necrosis factor α a trigger for the initiation of endometrial prostaglandin F2α release at luteolysis in cattle? Biol Reprod 2000; 62: 1109–1115 [DOI] [PubMed] [Google Scholar]
- 10.Okuda K, Miyamoto A, Sauerwein H, Schweigert FJ, Schams D. Evidence for oxytocin receptors in cultured bovine luteal cells. Biol Reprod 1992; 46: 1001–1006 [DOI] [PubMed] [Google Scholar]
- 11.Elger W, Bartley J, Schneider B, Kaufmann G, Schubert G, Chwalisz K. Endocrine pharmacological characterization of progesterone antagonists and progesterone receptor modulators with respect to PR-agonistic and antagonistic activity. Steroids 2000; 65: 713–723 [DOI] [PubMed] [Google Scholar]
- 12.Nishimura R, Shibaya M, Skarzynski DJ, Okuda K. Progesterone stimulation by LH involves the phospholipase-C pathway in bovine luteal cells. J Reprod Dev 2004; 50: 257–261 [DOI] [PubMed] [Google Scholar]
- 13.Okuda K, Korzekwa A, Shibaya M, Murakami S, Nishimura R, Tsubouchi M, Woclawek-Potocka I, Skarzynski DJ. Progesterone is a suppressor of apoptosis in bovine luteal cells. Biol Reprod 2004; 71: 2065–2071 [DOI] [PubMed] [Google Scholar]
- 14.Kondo-Nakamura M, Shintani-Ishida K, Uemura K, Yoshida K. Brief exposure to carbon monoxide preconditions cardiomyogenic cells against apoptosis in ischemia-reperfusion. Biochem Biophys Res Commun 2010; 393: 449–454 [DOI] [PubMed] [Google Scholar]
- 15.Sakumoto R, Komatsu T, Kasuya E, Saito T, Okuda K. Expression of mRNAs for interleukin-4, interleukin-6 and their receptors in porcine corpus luteum during the estrous cycle. Domest Anim Endocrinol 2006; 31: 246–257 [DOI] [PubMed] [Google Scholar]
- 16.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000; 132: 365–386 [DOI] [PubMed] [Google Scholar]
- 17.Osnes T, Sandstad O, Skar V, Osnes M, Kierulf P. Total protein in common duct bile measured by acetonitrile precipitation and a micro bicinchoninic acid (BCA) method. Scand J Clin Lab Invest 1993; 53: 757–763 [DOI] [PubMed] [Google Scholar]
- 18.Okuda K, Uenoyama Y, Fujita Y, Iga K, Sakamoto K, Kimura T. Functional oxytocin receptors in bovine granulosa cells. Biol Reprod 1997; 56: 625–631 [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Acosta TJ, Nakagawa Y, Okuda K. Role of nitric oxide in the regulation of superoxide dismutase and prostaglandin F2α production in bovine luteal endothelial cells. J Reprod Dev 2010; 56: 454–459 [DOI] [PubMed] [Google Scholar]
- 20.Tamm K, Suhorutshenko M, Room M, Simm J, Metsis M. The tissue specific role of estrogen and progesterone in human endometrium and mammary gland. Steroids-Basic Science 2012; 1: 35–64 [Google Scholar]
- 21.Spencer TE, Johnson GA, Burghardt RC, Bazer FW. Progesterone and placental hormone actions on the uterus: insights from domestic animals. Biol Reprod 2004; 71: 2–10 [DOI] [PubMed] [Google Scholar]
- 22.Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci 2002; 7: d1879–1898 [DOI] [PubMed] [Google Scholar]
- 23.Peters KE, Bergfeld EG, Cupp AS, Kojima FN, Mariscal V, Sanchez T, Wehrman ME, Grotjan HE, Hamernik DL, Kittok RJ, Kinder JE. Luteinizing hormone has a role in development of fully functional corpora lutea (CL) but is not required to maintain CL function in heifers. Biol Reprod 1994; 51: 1248–1254 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Ishigami T, Makino N, Sakamoto K. The down-regulation of glutathione peroxidase causes bovine luteal cell apoptosis during structural luteolysis. J Biochem 2001; 129: 937–942 [DOI] [PubMed] [Google Scholar]
- 25.Sugino N, Karube-Harada A, Sakata A, Takiguchi S, Kato H. Different mechanisms for the induction of copper-zinc superoxide dismutase and manganese superoxide dismutase by progesterone in human endometrial stromal cells. Hum Reprod 2002; 17: 1709–1714 [DOI] [PubMed] [Google Scholar]
- 26.Sugino N, Karube-Harada A, Kashida S, Takiguchi S, Kato H. Differential regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase by progesterone withdrawal in human endometrial stromal cells. Mol Hum Reprod 2002; 8: 68–74 [DOI] [PubMed] [Google Scholar]
- 27.Sugino N, Telleria CM, Gibori G. Differential regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase in the rat corpus luteum: induction of manganese superoxide dismutase messenger ribonucleic acid by inflammatory cytokines. Biol Reprod 1998; 59: 208–215 [DOI] [PubMed] [Google Scholar]