Abstract
A high incidence (about 70%) of alteration in endometrial epidermal growth factor (EGF) profile, i.e., loss of 2 peaks on days 2–4 and 13–14, has been linked to a reduced fertility in multiparous repeat breeder Holstein cows. However, the EGF profile in Holstein heifers and other breeds (types) of cattle has not been investigated. In study 1, EGF concentrations were determined using endometrial tissues obtained by biopsy on days 3, 7 and 14 from 84 fertile Holstein heifers to obtain a normal range and 53 repeat breeder Holstein heifers to estimate incidence of alterations in the EGF profile. In repeat breeder heifers, EGF concentrations were similar to fertile controls on 3 days and five animals (9.4%) had an altered EGF profile with EGF concentrations below the normal range on days 3 and 14. In study 2, EGF concentrations on day 3 were repeatedly examined from the nulliparous period to the third postpartum period in 28 Holstein (dairy) and 47 Japanese Black (beef) cattle. The effect of parity on EGF concentrations on day 3 was different between Holstein and Japanese Black cattle. In Japanese Black cows, the EGF concentrations were consistently high throughout the study period, while in Holstein cows, the EGF concentrations decreased after the second calving. In conclusion, unlike multiparous repeat breeder Holstein cows, an altered EGF profile may not be a major cause of repeat breeding in Holstein heifers, and the peak EGF concentrations around day 3 may decrease even in fertile populations of multiparous dairy cows, but not in beef cows.
Keywords: Biopsy, Breeds, EGF, Fertility, Parity
Repeat breeding has been one of the major causes of economic loss in the dairy and beef industries. Although fertilization failure and embryonic death have been suggested to be major causes of repeat breeding, mechanisms of infertility in repeat breeders are not clearly understood [1,2,3,4]. We have previously reported an alteration in the cyclic change of endometrial EGF concentrations in repeat breeder Holstein cows [5, 6]. In control cows, endometrial EGF concentrations show 2 peaks, on days 2 to 4 and days 13 to 14, with low concentrations of EGF around day 7. These 2 peaks in the EGF profile were missing in about 70% of repeat breeder cows [5, 6], and normalization of the EGF profile resulted in the restoration of fertility [6, 7]. This alteration in endometrial EGF concentrations has been linked to embryonic death due to the potential role of EGF in the uterus [8, 9] and decreased conception rates after embryo transfer to recipient cows having low EGF peaks on day 3 [10]. Thus, the properties and significance of the alteration in the endometrial EGF profile of multiparous repeat breeder Holstein (dairy) cows are being investigated; however, the EGF profiles in Holstein heifers and other breeds (types) of cattle remain to be determined. Therefore, the present study, first, examined endometrial EGF concentrations in fertile Holstein heifers to determine a normal range of endometrial EGF concentrations. Then, using the normal range, the incidence of the alteration in the EGF profile in repeat breeder Holstein heifers was estimated for comparison with that in multiparous repeat breeder Holstein cows reported in our previous study [6] (Study 1). In a separate study, EGF concentrations on day 3 were examined in Holstein (dairy) and Japanese Black (beef) cattle in 4 consecutive parities to examine the effects of parity and breed (type) of cattle on the EGF profile (Study 2).
Materials and Methods
Biopsy of the uterine endometrium
Uterine endometrial tissues were obtained by biopsy using a biopsy instrument (Fujihira, Tokyo, Japan) under caudal epidural anesthesia with 3 ml of 2% lidocaine (2% Xylocaine, Fujisawa Pharmaceutical, Osaka, Japan) as described previously [6]. The uterine endometrial tissue of the intercaruncle region (about 0.1 g) was obtained from the middle section of 3 sections of the uterine horns, which were equally divided on the longitudinal axis. The tissues were weighed, frozen in liquid nitrogen and stored at –80 C.
EGF assay
Uterine endometrial tissue was processed by the method described previously [11] with a modification to increase the ratio of tissue (g) to extraction solution (ml) from 1:5 to 1:15 [6]. The EGF concentrations in uterine extracts were determined by a double-antibody sandwich EIA using 96-well microtiter plates [5]. Anti-human EGF mouse monoclonal antibody (R & D Systems, Minneapolis, MN, USA) was used for solid-phase antibody, and anti-human EGF rabbit antiserum (Biogenesis, Poole, UK) was used for detection with anti-rabbit IgG goat antibody (Seikagaku, Tokyo, Japan) labeled with peroxidase. The sensitivity of the assay was 10 pg/well. The intra- and interassay CVs at 50 pg/well were 4.2 and 5.3%, respectively.
Study design
Study 1: Clinically healthy, sexually mature, cyclic Holstein heifers between 13 and 16 months of age that were housed in 4 commercial dairy farms were used to determine a normal range of the endometrial EGF concentrations. Heifers were observed for estrus (day 0) twice daily, and endometrial tissues were obtained for EGF assays by biopsy on days 3, 7 and 14 of the same estrous cycle. The normal ranges (means ± 2 SD) of endometrial EGF concentrations on the 3 days were calculated using EGF concentrations of 84 fertile heifers that became pregnant by the artificial insemination (AI) during the next 2 estrous cycles.
Repeat breeder Holstein heifers were examined for endometrial EGF concentrations on days 3, 7 and 14 to determine the incidence of alterations in the endometrial EGF profile. Heifers that were clinically normal but had failed to conceive after 3 or more AIs without any abnormality in the cyclicity and in the genital organs according to routine examinations by field practitioners were recruited for the study. All animals were then examined by one of the authors for regularity of the estrous cycle, time of ovulation (<48 h after onset of estrus), subclinical inflammation of the uterus by cytology or histology and oviductal patency. Fifty-three heifers at 18 to 26 months of age without any sign of abnormality by the examinations were used as repeat breeders in the present study. An endometrial EGF profile was judged as being altered when EGF concentrations on any of the 3 days were out of the normal range.
Study 2: Sixty-four Holstein and 56 Japanese Black heifers housed in 4 and 2 farms, respectively, were recruited for the Study 2 to examine the effects of parity and type of cattle (dairy and beef) on the endometrial EGF profile. The heifers had their endometrial EGF concentrations examined on day 3 and were subjected to AI at estrus up to 2 times. The same animals were again examined for endometrial EGF concentrations on day 3 of the first estrous cycle, beginning after 60 days postpartum, and then subjected to AIs. Measurement of the EGF concentrations on day 3 was repeated until the third postpartum period (i.e., 4 times including the first examination in heifers). Animals were excluded from the study in any parity in which they failed to conceive after the second AI after biopsy of the endometrial tissues for EGF assay. Animals were also excluded from the study when they failed to show an estrus by 90 days postpartum. The proportions of dairy and beef cattle remaining in the study were compared by Fisher’s exact test. The effects of parity and type of cattle were analyzed using 2-way ANOVA with repeated measurement followed by Scheffe’s test as a post hoc test. Statistical analyses were performed using SPSS for Windows (Ver. 18, SPSS, Tokyo, Japan).
Results
Study 1
The endometrial EGF concentrations of fertile and repeat breeder Holstein heifers and the calculated normal ranges are shown in Table 1. Endometrial EGF concentrations were similar between fertile and repeat breeder heifers on all 3 days of the estrous cycle. Five (9.4%) repeat breeder heifers showed an altered EGF profile with low EGF concentrations on days 3 and 14.
Table 1. Endometrial EGF concentrations (ng/g of tissue weight) in fertile and repeat breeder Holstein heifers.
| Group | (n) | Days of the estrous cycle | ||
| 3 | 7 | 14 | ||
| Fertile* (range) | (84) | 11.5 ± 2.6 (3.8–17.6) | 2.1 ± 0.4 (1.3–3.0) | 12.5 ± 2.8 (1.4–19.7) |
| Repeat breeders (range) | (53) | 11.3 ± 2.1 (1.7–9.7) | 2.8 ± 1.1 (1.3–2.7) | 13.1 ± 2.4 (0.8–12.4) |
Values are means ± SD. *A normal range was determined by using endometrial EGF concentrations from 84 fertile Holstein heifers that conceived within 2 AIs after biopsy of the endometrial tissues. Calculated normal ranges (means ± 2 SD) were 6.3–16.7, 1.3–2.9 and 6.9–18.1 ng/g tissue weight on days 3, 7 and 14 of the estrous cycle, respectively. Five out of 53 repeat breeder cows (9.4%) showing EGF concentrations lower than the normal range on days 3 and 14 were judged to have an altered EGF profile.
Study 2
Twenty-eight (43.8%) out of 64 Holstein cattle and 47 (83.9%) out of 56 Japanese Black cattle remained in the study; they became pregnant with up to 2 AIs at 4 consecutive parities (Table 2). The proportion of animals remaining at the end of the study was greater in Japanese Black than Holstein cattle (P<0.01). In Holstein cattle, 27 (42.2%) and 9 (14.1%) cattle were withdrawn by not conceiving with 2 AIs and by being culled for reasons other than fertility, respectively (Table 3). Endometrial EGF concentrations of animals that failed to conceive with 2 AIs from the nulliparous period to the third postpartum period were 7.8 ± 1.4, 6.2 ± 1.9, 5.6 ± 1.9 and 4.8 ± 1.7 ng/g tissue weight, respectively, and were lower than those of the fertile population at comparable parities (Table 2). In Japanese Black cattle, 2, 1, 3 and 3 cattle were excluded from the study at each parity: from the nulliparous period to the third postpartum period. All cattle, except for 2 cows that were culled due to accidents at the first and third parturition, were excluded based on not conceiving with 2 AIs.
Table 2. Endometrial EGF concentrations (ng/g of tissue weight) on day 3 at different parities in Holstein and Japanese Black cattle.
| Breed | (n) | Parity | |||
| 0 | 1 | 2 | 3 | ||
| Holstein | (28) | 13.0 ± 3.4a | 12.4 ± 3.3a | 9.0 ± 2.0ab,A | 7.5 ± 2.0b,A |
| Japanese Black | (47) | 13.5 ± 2.1 | 14.2 ± 2.8 | 13.7 ± 2.0B | 11.8 ± 1.7B |
Values are means ± SD. ab Values with different letters within the same type of cattle differ significantly (P<0.05). AB Values with different letters within the same parity differ significantly (P<0.05).
Table 3. Numbers of Holstein cattle withdrawn in Study 2 and reasons for withdrawal.
| Parity | 0 | 1 | 2 | 3 | |
| Remaining in the study at indicated parity* | 61 (95.3) | 50 (78.1) | 35 (54.7) | 28 (43.8) | |
| Withdrawn from the study at indicated parity$ | 3 (4.7) | 11 (18.0) | 15 (30.0) | 7 (20.0) | |
| Not conceived by the second AI | 3 (4.7) | 7 (11.5) | 11 (22.0) | 6 (17.1) | |
| Mastitis | 0 (0) | 2 (3.3) | 2 (4.0) | 1 (2.9) | |
| Accidents# | 0 (0) | 2 (3.3) | 1 (2.0) | 0 (0) | |
| Displacement of abomasum | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | |
* Percentage based on 64 virgin Holstein heifers recruited for the study. $ Percentage based on the number of animals recruited for the nulliparous heifers and based on the number of animals remaining in the study at the previous parity for the primiparous and multiparous cows. # One cow each was culled due to accidents at the second and third parturitions. One cow at the first parity was culled due to hip dislocation.
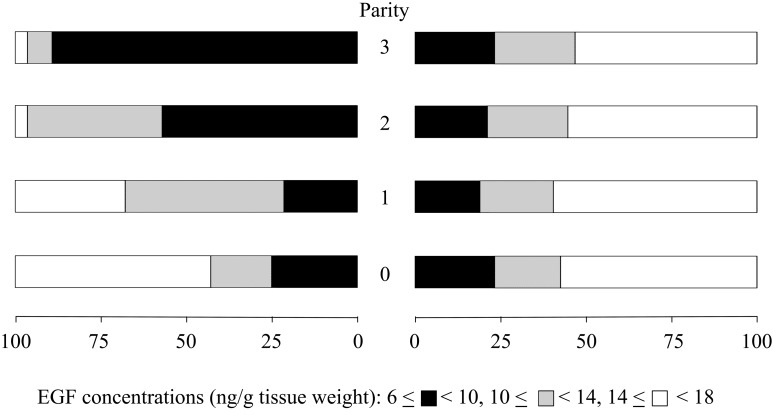
Data from animals remaining at the end of the study were used to determine the effects of parity and breed (type) of cattle on the EGF profile in the fertile population. All animals remaining at the end of the study had peak EGF concentrations on day 3 within the normal range at all parities. Endometrial EGF concentrations were similar in Holstein and Japanese Black heifers (Table 2). However, the effect of parity on EGF concentrations on day 3 was different between Holstein and Japanese Black cattle (P<0.01). In Japanese Black cattle, EGF concentrations remained high throughout the study period, while in Holstein cattle, EGF concentrations decreased after the second calving. Consequently, the EGF concentrations of Holstein cattle were similar to those of Japanese Black cattle in the nulliparous period and the first postpartum period and became lower than those of Japanese Black in the second and third postpartum periods. Accordingly, the proportions of animals having EGF concentrations at different levels were similar between Holstein and Japanese Black heifers (Fig. 1). However, the proportion changed in Holstein cows and became different from that of Japanese Black cattle after the first parturition.
Fig. 1.
Proportion of cattle with different levels of peak EGF concentrations on day 3 in the endometrium at each parity in fertile Holstein (left) and Japanese Black (right) cattle.
Discussion
Alterations in the endometrial EGF profile are found in about 70% of multiparous repeat breeder Holstein cows [6], and these abnormalities have been linked to reduced fertility in lactating multiparous Holstein repeat breeder and apparently normal cows [9]. However, the incidence of the altered EGF profile in repeat breeder Holstein heifers, which was determined based on the EGF concentrations on day 3 in the present study, was about 10%. This low incidence of the altered EGF profile in repeat breeder Holstein heifers suggests that alterations in the endometrial EGF profile may be less important as a cause of reduced fertility in repeat breeder Holstein heifers. This may also partially explain a lower incidence of repeat breeding in heifers than lactating multiparous Holstein cows.
The present study also demonstrated a clear difference between Holstein (dairy) and Japanese Black (beef) cattle in the effect of parity on the endometrial EGF profile. The EGF concentrations on day 3 of the first estrous cycle starting after 60 days postpartum were consistently high up to the third postpartum period in Japanese Black cattle, while the peaks decreased in Holstein cattle after the second parturition. The present study was carefully designed to select fertile animals by using animals that conceived by the first and second AI after biopsy of the endometrium at the every parity. This design largely reduced the number of animals, especially in dairy cattle, but allowed us to conclude that, even in the fertile population, peak concentrations of endometrial EGF decreased at the time when lactating Holstein cows were subjected to AI for the first time after parturition. Lactating dairy cows with an advanced parity may take a longer period to return the peak EGF concentrations to the levels found in heifers than dairy cows with lower numbers of parities and beef cows with similar parities. Alternatively, some physiological changes associated with high levels of lactation and repeated parturition may prevent the peak EGF concentrations from making a full recovery. It is of interest to examine how and when the peak EGF concentrations are restored after parturition to the levels found in heifers in lactating dairy cows.
A low incidence of alterations in the EGF profile in repeat breeder Holstein (dairy) heifers and Japanese Black (beef) cows may give us insights into the etiology of the alteration in the endometrial EGF profile. A number of studies of repeat breeder cows have shown endocrine disorders in the periovulatory and metestrus periods [9]. Some of these changes are also found in high-producing cows and have been linked to reduced fertility [12]. In the periovulatory period, a slow rise and low peak of plasma estradiol concentrations and a delayed LH surge have been found in repeat breeder [12, 13] and high-producing cows [12, 13]. In metestrus, a slow rise and low plasma progesterone level have also been described in repeat breeders [12, 14, 15]. In high-producing cows, disorders in the ovarian steroid hormone environment during the periovulatory period have been attributed to increased clearance of steroid hormones from the circulation, due to an increased blood flow to the liver resulting from high food intake for high levels of milk production [12]. These changes in the ovarian steroid environment during the periovulatory period may cause a loss or decrease in the EGF peak levels in lactating dairy cattle because synthesis of EGF is regulated by estrogen and progesterone in the endometrium [16,17,18]. Our previous findings may also support this hypothesis. Treatment with a high dose of estradiol benzoate (5 mg) with an intravaginal progesterone device normalized EGF profiles and restored fertility in lactating repeat breeder Holstein cows [7]. Plasma progesterone concentrations on day 5, but not at peaks, correlated with peak EGF concentrations on days 3 and 14 [8]. These findings have been interpreted to mean that insufficient stimulation of the uterine endometrium by estrogen and progesterone at estrus and metestrus, respectively, may be primary causes of alterations in the EGF profile.
However, some of these changes in endocrine disorders have also been found in repeat breeder heifers [19]. The degree and/or incidence of the alteration in the steroid hormone environment in lactating cows may be greater than those in heifers, or loss of the EGF peak may require some factors or physiological changes that are associated with high levels of lactation and parturition, in addition to disorders in the ovarian steroid hormone environment.
In Study 2, endometrial EGF concentrations were examined on day 3 only. This study design was employed after our experience in the diagnosis of the endometrial EGF profiles in more than 1000 cows [5,6,7, 10] by measuring EGF concentrations on days 3, 7 and 14 of the estrous cycle. In fertile cattle, endometrial EGF concentrations show 2 peaks, on days 2–4 and days 13–14, with low concentrations of EGF around day 7. Alterations of the endometrial EGF profile in lactating repeat breeder Holstein cows can be characterized by the absence of the 2 peaks in the EGF concentrations [5]. Few cows show an alteration in EGF concentrations around day 7, at which point the endometrial EGF concentrations show a basal level. Importantly, cows losing the first peak on days 2–4 also lose the second peak on days 13–14. About 85 and 10% of cows having low EGF peaks on day 3 have the EGF concentrations below or slightly above (within 0.5 ng/g tissue weight), respectively, the lower limit of the normal range on day 14 (4.9 ng/g tissue weight [6]). Accordingly, EGF concentrations on day 3 have been shown to be a good marker of fertility after embryo transfer in beef and dairy cows. In our previous study [10], a total of 437 cows were examined for endometrial EGF concentrations on day 3 and were transferred an embryo on day 7 of the same cycle. Recipient cows having EGF concentrations within a normal range on day 3 had a greater conception rate (76.9%) than those with low EGF concentrations (33.3%). Thus, a single examination of endometrial EGF concentrations on day 3 could be used in place of a complete examination of the EGF profile, with concentrations determined on days 3, 7 and 14. This finding may provide a great advantage when one wants to use the measurement of endometrial EGF concentrations as a tool to diagnose repeat breeder cows under field conditions because repeated biopsy is time- and labor-intensive for practitioners.
Acknowledgment
This study was supported by Grants-in-Aid for Scientific Research (No. 23580446, 20580350 and 15580284) from the Japan Society for the Promotion of Science.
References
- 1.Ayalon N. A review of embryonic mortality in cattle. J Reprod Fertil 1978; 54: 483–493 [DOI] [PubMed] [Google Scholar]
- 2.Levine HD. The repeat breeder cow. Bov Prac 1999; 33: 97–105 [Google Scholar]
- 3.Parkinson T. Infertility and subfertility in the cow: structural and functional abnormalities, management deficiencies and non-specific infections. In: Noakes DE, Parkinson TJ, England GCW (eds.), Veterinary Reproduction and Obstetrics, 9th ed. London: Saunders Elsevier; 2009: 393–475.
- 4.Peters AR. Embryo mortality in the cow. Anim Breed Abstr 1996; 64: 587–598 [Google Scholar]
- 5.Katagiri S, Takahashi Y. Changes in EGF concentrations during estrous cycle in bovine endometrium and their alterations in repeat breeder cows. Theriogenology 2004; 62: 103–112 [DOI] [PubMed] [Google Scholar]
- 6.Katagiri S, Takahashi Y. Potential relationship between normalization of endometrial epidermal growth factor profile and restoration of fertility in repeat breeder cows. Anim Reprod Sci 2006; 95: 54–66 [DOI] [PubMed] [Google Scholar]
- 7.Katagiri S, Takahashi Y. A progestin-based treatment with a high dose of estradiol benzoate normalizes cyclic change in endometrial EGF concentrations and restores fertility in repeat breeder cows. J Reprod Dev 2008; 54: 473–479 [DOI] [PubMed] [Google Scholar]
- 8.Katagiri S, Takahashi Y. Relationship between endometrial concentrations of epidermal growth factor (EGF) and preimplantation embryo development in dairy cattle. In: The 37th Annual Meeting of Society for the Study of Reproduction; 2004; Vancouver, Canada. Abstract 518.
- 9.Katagiri S, Moriyoshi M. The role of endometrial EGF to connect the alterations of the ovarian steroid hormone profile and embryonic loss in the repeat breeder and high producing dairy cows. J Reprod Dev 2013; 59, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katagiri S. Relationship between endometrial epidermal growth factor and fertility after embryo transfer. J Reprod Dev 2006; 52(Suppl): 133–137 [Google Scholar]
- 11.Katagiri S, Ho Yuen B, Moon YS. The role for the uterine insulin-like growth factor I in early embryonic loss after superovulation in the rat. Fertil Steril 1996; 65: 426–436 [DOI] [PubMed] [Google Scholar]
- 12.Wiltbank M, Lopez H, Sartori R, Sangsritavong S, Gumen A. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology 2006; 65: 17–29 [DOI] [PubMed] [Google Scholar]
- 13.Erb RE, Gaverick HA, Randel RD, Brown BL, Callahan CJ. Profile of reproductive hormones associated with fertile and non-fertile inseminations of dairy cows. Theriogenology 1976; 5: 227–242 [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Nakao T, Moriyoshi M, Kawata K. Luteal phase deficiency as a possible cause of repeat breeding in dairy cows. Br Vet J 1987; 143: 560–566 [DOI] [PubMed] [Google Scholar]
- 15.Shelton K, Gayerie de Abreu MF, Hunter MG, Parkinson TJ, Lamming GE. Luteal inadequacy during the early luteal phase of subfertile cows. J Reprod Fertil 1990; 90: 1–10 [DOI] [PubMed] [Google Scholar]
- 16.Brigstock DR. Growth factors in the uterus: steroidal regulation and biological actions. Baillieres Clin Endocrinol Metab 1991; 5: 791–808 [DOI] [PubMed] [Google Scholar]
- 17.DiAugustine RP, Petrusz P, Bell GI, Brown CF, Korach KS, McLachlan JA, Teng CT. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology 1988; 122: 2355–2363 [DOI] [PubMed] [Google Scholar]
- 18.Paria BC, Song H, Dey SK. Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol 2001; 45: 597–605 [PubMed] [Google Scholar]
- 19.Båge R, Gustafsson H, Larsson B, Forsberg M, Rodriguez MH. Repeat breeding in dairy heifers: Follicular dynamics and estrous cycle characteristics in relation to sexual hormone patterns. Theriogenology 2002; 57: 2257–2269 [DOI] [PubMed] [Google Scholar]