Abstract
Transforming growth factor (TGF) β and its receptors are expressed at the conceptus-maternal interface during early pregnancy in the pig. The present studies were conducted to examine: (1) the effect of conceptus products on TGFβ1 mRNA expression and protein concentration in the porcine endometrium using in vivo and in vitro models, and (2) the effect of TGFβ1 on proliferation of porcine trophoblast cells in vitro. During in vivo experiments, gilts with one surgically detached uterine horn were slaughtered on days 11 or 14 of the estrous cycle and pregnancy. For in vitro studies, endometrial explants and luminal epithelial (LE) cells co-cultured with stromal (ST) cells were treated with conceptus-exposed medium (CEM). Moreover, porcine trophoblast cells were treated with TGFβ1, and the number of viable cells was measured. On day 11, the presence of conceptuses had no effect on TGFβ1 mRNA expression, but decreased the TGFβ1 protein concentration in the connected uterine horn compared with the detached uterine horn. In contrast to day 11, on day 14 after estrus, TGFβ1 mRNA expression and protein content in the endometrium collected from the gravid uterine horn were greater when compared with the contralateral uterine horn. The treatment of endometrial slices with CEM resulted in greater TGFβ1 mRNA expression and protein secretion. LE cells responded to CEM with an increased TGFβ1 mRNA level. Moreover, TGFβ1 stimulated the proliferation of day 14 trophoblast cells. In summary, porcine conceptuses may regulate TGFβ1 synthesis in the endometrium at the time of implantation. TGFβ1, in turn, may promote conceptus development by increasing the proliferation of trophoblast cells.
Keywords: Conceptus, Endometrium, Pig, Pregnancy, TGFβ1
Successful implantation requires coordinated development of an embryo and the receptive endometrium, followed by an intimate dialogue between the conceptus and maternal cells [1,2,3]. Cytokines and their receptors belong to the factors involved in embryo-maternal interactions during early pregnancy [4, 5].
Transforming growth factor β (TGFβ) represents a family of structurally related cytokines, which also includes activins and bone morphometric proteins. Members of the TGFβ superfamily induce multiple cellular effects and have been shown to control proliferation, migration and apoptosis [6]. TGFβ participates in steroidogenesis, immunotolerance, embryogenesis and tissue remodeling [5, 7]. Moreover, TGFβ controls the expression of integrins and extracellular matrix proteins [8]. In mammals, three isoforms of TGFβ (TGFβ1, TGFβ2 and TGFβ3) have been identified and well characterized. Each of them is released from cells in a latent form, non-covalently associated with latency associated peptide (LAP). Once active, TGFβ acts via transmembrane, serine-threonine kinase type I and type II TGFβ receptors [9].
TGFβs are localized to the maternal-conceptus interface in numerous species and have been implicated in maternal-conceptus interactions during pregnancy [for a review, see: 10]. TGFβ1, TGFβ2 and TGFβ3, as well as TGFβ-RI and TGFβ-RII, are localized in embryonic and extraembryonic cells of porcine conceptuses on days 10 to 14 of pregnancy [11, 12]. Expression of all TGFβ isoforms is greater in the trophectoderm of day 14 filamentous conceptuses than in day 11 spherical ones [12]. Maternal expression of TGFβ1, TGFβ2 and TGFβ3 transcripts increases progressively in the uterine luminal epithelium and underlying stroma from days 10 to 14 of gestation [12] and is accompanied by increased expression of TGFβ proteins and type I and type II receptors [13]. Profiles of TGFβs and the expression of their receptors in the endometrium suggest a possible regulation by conceptus products; however, there is no data available demonstrating such mechanism. Therefore, the present studies were conducted to examine (1) the effect of conceptus presence on TGFβ1 mRNA expression and protein concentration in the porcine endometrium using an in vivo model, (2) the effect of conceptus secretions on TGFβ1 mRNA expression and protein secretion by the porcine endometrium in vitro and (3) the effect of TGFβ1 on the proliferation of porcine trophoblast cells in vitro. We focused on TGFβ1 because this isoform is the best characterized among mammals and its important role in the attachment of porcine conceptuses was previously demonstrated [14].
Materials and Methods
Animals and sample collection
All procedures involving the use of animals were conducted in accordance with the national guidelines for agricultural animal care and were approved by the Animal Ethics Committee, University of Warmia and Mazury in Olsztyn, Poland. In all experiments, 35 crossbred gilts (Large White × Polish Landrace) of similar genetic background from one commercial herd were used.
To examine the effect of conceptus presence (in vivo study) on TGFβ1 mRNA expression and protein concentration in the endometrium, 19 prepubertal gilts at 6.0–6.5 months of age with an average body weight of 100 kg were subjected to the surgical procedure described previously [15], with some modifications. Under general anesthesia, one uterine horn of each gilt was cut transversely, and the cut ends were closed with sutures. In this way, the uterus consisted of one intact uterine horn and one horn detached from the uterine corpus. After first estrus, gilts were injected with 10 mg PGF2α (Dinolytic; Pfizer, Puurs, Belgium) on day 14 of the estrous cycle. The next day, 10 mg of PGF2α was injected simultaneously with 750 IU PMSG (Folligon; Intervet, Boxmeer, The Netherlands), followed by 500 IU hCG (Chorulon; Intervet) 72 h later. Subsequently, gilts assigned to the pregnant group were inseminated 24 and 48 h after hCG injection. The day of the second insemination was designated as the first day of pregnancy. Gilts were slaughtered on days 11 (n=6) or 14 (n=5) of pregnancy. The remaining gilts were not inseminated and used as a control group to exclude the effect of surgery. These gilts were slaughtered on days 11 (n=4) or 14 (n=4) of the estrous cycle. After slaughter, each uterine horn of all gilts was washed with 20 ml of PBS. Pregnancy was confirmed by the morphology of conceptuses, which were flushed only from the connected uterine horn of pregnant animals. Conceptuses collected on day 11 were spherical (from 3 to 8 mm in diameter) or tubular (from 12 to 30 mm in length) in shape, while those obtained on day 14 were elongated. Endometrial tissue was dissected from the myometrium, snap-frozen in liquid nitrogen and stored at –80 C for further use.
To examine the effect of conceptus secretions (in vitro study) on TGFβ1 synthesis in the endometrium, 12 pubertal gilts of similar age (8–8.5 months) and weight (140–150 kg) were used. After exhibiting two estrous cycle, gilts assigned to be pregnant (n=6) were bred 12 and 24 h after detection of their third estrus. All these gilts were slaughtered on day 14 of pregnancy and used as a source of conceptuses for incubation. Therefore, uterine horns were closed with clamps and transported to the laboratory immediately. Gilts assigned to be cyclic (n=6) were slaughtered on day 12 of their third estrous cycle and used as a source of endometrial explants for incubations or LE and ST cells for culture.
Incubation of conceptuses
Day 14 conceptuses were collected from uteri by gentle flushing of each uterine horn with sterile phenol red-free Medium 199 (M3769; Sigma-Aldrich, St. Louis, MO, USA) containing 5% (v/v) of steroid-free newborn calf serum (NCS; Sigma-Aldrich) and antibiotics (100 IU/ml penicillin and 100 µg/ml streptomycin), as described previously [16]. All flushed conceptuses were elongated. Conceptuses were weighted and placed separately in culture flasks containing an appropriate amount (3 ml of medium per 40 mg of conceptus) of phenol red-free Medium 199 supplemented with 5% steroid-free NCS and antibiotics. Three conceptuses from each gilt were used. The incubation was performed for 24 h at 37 C in a humidified atmosphere of 95% air and 5% CO2 with gentle shaking. After incubation, media from all conceptuses obtained from each gilt were pooled together, centrifuged at 500 × g for 5 min and used as conceptus-exposed medium (CEM).
Endometrial explant incubation
Endometrial explants (100–110 mg per vial) were collected and preincubated in 2 ml of Medium 199 supplemented with 5% of charcoal-stripped NCS and antibiotics for 2 h, as described recently [17]. Then, the medium was removed, and endometrial tissue was treated with the control medium (phenol red-free Medium 199 containing 5% of charcoal-stripped NCS and antibiotics) or CEM mixed 3:1 with the control medium for 6 and 24 h. All treatments were performed in duplicate for each of six gilts. After incubation, the medium was collected and stored at –40 C for analysis of TGFβ1. Endometrial strips were snap-frozen in liquid nitrogen and stored at –80 C for RNA extraction.
Endometrial cell isolation and culture
LE and ST cells of the porcine endometrium were isolated as described in detail previously [16]. The viability of the cells was higher than 90% as assessed by 0.5% (w/v) trypan blue dye exclusion. Cells were plated in a co-culture system, in which ST cells were cultured at the bottom of wells in 6-well culture plates (basal compartment) and LE cells were cultured on collagen-coated inserts (BioCoat® Collagen I Cell Culture Inserts, BD Biosciences, Bedford, MA, USA; apical compartment). In the co-culture system, interactions between LE and ST cells are possible, and LE cells may mediate the effect of conceptus products on ST cells. Both cell types were cultured at a density of 2 × 106 cells per well in phenol red-free Medium 199 containing 10% NCS and antibiotics supplemented with estradiol (10 nM) and progesterone (100 nM). Cells were cultured for 72 h before initiation of the experiment, when monolayers were estimated to be 100% confluent. The homogeneity of cells was evaluated by immunofluorescent staining of cultured cells for the presence of cytokeratin and vimentin [18]. The purities of the LE and ST cell cultures were 95% and 90–95%, respectively.
To study the effect of conceptus products on TGFβ1 mRNA expression and protein secretion, LE cells cultured in the apical compartment were treated with the control medium (phenol red-free Medium 199 containing 5% steroid-free NCS and antibiotics) or CEM mixed 3:1 with the control medium. In the basal compartment, ST cells were treated with the control medium only. Incubation was performed for 24 h, because this period was effective in increasing the expression of endometrial receptivity markers in LE cells treated with CEM [16]. Then, culture media were collected from the apical and basal compartments and stored until further ELISA of TGFβ1. Cells were treated with Fenozol buffer (A&A Biotechnology, Gdansk, Poland) and stored until total RNA extraction.
Trophoblast cell isolation
Trophoblast cells were isolated from conceptuses collected from day 14 pregnant gilts (n=4), according to the method described recently [19]. The trophoblast was cut into small pieces and digested in 0.25% trypsin solution (Biomed, Lublin, Poland) for 30 min at 37 C. The cell suspension was filtered through two layers of gauze and centrifuged at 200 × g for 10 min. The obtained cells were washed three times, counted and resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM)/Nutrient Mixture F-12 Ham (Sigma-Aldrich), supplemented with 10% of NCS and antibiotics.
Proliferation assay
Trophoblast cells were seeded in 96-well plates at a density of 5 × 105 cells per well and cultured for 48 h, when monolayers reached 60–70% confluence. Then, the cells were incubated in serum-free DMEM/F-12 (control) or DMEM/F-12 supplemented with TGFβ1 (10 ng/ml; 240-B; R&D Systems, Minneapolis, MN, USA) or 20% NCS (used as a positive control based on previous results [19]). All treatments were performed in triplicate, using cells isolated from four separate gilts. After 24 h of treatment, 0.2% crystal violet in 10% ethanol were used to stain viable cells. The absorbance was determined colorimetrically at a wavelength of 550-nm.
Total RNA isolation and real-time PCR
Total RNA was extracted using a Total RNA Prep Plus kit (A&A Biotechnology) and treated with DNase I (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Samples were reverse transcribed using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA).
Real-time PCR was performed with an ABI Prism 7300 sequence detection system using Power SYBR Green PCR Master Mix (Applied Biosystems), as previously described [20]. To evaluate mRNA levels, specific primers were used (Table 1). For quantification, standard curves consisting of serial dilutions of the appropriate purified cDNA were included. The following PCR conditions were used: initial denaturation for 10 min at 95 C, followed by 37 cycles of 15 sec of denaturation at 95 C and 30 sec of annealing at 59 C, and then 60 sec of elongation at 72 C. After each PCR reaction, melting curves were obtained by stepwise increases in the temperature from 60 to 95 C to ensure single product amplification. Data obtained from the real-time PCR were normalized against GAPDH.
Table 1. Primers used for real-time PCR.
| Gene | Sequence (5’–3’) | Product size | EMBLa/Reference |
| TGFβ1 | Forward: GGA AAG CGG CAA CCA AAT Reverse: TCT GCC CGA GAG AGC AAT ACA |
120 | AF281156 / [20] |
| GAPDH | Forward: CCT TCA TTG ACC TCC ACT ACA TGG T Reverse: CCA CAA CAT ACG TAG CAC CAC GAT C |
183 | U48832 / [21] |
a GenBank Accession Number.
Preparation of homogenates of endometrial tissue
Endometrial tissue was homogenized in 500 μl of ice-cold homogenization buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl, 1% Triton X-100, 1 mM EDTA) containing 10 μl/ml Protease Inhibitor Cocktail (Sigma-Aldrich) in Lysing Matrix D (MP Biomedicals, Solon, OH, USA) with a FastPrep®-24 instrument (MP Biomedicals). Homogenates were then centrifuged for 10 min at 700 × g, and the supernatant was stored at –80 C for further analysis. The protein content was determined by the method of Bradford [22].
ELISA of TGFβ1
Concentrations of TGFβ1 in CEM, endometrial tissue homogenates and incubation medium were determined using a Quantikine Porcine TGFβ1 ELISA kit (MB100B; R&D Systems) according to the manufacturer’s protocol. Before assay, all samples were activated by the addition of 20 µl of 1 M HCl per 100 µl of sample. The sensitivity of the assay was 31.5 pg/ml.
Statistical analysis
Statistical analyses were conducted using GraphPad PRISM v. 5.0 (GraphPad Software, San Diego, CA, USA). To test the effect of conceptus presence in the uterine horn on TGFβ1 mRNA expression and protein concentration in the endometrium of gilts, statistical analysis was conducted using two-way ANOVA followed by Bonferroni’s post hoc test. This analysis included the effect of conceptus presence, reproductive status and conceptus presence x reproductive status interaction. To test the effect of CEM on TGFβ1 mRNA expression and protein secretion from endometrial explants and LE and ST cells, the paired t-test was performed. To test the effect of TGFβ1 and NCS on proliferation of trophoblast cells, one-way ANOVA followed by Bonferroni’s post hoc test was performed. Pig conceptuses produce and secrete TGFβs. Therefore, the concentration of TGFβ1 in CEM was subtracted from the amount of TGFβ1 determined in the culture medium and expressed as pg/ml. All numerical data are expressed as mean ± SEM, and differences were considered to be statistically different at P<0.05.
Results
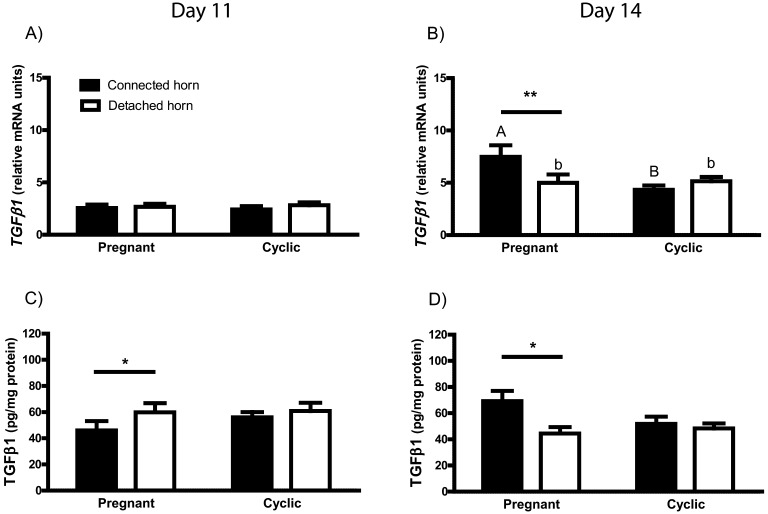
Neither the presence of conceptuses nor reproductive status affected TGFβ1 mRNA expression in the endometrium of gilts with one detached uterine horn that were slaughtered on day 11 after estrus (Fig. 1A). However, a decreased TGFβ1 protein concentration was observed in the gravid uterine horn when compared with the nongravid uterine horn of day 11 pregnant animals (P<0.05; Fig. 1C). In contrast to day 11, on day 14 after estrus, TGFβ1 mRNA expression in the endometrium collected from the gravid uterine horn was higher compared with both the contralateral uterine horn (P<0.01) and the respective horn of cyclic (control) animals (P<0.05). Moreover, a greater concentration of TGFβ1 protein was observed in the endometrium of the connected uterine horn containing developing conceptuses compared with the detached uterine horn of pregnant gilts (P<0.05; Fig. 1D).
Fig. 1.
TGFβ1 mRNA expression (A and B) and protein concentration (C and D) in the endometrium of gilts subjected to surgical procedure. Gilts were slaughtered on days 11 or 14 of the estrous cycle and early pregnancy. Values from real-time PCR were normalized to GAPDH. Data are expressed as means ± SEM (n=4–5). Letters above bars (capital letters for connected uterine horn and small letters for detached uterine horn) indicate differences between cyclic and pregnant gilts. Asterisks indicate differences between the connected and detached uterine horn in cyclic and pregnant gilts (*, P<0.05; **, P<0.01).
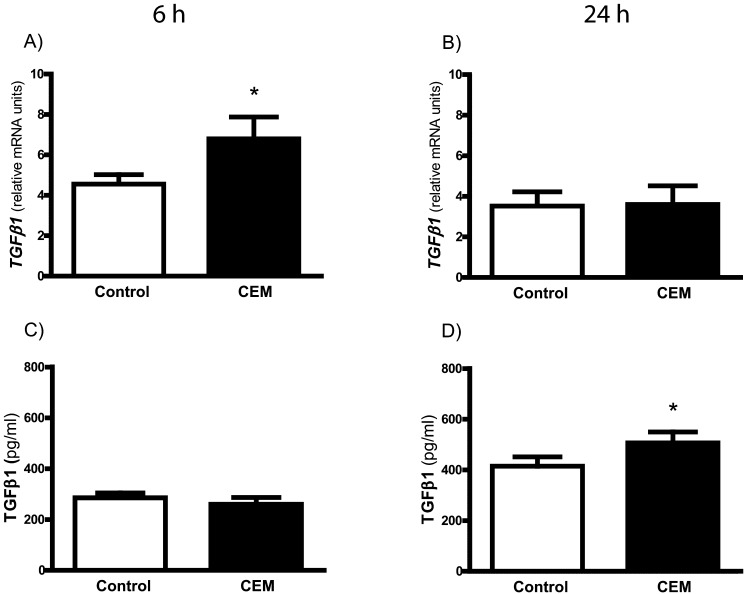
Figure 2 shows the effect of CEM on TGFβ1 mRNA expression in endometrial explants and protein content in the incubation medium. CEM treatment of endometrial slices for 6 h resulted in a greater mRNA level in the tissue when compared with the control value (P<0.05). No difference in TGFβ1 protein accumulation in the medium was observed. Longer period of incubation with CEM did not affect endometrial TGFβ1 mRNA expression but increased the concentration of TGFβ1 in the medium (414.7 ± 36.7 vs. 506.9 ± 42.8 pg/ml; P<0.05).
Fig. 2.
Effect of the conceptus-exposed medium (CEM) on TGFβ1 mRNA expression in endometrial explants (A and B) and protein concentration in the incubation medium (C and D). Endometrial slices were collected on day 12 of the estrous cycle and exposed to CEM for 6 and 24 h. Values from real-time PCR were normalized to GAPDH. Data are expressed as means ± SEM obtained from six experiments (gilts), each performed in duplicate. An asterisk indicates differences between the control and CEM-treated endometrium (*, P<0.05).
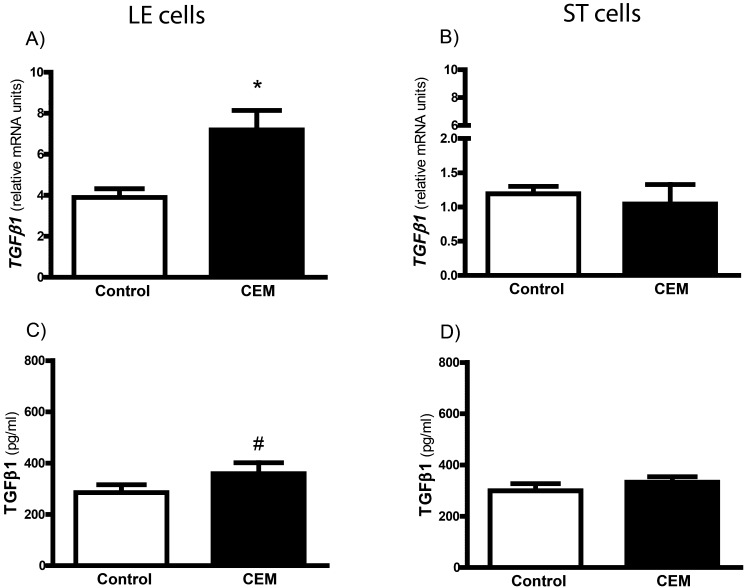
As demonstrated in Fig. 3, the addition of CEM to the apical compartment stimulated TGFβ1 mRNA expression in LE cells (P<0.05). It was accompanied by a tendency for greater TGFβ1 accumulation in the incubation medium (285.1 ± 30.6 vs. 358.9 ± 42.8 pg/ml; P=0.07; Fig. 3C). In contrast to LE cells, TGFβ1 mRNA expression in ST cells was not affected by CEM treatment (P=0.65). Moreover, no difference in TGFβ1 protein level in the culture medium collected from the basal compartment was detected (299.2 ± 27.8 vs. 332.7 ± 21.5 pg/ml; P=0.45; Fig. 3D).
Fig. 3.
Effect of the conceptus-exposed medium (CEM) on TGFβ1 mRNA expression in co-cultured LE and ST cells (A and B) and protein concentration in the incubation medium (C and D). Values from real-time PCR were normalized to GAPDH. Data are expressed as means ± SEM obtained from four experiments (gilts), each performed in duplicate. An asterisk indicates differences between the control and CEM-treated cells (*, P<0.05). A tendency for greater TGFβ1 secretion from LE cells after treatment with CEM is also indicated (#; P=0.07).
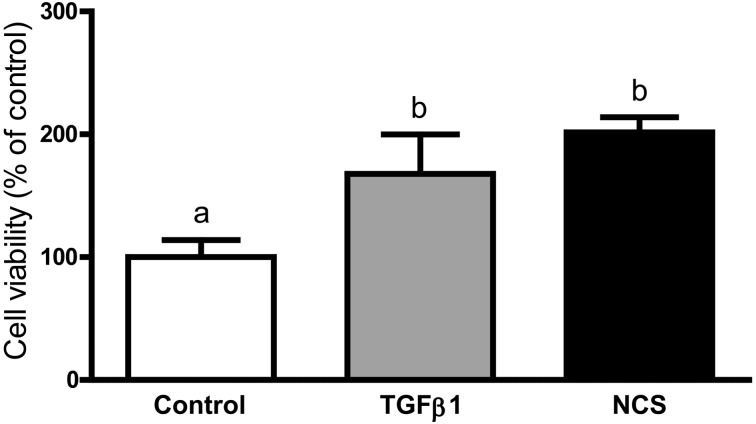
A significant increase in proliferation, measured as the number of viable trophoblast cells, was observed between the control group (serum-free medium) and cells exposed to 10 ng/ml of TGFβ1 (68% increase; P<0.05) or 20% NCS (100% increase; P<0.01; Fig. 4).
Fig. 4.
Effect of TGFβ1 (10 ng/ml) and 20% NCS on the proliferation of trophoblast cells. Cells were treated with medium only, TGFβ1 or NCS for 24 h. Then, cells were stained with 0.2% crystal violet. Results are expressed as the percentage of viable cells relative to the control value. Data are expressed as means ± SEM obtained from four experiments (gilts), each performed in triplicate. Letters above bars indicate differences.
Discussion
In pigs, a progressive increase in the expression of TGFβs in the endometrium between days 10 and 14 was demonstrated for pregnant animals [12, 13]. Moreover, bioactive TGFβs were detected in uterine luminal flushings on days 12 to 14 of gestation, but not on days 10 and 11 [13]. This indicates conceptus-dependent TGFβ synthesis and release from the porcine endometrium. This hypothesis was verified in the present study. We clearly demonstrated that conceptus presence (in vivo model) affected TGFβ1 mRNA and/or protein expression in the endometrium and that the effect was dependent on the morphology of conceptuses. On day 11 of pregnancy, when spherical or tubular conceptuses were found in the connected uterine horn, no difference in TGFβ1 mRNA expression was detected, but the protein concentration in the tissue decreased in the gravid uterine horn when compared with the nongravid uterine horn. In contrast to day 11, on day 14 of pregnancy, both mRNA and protein concentrations were greater in the endometrium of the uterine horn bearing conceptuses than in the endometrium of the detached uterine horn. Moreover, the mRNA level in the connected uterine horn of pregnant gilts was higher than in the respective horn of cyclic animals. Additionally, endometrial TGFβ1 mRNA expression in the gravid uterine horn was 3-fold greater on day 14 of pregnancy compared with day 11. This is consistent with the profile of TGFβ1 mRNA level on days 10 to 14 of gestation demonstrated previously [12, 13] and indicates that conceptuses may influence TGFβ1 synthesis in the endometrium during early pregnancy in the pig.
Our in vivo results were confirmed during in vitro experiments. A co-culture model of endometrial LE and ST cells and incubation of endometrial explants were used to study the effect of CEM on TGFβ1 synthesis. The addition of conditioned medium after incubation of day 14 conceptuses to endometrial explants resulted in greater TGFβ1 mRNA expression in the endometrium and protein content in the medium. LE cells responded to CEM with an increased TGFβ1 mRNA level, but only a tendency for greater protein secretion was observed. Moreover, the addition of CEM to LE cells resulted in greater TGFβ1 mRNA expression after 24 h, while endometrial explants responded to CEM earlier, after 6 h. All these differences between endometrial explants and LE cells may be due to experimental procedure. Strips of endometrium were treated with CEM on the same day of collection from slaughtered gilts, while LE cells were first isolated enzymatically and treated with CEM 72 h after seeding. Moreover, endometrial slices consisted of epithelial and stromal cells but also endothelial cells, macrophages. All these cells produce and secrete different proteins, which may affect other cells function, including sensitivity to exogenous factors. The co-culture system for LE and ST cells enables interactions between both cell types, which is more physiologically appropriate. However, neither mRNA expression nor protein concentration were changed in ST cells cultured in the basal compartment and not directly exposed to CEM. It indicates 1) that the effect of conceptus secretions is direct, not mediated by other cells, and/or 2) that the epithelium rather than the underlying stroma is the main source of increased concentrations of TGFβ1 in the uterine lumen during the early stage of implantation. Nevertheless, stromal cells also produce TGFβs [12, 13]. Therefore, epithelial-derived TGFβ1 could participate in conceptus-epithelial interactions, while TGFβ1 secreted by the stroma may play a role in regulation of tissue proliferation and apoptosis.
Despite the important role of TGFβ1 in conceptus-endometrial interactions crucial for pregnancy establishment, little is known about mechanisms regulating this cytokine expression in the endometrium. In women, TGFβs occur in all cell types of the endometrium, and their expression is upregulated during the period of increasing plasma progesterone concentrations and downregulated during progesterone withdrawal [7, 23]. In ewes, endometrial expression of TGFβs was greatest during proestrus, which coincides with a low plasma progesterone level and increasing estrogen concentrations [24, 25]. Similarly, estrogen administration results in a rapid increase of TGFβ2 expression in mice and rats [26, 27].
In pigs, the expression of TGFβ mRNA in uterine cells may also be regulated by conceptus-secreted estrogens [12]. In fact, a more than 3-fold increase in mRNA expression of all three isoforms was observed between days 10 and 12 of gestation in LE and ST cells [12]. This was accompanied by a progressive immunostaining of TGFβs proteins [13]. Similarly, conceptus secretion of estradiol increases more than 6-fold between the day 10.5 spherical and day 12 filamentous forms [28]. However, we did not confirm a stimulatory effect of developing conceptuses on day 11 of pregnancy, since no changes in mRNA level were observed and even lower protein content was detected in the endometrium of the gravid vs. nongravid uterine horn. The discrepancy between the present results and those obtained previously [12, 13] may be explained, in part, by the experimental animal models. In our study, gilts were inseminated at their hormonally-induced estrus, while in that performed by Gupta et al., gilts were bred at their natural estrus. Decreased expression of genes important for pregnancy establishment, including TGFβ1, was previously demonstrated in pregnant gilts with gonadotropin-induced estrus [20]. Thus, the effect of conceptus estrogens was not visible in the present results, probably due to decreased endometrial receptivity. Another explanation may be the amount of estrogens synthesized by conceptuses needed for upregulation of TGFβ1. Gupta et al. [12] showed a substantial increase in endometrial TGFβs between days 10 and 12 of pregnancy, but no comparison was made with day 11 of gestation. Moreover, no bioactive TGFβs were found on day 11 in the uterine lumen [13]. The uterine concentration of estradiol is greater on day 12 than on day 11 of pregnancy [29]. Thus, no effect of conceptus presence on TGFβ1 mRNA expression on day 11 of pregnancy observed in the present study may result from insufficient secretion of estrogens. Interestingly, a decreased protein level in the endometrium was observed in the gravid vs. nongravid uterine horn on day 11. Posttranscriptional regulation of TGFβ1 gene expression due to the presence of a stem-loop structure in the 5’ flanking region of this gene [30] may be responsible for the lower protein content.
Greater TGFβ1 mRNA and protein expression in the endometrium of the gravid uterine horn than in the nongravid uterine horn observed on day 14 of pregnancy may be a result of cell-to-cell contact between conceptuses and maternal tissue as well as of soluble mediators secreted by conceptuses. In the pig, initial attachment of the conceptus trophectoderm to the uterine epithelium starts at approximately day 13 of pregnancy, followed by more stable adhesions observed on day 16 [31]. These stable adhesions require both integrin receptors expressed on the trophectoderm and maternal LE and their ligands belonging to extracellular matrix (ECM). This includes LAP linked to TGFβ isoforms. Interestingly, mechanotransduction involving integrins and ECM proteins plays an important role in adhesion and remodeling of the conceptus [3]. Integrins expressed at the apical surfaces of the porcine uterine LE are rapidly activated by several ECM proteins, resulting in formation of focal adhesions, which serve to stimulate numerous intracellular pathways, including gene expression [3, 32]. Moreover, integrins can activate TGFβ by both conformational changes in the latent complex and traction forces such as cellular contractions and external stretching [33]. Nevertheless, our in vitro experiments showed that besides cell-to-cell contact, some soluble factor(s) secreted by day 14 conceptuses increased TGFβ1 expression. Since the estradiol concentration in the uterine lumen on day 14 of gestation is almost 8-fold lower than on day 12 [29], we suggest that estradiol of conceptus origin is not the main factor responsible for greater TGFβ1 expression found in our study. Among several proteins produced by conceptuses are interferons (IFN). Abundant IFNγ mRNA is detected in the trophectoderm between days 13 and 20, whereas IFNδ is expressed in day 14 conceptuses [34]. Moreover, IFN activity in uterine flushings increases significantly between days 12 and 16 of pregnancy [35], and IFN-dependent expression of several genes in the porcine endometrium during the peri-implantation period was demonstrated [36]. Therefore, IFNs may be involved in regulation of the expression of TGFβs in this tissue. However, further studies should be performed to define mechanisms responsible for greater endometrial TGFβ1 expression during early pregnancy.
Proposed regulatory roles of uterine TGFβ during early pregnancy include decidualization, trophoblast attachment, invasion, differentiation and embryogenesis [7]. Because TGFβ and its receptors are present at the fetal-maternal interface [12, 13, 26, 37,38,39], both auto- and paracrine actions are possible. TGFβ1 stimulates human cytotrophoblast cells [40] and porcine trophectoderm cells [14] to produce oncofetal fibronectin, which is important for trophoblast attachment to uterine tissue. TGFβ1 and integrins are involved in conceptus elongation and placental and fetal size [41]. Our present study demonstrated that TGFβ1 may stimulate porcine trophoblast cells proliferation, thus supporting conceptus survival and implantation. These results differ from those obtained in human, in which TGFβ1 inhibited proliferation of cytotrophoblast cell lines in vitro [42, 43]. On the other hand, exogenous TGFβ1 has a mitogenic effect on embryonic cells [44] and stimulates in vitro trophoblast outgrowth [45] in mice. Moreover, both TGFβ1 and TGFβ2 increase proliferation of bovine trophoblast cells [46]. Differences in the response to TGFβ among species may be explained, in part, by different types of implantation and placentation. Additionally, cellular response to TGFβs may be variable, promoting as well as antagonizing a variety of responses including proliferation, apoptosis and differentiation depending on the cell type and stimulation context [47]. Interestingly, TGFβ induces these responses via the same type I and II receptors and the same SMAD proteins. Moreover, integration of SMAD and non-SMAD signaling pathways determines the cellular response to TGFβ [48]. Therefore, the detailed mechanism of TGFβ1 action in porcine trophoblast cells needs further studies.
Besides its important role in conceptus development and attachment, TGFβ1 regulates the function of endometrial cells. It modulates maternal immunotolerance, regulates cytokine and hormone production and affects cell apoptosis during implantation [5, 7, 10]. In the pig, TGFβ receptors are present in endometrial cells [13], and phosphorylated-SMAD2/3 proteins were detected in nuclei of luminal and glandular epithelial cells, fibroblasts and endothelial cells on days 10 to 24 of pregnancy [33]. Thus, TGFβs may activate intracellular signaling leading to the expression of different genes and in this way control uterine function during early pregnancy in the pig.
In conclusion, to our knowledge, this is the first report demonstrating the direct effect of conceptus presence on TGFβ1 expression in the porcine endometrium. Day 14 elongated conceptuses increased TGFβ1 mRNA and protein content in the endometrium, indicating an important role of this cytokine during implantation. In addition to the significant role of TGFβ1 in the attachment of conceptuses to the maternal epithelium [14], we demonstrated that this cytokine may increase the proliferation of pig trophoblast cells.
Acknowledgments
This research was supported by grants DWM/N106/COST/2008 and 717/N-COST/2010/0 from the Ministry of Science and Higher Education of Poland and by a basic grant of the Polish Academy of Sciences.
References
- 1.Paria BC, Song H, Dey SK. Implantation: molecular basis of uterine dialogue. Int J Dev Biol 2001; 45: 597–605 [PubMed] [Google Scholar]
- 2.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 2006; 12: 731–746 [DOI] [PubMed] [Google Scholar]
- 3.Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction 2009; 138: 195–209 [DOI] [PubMed] [Google Scholar]
- 4.Ziecik AJ, Blitek A, Kaczmarek MM, Waclawik A, Bogacki M. Inhibition of luteolysis and embryo-uterine interactions during the peri-implantation period in pigs. Soc Reprod Fertil Suppl 2006; 62: 147–161 [PubMed] [Google Scholar]
- 5.Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med 2009; 27: 62–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β). Growth Factors 1993; 8: 1–9 [DOI] [PubMed] [Google Scholar]
- 7.Godkin JD, Doré JJ. Transforming growth factor beta and the endometrium. Rev Reprod 1998; 3: 1–6 [DOI] [PubMed] [Google Scholar]
- 8.Roberts AB, Heine UI, Flanders KC, Sporn MB. Transforming growth factor-β. Major role in regulation of extracellular matrix. Ann NY Acad Sci 1990; 580: 225–232 [DOI] [PubMed] [Google Scholar]
- 9.Massagué J. TGF-β signal transduction. Ann Rev Biochem 1998; 67: 753–791 [DOI] [PubMed] [Google Scholar]
- 10.Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-β superfamily expression and actions in the endometrium and placenta. Reproduction 2006; 132: 217–232 [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Bazer FW, Jaeger LA. Differential expression of beta transforming growth factors (TGFβ1, TGFβ2, and TGFβ3) and their receptors (type I and type II) in peri-implantation conceptuses. Biol Reprod 1996; 55: 796–802 [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Ing NH, Bazer FW, Bustamante LS, Jaeger LA. Beta transforming growth factors (TGFβ) at the porcine conceptus-maternal interface. Part I: Expression of TGFβ1, TGFβ2, and TGFβ3 messenger ribonucleic acids. Biol Reprod 1998; 59: 905–910 [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Dekaney CM, Bazer FW, Madrigal MM, Jaeger LA. Beta transforming growth factors (TGFβ) at the porcine conceptus-maternal interface. Part II: Uterine TGFβ bioactivity and expression of immunoreactive TGFßs (TGFβ1, TGFβ2, and TGFβ3) and their receptors (type II and type II). Biol Reprod 1998; 59: 911–917 [DOI] [PubMed] [Google Scholar]
- 14.Jaeger LA, Spiegel AK, Ing NH, Johnson GA, Bazer FW, Burghardt RC. Functional effects of transforming growth factor β on adhesive properties of porcine trophectoderm. Endocrinology 2005; 146: 3933–3942 [DOI] [PubMed] [Google Scholar]
- 15.Kamińska K, Wasielak M, Bogacka I, Blitek M, Bogacki M. Quantitative expression of lysophosphatidic acid receptor 3 gene in porcine endometrium during the periimplantation period and estrus cycle. Prostag Other Lipid M 2008; 85: 26–32 [DOI] [PubMed] [Google Scholar]
- 16.Blitek A, Morawska E, Kiewisz J, Ziecik AJ. Effect of conceptus secretions on HOXA10 and PTGS2 gene expression, and PGE2 release in co-cultured luminal epithelial and stromal cells of the porcine endometrium at the time of early implantation. Theriogenology 2011; 76: 954–966 [DOI] [PubMed] [Google Scholar]
- 17.Morawska E, Kaczmarek MM, Blitek A. Regulation of prostacyclin synthase expression and prostacyclin content in the pig endometrium. Theriogenology 2012; 78: 2071–2086 [DOI] [PubMed] [Google Scholar]
- 18.Blitek A, Ziecik AJ. Prostaglandins F2α and E2 secretion by porcine epithelial and stromal endometrial cells on different days of the estrous cycle. Reprod Domest Anim 2004; 39: 340–346 [DOI] [PubMed] [Google Scholar]
- 19.Blitek A, Morawska E, Ziecik AJ. Regulation of expression and role of leukemia inhibitory factor (LIF) and interleukin (IL)-6 in the uterus of early pregnant pigs. Theriogenology 2012; 78: 951–964 [DOI] [PubMed] [Google Scholar]
- 20.Blitek A, Kaczmarek MM, Kiewisz J, Ziecik AJ. Endometrial and conceptus expression of HoxA10, transforming growth factor β1, leukaemia inhibitory factor, and prostaglandin H synthase-2 in early pregnant pigs with gonadotropin-induced estrus. Domest Anim Endocrinol 2010; 38: 222–234 [DOI] [PubMed] [Google Scholar]
- 21.Bogacka I, Przala J, Siawrys G, Kamiński T, Smolinska N. The expression of short form of leptin receptor gene during early pregnancy in the pig examined by quantitative real time RT-PCR. J Physiol Pharmacol 2006; 57: 479–489 [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254 [DOI] [PubMed] [Google Scholar]
- 23.Chegini N, Zhao Y, Williams RS, Flanders K. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-β1 (TGF-β1), TGF-β2, TGF-β3, and TGF-β type II receptor messenger ribonucleic acid and protein and contains [125I] TGF-β1-binding sites. Endocrinology 1994; 135: 439–449 [DOI] [PubMed] [Google Scholar]
- 24.Doré JJ, Jr, Wilkinson JE, Godkin JD. Ovine endometrial expression of transforming growth factor-β isoforms during the peri-implantation period. Biol Reprod 1996; 54: 1080–1087 [DOI] [PubMed] [Google Scholar]
- 25.Doré JJ, Jr, Eberhardt DM, Jacobs WG, Godkin JD. Regulation of ovine endometrium transforming growth factor-βs by steroids. Am J Reprod Immunol 1996; 35: 459(P209) [Google Scholar]
- 26.Das SK, Flanders KC, Andrews GK, Dey SK. Expression of transforming growth factor-β isoforms (β2 and β3) in the mouse uterus: analysis of the pre-implantation period and effects of ovarian steroids. Endocrinology 1992; 130: 3459–3466 [DOI] [PubMed] [Google Scholar]
- 27.Schneider SL, Gollnick SO, Grande C, Pazik JF, Tomasi TB. Differential regulation of TGF-β2 by hormones in rat uterus and mammary glands. J Reprod Immunol 1996; 32: 125–144 [DOI] [PubMed] [Google Scholar]
- 28.Geisert RD, Renegar RH, Thatcher WW, Robrts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod 1982; 27: 925–939 [DOI] [PubMed] [Google Scholar]
- 29.Stone BA, Seamark RF. Steroid hormones in uterine washings and in plasma of gilts between days 9 and 15 after oestrus and between days 9 and 15 after coitus. J Reprod Fertil 1985; 75: 209–221 [DOI] [PubMed] [Google Scholar]
- 30.Kim S-J, Park K, Koeller D, Kim KY, Wakefield LM, Sporn MB, Roberts AB. Post-transcriptional regulation of the human transforming growth factor β1 gene. J Biol Chem 1992; 267: 13702–13707 [PubMed] [Google Scholar]
- 31.Keys JL, King GJ. Microscopic examination of porcine conceptus-maternal interface between days 10 and 19 of pregnancy. Am J Anat 1990; 188: 221–238 [DOI] [PubMed] [Google Scholar]
- 32.Jaeger LA, Johnson GA, Ka H, Garlow JG, Burghardt RC, Spencer TE, Bazer FW. Functional analysis of autocrine and paracrine signalling at the uterine-conceptus interface in pigs. Reproduction Suppl 2001; 58: 191–207 [PubMed] [Google Scholar]
- 33.Massuto DA, Kneese EC, Johnson GA, Burghardt RC, Hooper RN, Ing NH, Jaeger LA. Transforming growth factor beta (TGFβ) signaling is activated during porcine implantation: proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction 2010; 139: 465–478 [DOI] [PubMed] [Google Scholar]
- 34.Joyce MM, Burghardt RC, Geisert RD, Burghardt JR, Hooper RN, Ross JW, Ashworth MD, Johnson GA. Pig conceptuses secrete estrogen and interferons to differentially regulate uterine STAT1 in a temporal and cell type-specific manner. Endocrinology 2007; 148: 4420–4431 [DOI] [PubMed] [Google Scholar]
- 35.La Bonnardière C, Martinat-Botte F, Terqui M, Lefèvre F, Zouari K, Martal J, Bazer FW. Production of two species of interferon by Large White and Meishan pig conceptuses during the peri-attachment period. J Reprod Fertil 1991; 91: 469–478 [DOI] [PubMed] [Google Scholar]
- 36.Johnson GA, Bazer FW, Burghardt RC, Spencer TE, Wu G, Bayless KJ. Conceptus-uterus interactions in pigs: endometrial gene expression in response to estrogens and interferons from conceptuses. Soc Reprod Fertil Suppl 2009; 66: 321–332 [PubMed] [Google Scholar]
- 37.Chen HL, Yelavarthi KK, Hunt JS. Identification of transforming growth factor-β1 mRNA in virgin and pregnant rat uterus by in situ hybridization. J Reprod Immunol 1993; 25: 221–233 [DOI] [PubMed] [Google Scholar]
- 38.Selick CE, Horowitz GM, Gratch M, Scott RT, Jr, Novot D, Hofmann GE. Immunohistochemical localization of transforming growth factor-β in human implantation sites. J Clin Endocrinol Metab 1994; 78: 592–596 [DOI] [PubMed] [Google Scholar]
- 39.Lennard SN, Stewart F, Allen WR. Transforming growth factor-β1 expression in the endometrium of the mare during placentation. Mol Reprod Dev 1995; 42: 131–140 [DOI] [PubMed] [Google Scholar]
- 40.Feinberg RF, Kliman HJ, Wang GL. Transforming growth factor-β stimulates trophoblast oncofetal fibronectin synthesis in vitro: implications for trophoblast implantation in vivo. J Clin Endocrinol Metab 1994; 78: 1241–1248 [DOI] [PubMed] [Google Scholar]
- 41.Massuto DA, Hooper RN, Kneese EC, Johnson GA, Ing NH, Weeks BR, Jaeger LA. Intrauterine infusion of latency-associated peptide (LAP) during early porcine pregnancy affects conceptus elongation and placental size. Biol Reprod 2010; 82: 534–542 [DOI] [PubMed] [Google Scholar]
- 42.Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-β at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod 1992; 46: 561–572 [DOI] [PubMed] [Google Scholar]
- 43.Smith AN, Carter QL, Kniss DA, Brown TL. Characterization of a TGFβ-responsive human trophoblast-derived cell line. Placenta 2001; 22: 425–431 [DOI] [PubMed] [Google Scholar]
- 44.Lim J, Bongso A, Ratnam S. Mitogenic and cytogenetic evaluation of transforming growth factor-beta on murine preimplantation embryonic development in vitro. Mol Reprod Dev 1993; 36: 482–487 [DOI] [PubMed] [Google Scholar]
- 45.Nowak RA, Haimovici F, Biggers JD, Erbach GT. Transforming growth factor-beta stimulates mouse blastocyst outgrowth through a mechanism involving parathyroid hormone-related protein. Biol Reprod 1999; 60: 85–93 [DOI] [PubMed] [Google Scholar]
- 46.Munson L, Wilhite A, Boltz VF, Wilkinson JE. Transforming growth factor β in bovine placenta. Biol Reprod 1996; 55: 748–755 [DOI] [PubMed] [Google Scholar]
- 47.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003; 3: 807–821 [DOI] [PubMed] [Google Scholar]
- 48.Rahimi RA, Leof EB. TGF-β signaling: a tale of two responses. J Cell Biochem 2007; 102: 593–608 [DOI] [PubMed] [Google Scholar]