Abstract
Mitochondria are reported to be critical in in vitro maturation of oocytes and subsequent embryo development after fertilization, but their contribution for fertilization has not been investigated in detail. In the present study, we investigate the contribution of mitochondria to fertilization using reconstructed porcine oocytes by fusion of ooplasmic fragments produced by serial centrifugations (centri-fusion). Firstly, we evaluated the characteristics of ooplasmic fragments. Three types of fragments were obtained by centrifugation of porcine oocytes matured in vitro for 46 h: brownish (B), transparent (T) and large (L) fragments containing both B and T parts in a fragment. The production efficiencies of these types of fragments were 71.7, 91.0 and 17.8 fragments/100 oocytes, respectively. In experiments, L fragments were excluded because they contained both brownish and transparent components that were apparently intermediate between B and T fragments. Observations by confocal microscopy after staining with MitoTracker Red CMXRos® and transmission electron microscopy revealed highly condensed active mitochondria in B fragments in contrast to T fragments that contained only sparse organelles. We reconstructed oocytes by fusion of a karyoplast and two cytoplasts from B and T fragments (B and T oocytes, respectively). The B oocytes showed higher sperm penetration (95.8%) and male pronuclear formation rates (94.2%) by in vitro fertilization than T oocytes (66.7% and 50.0%, respectively). These results suggest that the active mitochondria in oocytes may be related to their ability for fertilization.
Keywords: Fertilization, Fusion, Mitochondria, Ooplasmic fragment, Pig
Mitochondria in oocytes play important roles in energy production and in regulation of reactive oxygen species and Ca2+ during maturation and embryonic development [1]. As both meiotic progression and cytoplasmic maturation consume intracellular energy in the form of ATP, both are dependent on the activity and distribution of mitochondria, which generate ATP [2]. ATP is also necessary for the process of oocyte activation during fertilization [3], and therefore, its quantity is considered to be an indicator of the quality of preimplantation mammalian oocytes/embryos [2, 4, 5, 6]. Changes in the distribution of mitochondria and their metabolic activity during oogenesis, maturation and embryo development have been reported in some species, including the mouse [7, 8], hamster [9], cattle [10], human [11] and pig [12, 13]. In porcine oocytes at the germinal vesicle stage, mitochondria are distributed in the peripheral region and thereafter migrate to the inner region of the oocyte during maturation; this phenomenon occurs both in vitro [12, 14] and in vivo [15]. This redistribution of mitochondria seems to be necessary for the ATP burst in oocytes that supplies energy for maturation [16]. In vitro maturation (IVM) may cause incomplete movement of mitochondria to the inner cytoplasm; therefore, this may affect cytoplasmic maturation [12]. Furthermore, it has been reported that extensive relocation of mitochondria to the inner cytoplasm during IVM of porcine oocytes is associated with high developmental competence [13, 17]. However, as no significant differences in ATP content between porcine oocytes with high and low competence have been detected, relocation of mitochondria may not necessarily be related to changes in overall mitochondrial activity [13]. El Shourbagy et al. [18], on the other hand, have suggested that mitochondrial content has an important bearing on fertilization outcome and reflects ooplasmic maturation status in pigs. However, the contribution of mitochondria to fertilization has not been documented in any previous report.
Recently, we have reported a novel method for transfer of cytoplasm between oocytes without the use of micromanipulation. This method, called “centri-fusion,” has been applied for reconstruction of oocytes and embryos from cytoplasmic fragments produced by serial centrifugation of IVM oocytes with full cytoplasmic maturity [19, 20, 21]. The fragments have also proven to be effective for restoration of cytoplasmic maturity in oocytes with compromised developmental competence [22]. Using this serial centrifugation approach, two morphologically different types of fragments (brownish and transparent fragments, B and transparent T fragments, respectively) can be distinguished, and our preliminary evaluation suggested that the only difference between them is in the distribution of their mitochondrial contents (K Kikuchi & Q Y Sun 2012, unpublished observations). However, their characteristics have not yet been evaluated in detail. It may be possible, after the characterization of the fragments, to reconstruct oocytes with different amounts of mitochondria.
The aim of the present study was to obtain direct evidence of the contribution of mitochondria to fertilization. For this purpose, we reconstructed oocytes with rich and poor mitochondria using B and T fragments, respectively. Prior to the main fertilization experiment, we assessed the mitochondria distribution in different types of fragments. Then, oocytes were reconstructed from a karyoplast and cytoplasts using either mitochondria-rich or mitochondria-poor fragments, and then their capacities for fertilization were compared.
Materials and Methods
Preparation and characterization of ooplasmic fragments
Collection and IVM of porcine oocytes were carried out as reported previously [23]. Briefly, porcine ovaries were obtained from prepubertal crossbred gilts (Landrace × Large White × Duroc) at a local slaughterhouse and transported to the laboratory at 33 C. Cumulus-oocyte complexes (COCs) were collected from follicles 2−6 mm in diameter in Medium 199 (M199; with Hanks’ salts, Sigma Chemical, St Louis, MO, USA) supplemented with 5% (v/v) fetal bovine serum (Tissue Culture Biologicals, Tulare, CA, USA), 20 mM HEPES (Dojindo Laboratories, Kumamoto, Japan), 100 U/ml penicillin G potassium (Sigma) and 0.1 mg/ml streptomycin sulfate (Sigma). About 40 COCs were cultured for 24 h in four-well dishes (Nunclon Multidishes; Nunc, Thermo Fisher Scientific, Roskilde, Denmark) containing 500 μl of IVM medium, which was a modified form of North Carolina State University (NCSU)-37 solution [24] containing 10% (v/v) porcine follicular fluid, 0.6 mM cysteine, 50 µM β-mercaptoethanol, 1 mM dibutyryl cAMP (Sigma), 10 IU/ml eCG (Serotropin; ASKA Pharmaceutical, Tokyo, Japan) and 10 IU/ml hCG (Puberogen 1500 U; Novartis Animal Health, Tokyo, Japan). They were subsequently cultured in IVM medium without dibutyryl cAMP and hormones for a further 22 h. Maturation culture was carried out under an atmosphere of 5% O2, 5% CO2 and 90% N2 at 39 C. Porcine follicular fluid was collected from ovaries of prepubertal crossbred gilts by aspiration with a syringe and centrifuged at 1,800 × g for 1.5 h, and the supernatant was stored at −20 C in advance. Then about l liter of the stock was thawed, mixed, centrifuged again, and stored at −20 C as a single batch until use.
Cytoplasmic fragments were obtained by serial centrifugation using the so-called centri-fusion method originally described by Fahrudin et al. [19]. Briefly, oocytes with a first polar body after IVM were transferred to 1.5-ml microcentrifuge tubes (approximately 100 oocytes per tube) and then centrifuged at 13,000 × g for 9 min in M199 at 36 C to stratify the cytoplasm (the 1st centrifugation). To remove the zona pellucida, the oocytes were exposed to 0.5% (w/v) pronase (Sigma, protease P-8811) in M199 for 20−30 sec. Those with an expanded and deformed zona pellucida were then transferred to M199 without pronase and freed completely from the zona pellucida by gentle pipetting. After several washings in M199, a group of approximately 100 zona-free oocytes was layered on a 300-μl discontinuous gradient (consisting of 100-μl layers of 7.5%, 30% and 45% solutions, from top to bottom, respectively) of Percoll (Amersham Biosciences, Uppsala, Sweden) in M199 supplemented with 5 μg/ml cytochalasin B (Sigma) in microcentrifuge tubes. These gradients were then subjected to centrifugation at 6,000 × g for 4 sec (the 2nd centrifugation). The resulting cytoplasmic fragments floating mainly on the 30% gradient were collected and then washed several times to remove the Percoll. Cytoplasmic fragments were selected and washed in M199. Fragments around 60−70 μm in size that were a light brownish (B fragments) or transparent (T fragments) color and those with a large size (more than 100 μm, L fragments; in many cases, L fragments contained both brownish and transparent parts) were selected and categorized into three groups (the B, T and L fragment groups, respectively) (Fig. 1A–C). Lipid-containing dark fragments were discarded. The groups were then stained with 5 μg/ml Hoechst 33342 (Calbiochem, La Jolla, CA, USA) for 20 min and briefly examined using a fluorescence microscope (Olympus BX-51, Tokyo, Japan). Those with and without a set of metaphase chromosomes were defined as karyoplasts and cytoplasts, respectively. The numbers of each fragment type were recorded. All types of fragments were kept separately in M199 until use. The exact diameters of fragments in all three groups were measured from images taken using an inverted microscope with a digital camera. The experiments to determine the number of fragments produced per 100 matured oocytes and the yields of cytoplasts from all the fragments obtained, were replicated at least 8 times.
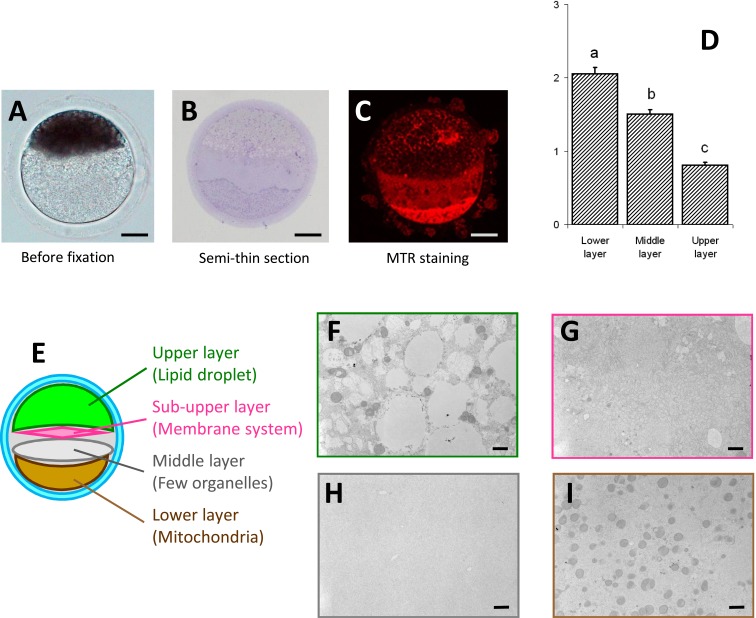
Fig. 1.
Matured oocytes were subjected to the first centrifugation at 13,000 × g for 9 min and photographed before fixation (A), after semi-thin sectioning (B) and after labeling of active mitochondria with MitoTracker Red CMXRos (MTR) (C). Images were taken using a light microscope without (A) and with (B) Hoffman modulation contrast and also by laser scanning confocal microscopy (C). Under these observation conditions, three layers (lower, middle and upper) were clearly distinguishable. The mitochondrial contents (D) of the three layers of centrifuged oocytes are shown as means ± SEM. a−c Significant differences (P<0.05) in relative fluorescence intensity of active mitochondria were evident among the three layers. Three trials were performed. The data in the chart are based on measurements of 31 oocytes. Observation using transmission electron microscopy (E) revealed a very thin additional layer (sub-upper layer) (G). The upper layer contained predominantly lipid droplets with mitochondria clustered around them (F). The lower layer contained condensed mitochondria (I). The sub-upper layer contained mainly membrane systems such as the endoplasmic reticulum (G). On the other hand, the middle layer contained only sparse organelles in comparison with the other three layers (H). Scale bars indicate 30 µm and 1 µm for plates A−C and F−I, respectively.
Evaluation of mitochondrial distribution in the fragments
Oocytes and B and T fragments were incubated with 200 nM MitoTracker Red CMXRos (MTR; Molecular Probes, Eugene, OR, USA), which selectively stains active mitochondria, for 30 min in Dulbecco’s phosphate-buffered saline (PBS; Nissui Pharmaceutical, Tokyo, Japan) containing 3% polyvinyl pyrrolidone (Sigma) under culture conditions. The oocytes and fragments were washed three times (10 min each) in pre-warmed PBS and then fixed for 20 min in 2% paraformaldehyde in PBS. The oocytes and fragments were then washed twice in PBS, mounted carefully with PBS on glass slides under cover slips with minimum pressure and examined immediately at room temperature in a dark room. Oocytes and fragments in all groups were processed simultaneously using the same batches of working solutions under minimum room light. The distribution of mitochondria stained with MTR was investigated in oocytes and fragments cut into 5-µm sections using a laser-scanning confocal microscope (Olympus FV-300) equipped with an argon-krypton-helium/neon ion laser using a 543-nm excitation barrier filter. To measure the distribution of mitochondria, an equatorial section image at the largest diameter of each oocyte or fragment was taken using the same setting. The abundance of active mitochondria in the equatorial section of the oocyte/fragment was evaluated by measuring numbers of pixels in inverted grayscale images using the NIH ImageJ (v. 1.40) software package. Relative fluorescence intensity of mitochondria expressed as the mean number of MTR-positive pixels in the total area of the oocyte/fragment equatorial section surface (mitochondrial fluoresce intensity) was measured. In all experiments, the intensity of intact oocytes that were not subjected to the centrifugation process was used as a comparative standard value (mean = 1) for the other groups. The experiments for mitochondrial fluorescence intensity were replicated three times using at least 10 oocytes in each replication.
Transmission electron microscopy (TEM) analysis of the fragments
Oocytes after the 1st centrifugation and B and T fragments after the 2nd centrifugation were fixed in 2.5% (v/v) glutaraldehyde in sodium cacodylate buffer (0.145 M, pH 7.2−7.4) overnight and stored in the same solution at 4 C until processing. After postfixation for 90 min in 1% (w/v) osmium tetroxide in the cacodylate buffer, specimens were dehydrated in an ethanol series (50−99%), equilibrated with propylene oxide and finally embedded in Epon 812 Resin (TAAB Laboratories Equipment, Berks, UK). Semi-thin sections and continuous ultrathin sections of oocytes and fragments were processed conventionally for TEM. Semi-thin sections for light microscopy were stained with toluidine blue. Thereafter, ultrathin sections were cut with a diamond knife (Sakai Electron Microscopy Application Laboratory, Saitama, Japan), stained with 2% uranyl acetate and 2% lead citrate and examined using a TEM (JEM-1010, JEOL, Tokyo, Japan) at 80 kV. At least five oocytes or fragments for each category were evaluated.
Oocyte reconstruction with a karyoplast and two cytoplasts
To produce reconstructed oocytes (B and T oocytes) in each group of B and T fragments, one karyoplast was initially aggregated with two cytoplasts that had been treated with 300 μg/ml phytohemagglutinin (PHA; Sigma L-2646). Prior to electro-fusion, pairs of aggregated complexes of the two groups were equilibrated stepwise with fusion solution consisting of 0.28 M mannitol, 0.05 mM CaCl2, 0.1 mM MgSO4 and 1 mg/ml bovine serum albumin (BSA, Fraction V, Sigma) [25]; the complexes in M199 were transferred sequentially to a 2:1 mixture of M199:fusion solution for 1 min and then to a 1:2 mixture for a further 1 min before final equilibration in the fusion solution. About ten pairs of aggregated complexes were aligned manually in a fusion chamber and given a single DC pulse for 30 min after aggregation. They were then incubated for 1 h in the same maturation medium. Fusion was confirmed at 1 h after stimulation if a karyoplast formed a single mass with the aggregated cytoplasts. Completely fused reconstructed oocytes (B and T oocytes) were subsequently subjected to in vitro fertilization (IVF).
IVF and culture
The oocytes or reconstructed oocytes were subjected to IVF [23]; they were coincubated with frozen-thawed epididymal spermatozoa from a Landrace boar [26] for 3 h under 5% CO2 and 5% O2 at 39 C in pig fertilization medium [27] supplemented with 2 mM caffeine and 5 mg/ml BSA. Thereafter, they were gently washed free of attached spermatozoa. In vitro culture was carried out in IVC-PyrLac [23] using the Well of the Well system [28]. Some oocytes were fixed at 10 h after insemination and further subjected to aceto-orcein staining to evaluate their fertilization status. The rates of female pronucleus (FPN) formation, monospermic fertilization (single-sperm penetration verified by the presence of a male pronucleus (MPN) and a sperm tail in the cytoplasm), MPN formation and normal fertilization (defined as FPN formation and a single MPN in the ooplasm) were scored. The experiment was replicated 5 times.
Statistical analysis
All data were expressed as means ± SEM. The data for parameters in different types of fragments were analyzed by Student’s t-test. The data for IVF were subjected to ANOVA followed by Tukey’s test. Percentage data were subjected to arcsine transformation before analysis. The analyses were carried out using the GLM procedure of the Statistical Analysis System (Ver. 9.2, SAS Institute, Cary, NC, USA). Differences at a probability value (P) of less than 0.05 were considered to be significant.
Results
Characterization of ooplasmic fragments
After the 1st centrifugation of matured oocytes, three layers (upper, middle and lower) were clearly distinguishable by light microscopy (Fig. 1A–C). Furthermore, TEM observations also revealed a very thin additional layer containing membrane systems such as the endoplasmic reticulum (sub-upper layer) (Fig. 1E–I). The upper layer contained predominantly lipid droplets with mitochondria clustered around them. The lower layer contained condensed mitochondria. The sub-upper layer contained mainly membrane systems such as the endoplasmic reticulum. On the other hand, the middle layer contained only a few organelles in comparison with the other three layers. We compared the active mitochondrial fluorescent intensity and found that the lower layer contained a significantly larger volume of mitochondria than those of the other layers (Fig. 1D).
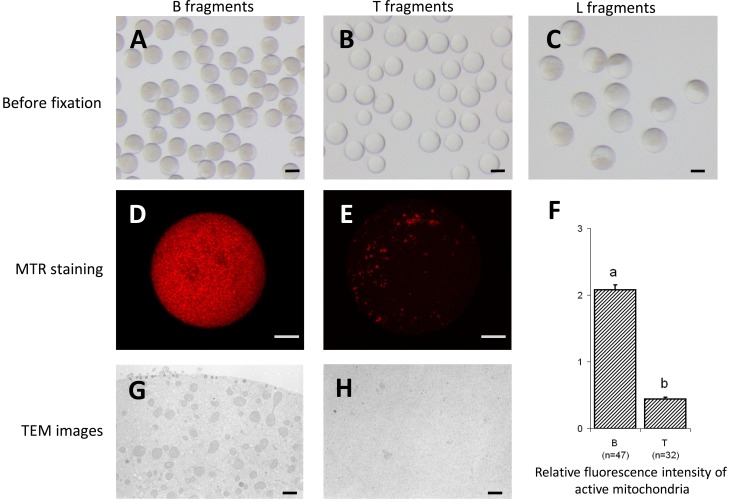
After the second centrifugation, we obtained three kinds of fragments (B, T and L fragments) that were distinguishable by stereomicroscopy (Fig. 2A–C, Table 1). The diameter of the fragments differed significantly among the groups (B, smallest; T, intermediate; and L, biggest). For every 100 matured oocytes that were serially centrifuged, the number of fragments obtained in the groups decreased significantly in the order of T > B > L. The percentages of cytoplasts (fragments without metaphase chromosomes) also decreased in the same order (P<0.05). In subsequent experiments, L fragments were excluded because of their low quantity and the fact that they contained both brownish and transparent components that were apparently intermediate between B and T fragments. We used both B and T fragments because the relative active mitochondrial fluorescent intensities of B fragments were much higher than those of T fragments (P<0.05) (Fig. 2D and E, respectively), indicating that the former had almost five times as many mitochondria as the latter (Fig. 2F). These characteristics were also confirmed by TEM observations (Fig. 2G and H). Based on these observations, the middle layer was considered to be the source of the T fragments after the 2nd centrifugation, and it had an intermediate mitochondrial fluorescent intensity (1.5 ± 0.1). The lower layer of the centrifuged oocytes might have contributed to the B fragments, and it had the highest mitochondrial fluorescent intensity (2.0 ± 0.1) among the layers (Fig. 1D).
Fig. 2.
After the 2nd centrifugation at 6,000 × g for 4 sec, three types of fragments – brownish (B), transparent (T) and large (L) – (A, B and C, respectively) were obtained. The distribution after staining with MitoTracker Red CMXRos (MTR) (D, B fragment; E, T fragment) and relative fluorescence intensities of active mitochondria in the B and T fragments are shown (F). Data for relative fluorescence intensity of active mitochondria are given as means ± SEM. a,b A significant difference (P<0.05) in relative fluorescence intensity of active mitochondria was evident between the two groups. Three trials were performed. Total numbers of oocytes measured in each group are given in parentheses. Transmission electron microscopy demonstrated that B fragments contained condensed mitochondria (G), similar to the lower layer of oocytes after the first centrifugation (see Fig. 1I), whereas T fragments had few organelles (H), suggesting that they originated from the middle layer (see Fig. 1H). Scale bars indicate 50 µm, 10 µm and 1 µm for plates A−C, D and F and G and I, respectively.
Table 1. Comparison between brownish, transparent and large fragments produced by serial centrifugation of porcine matured oocytes*.
| Fragment | Color | Diameter (µm) (Total number of oocytes examined) |
Number of fragments produced per 100 porcine DO46 oocytes (Total number of fragments/oocytes) |
Rate of cytoplasts (Total number of cytoplasts/fragments) |
| Brownish (B) | Brown | 61.2 ± 0.4a (122) | 71.7 ± 2.5a (1374/1915) | 67.6 ± 2.4a (926/1374) |
| Transparent (T) | Colorless | 72.1 ± 0.6b (81) | 91.0 ± 2.9b (1247/1915) | 75.2 ± 2.0b (935/1247) |
| Large (L) | Contain both brown and colorless areas |
148.5 ± 1.3c (25) | 17.8 ± 3.8c (202/1915) | 38.17 ± 8.2c (70/145) |
* Oocytes were cultured with intact cumulus cell layers for the full in vitro maturation period (46 h). Data are presented as means ± SEM. For diameter measurement, 3 replicated trials were carried out. For determining the number of fragments produced per 100 matured oocytes and the rates of cytoplasts derived from the fragments, 8 to 15 replications were used. Cytoplasts were defined as fragments without metaphase plates. For relative mitochondrial intensity, 3 replications were carried out in which images were taken at the equatorial plane of oocytes or fragments by laser scanning confocal microscopy after labeling active mitochondria with MitoTracker Red CMXRos (MTR) at ×400 magnification using the same setting, and data were compared with those for matured oocytes. Large fragments (L) were not included in this experiment due to their low numbers. In any row, data with different superscripts are significantly different (P<0.05).
Fertilization competence of B and T reconstructed oocytes
Sperm were able to penetrate into B and T oocytes, and an MPN(s) was formed successfully. Fertilization events were observable directly without any fixation or staining procedures like those in mice or human oocytes because the reconstructed oocytes did not contain lipid droplets (Fig. 3). To obtain solid data, however, we conducted evaluations after fixation and staining of the oocytes. It was found that the sperm penetration rate for B oocytes was significantly higher than that for T oocytes (P<0.05). Almost all B oocytes formed an MPN(s), whereas only half of the T oocytes did so, and the rate was significantly lower than that for B oocytes (P<0.05) (Table 2).
Fig. 3.
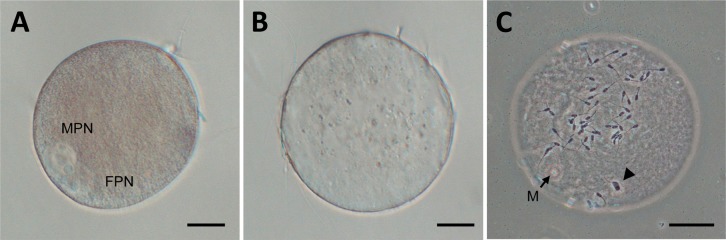
Oocytes reconstructed with B and T fragments (B and T oocytes, respectively) were in vitro fertilized and then subsequently in vitro cultured for 10 h (A, B oocyte; B and C, T oocyte). They were examined before fixation (A and B) or after fixation and staining (C). In a B oocyte, both male and female pronuclei (MPN and FPN, respectively) were clearly observed. In a T oocyte, no pronuclei were observed, but it was found that the oocyte was not activated but was arrested at the metaphase-II stage (M; metaphase chromosomes, out of focus); however, it was penetrated by a spermatozoon, which had undergone decondensation (indicated by an arrowhead). Scale bars indicate 30 µm.
Table 2. Fertilization and pronucleus formation in oocytes* reconstructed from brownish (B) and transparent (T) fragments after in vitro fertilization.
| Group of oocytes reconstructed | Number of reconstructed oocytes | Number (%) of oocytes penetrated | Number (%) of oocytes with MPN formation |
| B oocyte | 72 | 69 (95.8 ± 2.2)a | 65 (94.2 ± 3.8)a |
| T oocyte | 24 | 16 (66.7 ± 20.4)b | 8 (50.0 ± 19.1)b |
* Reconstruction of oocytes was carried out with one karyoplast and two cytoplasts for each of the B and T fragments (B and T oocytes). MPN: male pronucleus, defined by the presence of a sperm tail located nearby. In each row, data with different superscripts are significantly different (P<0.05).
Discussion
In our previous study, we demonstrated that fusion of ooplasmic fragments to incompetent oocytes (oocytes matured in vitro for 24 h, showing low developmental ability after IVF) contributed to enhancement of their fertilization and subsequent development [22]. However, in that study, we did not investigate the characteristics (i.e., organelle distribution and ATP content) of the fragments, and therefore, the cellular mechanism behind the improvement of cytoplasmic maturity remained unknown. In the present study, three types of fragments – brownish (B), transparent (T) and large (L) – were identified, showing differences in appearance (color), size (diameter) and mitochondrial content. As shown in Figs. 1 and 2, according to the similarity in the relative mitochondrial fluorescence intensities of the B and T fragments and those of the layers of centrifuged oocytes, it can be inferred that the B fragments originated from the lower layer of the centrifuged oocytes, where mitochondria were condensed, whereas T fragments originated from the middle layer of the oocytes, which contained very few or almost no mitochondria. TEM observations further confirmed the origin of the B and T fragments based on the similarity of the organelle composition of B to the lower mitochondria-rich layer and of T to the middle layer containing no organelles. L fragments, on the other hand, might have been derived from cytoplasm located near the border of the middle and lower layers. The intracellular layer structure of porcine oocytes revealed after the 1st centrifugation at 13,000 × g in the present study confirmed the results of previous studies using porcine and sheep oocytes [29] and bovine oocytes [29, 30]. The sub-upper layer in porcine oocytes could not be distinguished by light microscopy. A vesicular layer, which was reported in cattle by Tatham et al. [30], was not frequently observed in our study.
The contribution of mitochondria itself to fertilization has not been investigated well, because mitochondria have roles in many physiological events such as energy production, regulation of reactive oxygen species and Ca2+ regulation [1]. To perform investigation in physiological experiments, one of the methods involves use of an inhibitor; for example, rotenone is used to reduce electron transport, resulting in reduced ATP production [31]. However, the effect on other mitochondrial functions cannot be investigated in this manner. This kind of methodology sometimes causes difficulty in analysis of physiological events. In the present study, on the other hand, we could obtain ooplasmic fragments with rich and scare mitochondrial contents (B and T fragments, respectively) and also reconstruct two kinds of oocytes based on mitochondrial contents (B and T oocytes, respectively). Using these reconstructed oocytes, we could assess the contribution of mitochondria to fertilization directly. After IVF, the B oocytes showed higher rates of sperm penetration and MPN formation than the T oocytes (Fig. 3, Table 2). Our previous study [21] demonstrated that zona-free oocytes without further manipulation show higher rates for both categories (78.0% and 96.6% for penetration and MPN formation rates, respectively). The MPN formation rate especially is comparative to that for B oocytes in the present study. El Shourbagy stated that mitochondria directly influence fertilization outcome in the pig [18]. However, the mechanism responsible for the different rates between types of fragments in the present study cannot be explained well. Mitochondria generate energy (ATP) and contribute to events of oocyte maturation and embryonic development [10, 11, 14]. However, to our knowledge, there have been only limited reports showing direct evidence that mitochondria or ATP affects fertilization potential such as by affecting sperm penetration, oocyte activation and formation of the FPN and MPN after gamete membrane fusion. Elevating the ATP content in bovine oocytes by supplementation with bovine follicular fluid promoted fertilization even when mitochondrial electron transport was blocked with rotenone in oocytes prior to in vitro fertilization [32]. Some papers support the positive correlation between ATP accumulation and enhancement of fertilization in humans [33, 34]. In the present study, B cytoplasm contained a large amount of mitochondria, whereas T cytoplasts were proven to have only few organelles, including mitochondria. This difference in mitochondrial content might have been responsible for the lower fertilization potential in T oocytes. In other words, reconstructed ooplasm containing a low number of mitochondria may not contribute to sperm penetration and MPN formation after IVF, thus confirming a previous study that demonstrated the importance of mitochondria during fertilization events [34]. Mitochondria present in oocytes have been thought to act as a relay in Ca2+ signaling at fertilization [3, 35]. On the other hand, oocyte activation has a tremendous relationship with Ca2+ release from endoplasmic reticulum [36−38]. We consider that these organelles were located in the sub-upper layer after the first centrifugation; however, their fate after the second centrifugation has not yet been confirmed, even by TEM analysis in the present study. Activation potential resulting in completion of fertilization should be also necessary in relation to the distribution of these organelles in both types of cytoplasts. In addition, it has been reported that microinjection of ooplasm into bovine oocytes does not affect their parthenogenetic development [39, 40]. Developmental ability after fertilization of reconstructed porcine oocytes should be checked elsewhere.
In the present study, we could generate oocytes with almost all lipid droplets removed by the centri-fusion method. The droplets could be polarized into a big mass after the 1st centrifugation, the mass was automatically extruded after the 2nd centrifugation, and finally only lipid droplet-free ooplasmic fragments were obtained. As shown in Fig. 3, we could observe fertilization events (such as sperm penetration and pronuclear formation) clearly in a live status without fixation. A disadvantage of using porcine oocytes compared with mouse or human oocytes is the inability to directly observe the cytoplasm because of the existence of ooplasmic lipid droplets. Using this procedure, however, we can overcome this disadvantage for species having lipid droplet-rich oocytes such as pigs and cattle. Another advantage of ooplasm without lipid droplets seems to be for cryopreservation. Lipids, in general, are greatly harmed by peroxidation during freezing (cryopreservation) and thawing [41]. In porcine embryos, delipation (removal of lipid droplets by micromanipulation) has been advantageous for porcine embryo freezing [42−44]; if we can generate embryos from oocytes lacking lipids by this procedure, cryopreservation efficacy may be improved. Further experiments will be needed to check for potential advantages.
In conclusion, mitochondria-rich and mitochondria-poor ooplasmic fragments were produced successfully by the centri-fusion method. The results of accelerated fertilization in vitro of mitochondria-rich oocytes suggest that the mitochondria in oocytes may be related to their ability to be fertilized
Acknowledgments
This study was supported by scholarships from the Japanese Ministry of Education, Culture, Sports, Science and Technology for foreign students (VL N, DN TQ and M NT). The study was also supported in part by Grants-in-Aid for Scientific Research (22380153 to K K and 21380715 to H K) from the Japan Society for the Promotion of Science (JSPS) and also by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) (106.12-2012.93 to VL N). The authors would like to thank Ms F Yukuhiro for TEM analysis and also Ms K Kaneko, M Osaki and M Nagai for technical assistance.
References
- 1.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009; 20: 346–353 [DOI] [PubMed] [Google Scholar]
- 2.Krisher RL, Brad AM, Herrick JR, Sparman ML, Swain JE. A comparative analysis of metabolism and viability in porcine oocytes during in vitro maturation. Anim Reprod Sci 2007; 98: 72–96 [DOI] [PubMed] [Google Scholar]
- 3.Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 2004; 131: 3057–3067 [DOI] [PubMed] [Google Scholar]
- 4.Quinn P, Wales RG. The in vitro metabolism of [U-14C]-glucose by the preimplantation mouse embryo. Aust J Biol Sci 1973; 26: 653–667 [DOI] [PubMed] [Google Scholar]
- 5.Slotte H, Gustafson O, Nylund L, Pousette A. ATP and ADP in human pre-embryos. Hum Reprod 1990; 5: 319–322 [DOI] [PubMed] [Google Scholar]
- 6.Van Blerkom J, Davis P, Alexander S. Inner mitochondrial membrane potential (DeltaPsim), cytoplasmic ATP content and free Ca2+ levels in metaphase II mouse oocytes. Hum Reprod 2003; 18: 2429–2440 [DOI] [PubMed] [Google Scholar]
- 7.Van Blerkom J, Runner MN. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat 1984; 171: 335–355 [DOI] [PubMed] [Google Scholar]
- 8.Calarco PG. Polarization of mitochondria in the unfertilized mouse oocyte. Dev Genet 1995; 16: 36–43 [DOI] [PubMed] [Google Scholar]
- 9.Ludwig TE, Squirrell JM, Palmenberg AC, Bavister BD. Relationship between development, metabolism, and mitochondrial organization in 2-cell hamster embryos in the presence of low levels of phosphate. Biol Reprod 2001; 65: 1648–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod 2001; 64: 904–909 [DOI] [PubMed] [Google Scholar]
- 11.Wilding M, Dale B, Marino M, Di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 2001; 16: 909–917 [DOI] [PubMed] [Google Scholar]
- 12.Sun QY, Lai L, Wu GM, Park KW, Day BN, Prather RS, Schatten H. Microtubule assembly after treatment of pig oocytes with taxol: correlation with chromosomes, gamma-tubulin, and MAP kinase. Mol Reprod Dev 2001; 60: 481–490 [DOI] [PubMed] [Google Scholar]
- 13.Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod 2005; 72: 1218–1223 [DOI] [PubMed] [Google Scholar]
- 14.Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, Day BN, Prather RS, Schatten H. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction 2001; 122: 155–163 [PubMed] [Google Scholar]
- 15.Torner H, Brüssow KP, Alm H, Ratky J, Pöhland R, Tuchscherer A, Kanitz W. Mitochondrial aggregation patterns and activity in porcine oocytes and apoptosis in surrounding cumulus cells depends on the stage of pre-ovulatory maturation. Theriogenology 2004; 61: 1675–1689 [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol 2010; 224: 672–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brevini TA, Vassena R, Paffoni A, Francisci C, Fascio U, Gandolfi F. Exposure of pig oocytes to PCBs during in vitro maturation: effects on developmental competence, cytoplasmic remodelling and communications with cumulus cells. Eur J Histochem 2004; 48: 347–356 [PubMed] [Google Scholar]
- 18.El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction 2006; 131: 233–245 [DOI] [PubMed] [Google Scholar]
- 19.Fahrudin M, Kikuchi K, Karja NWK, Ozawa M, Maedomari N, Somfai T, Ohnuma K, Noguchi J, Kaneko H, Nagai T. Development to the blastocyst stage of porcine somatic cell nuclear transfer embryos reconstructed by the fusion of cumulus cells and cytoplasts prepared by gradient centrifugation. Cloning Stem Cells 2007; 9: 216–228 [DOI] [PubMed] [Google Scholar]
- 20.Nagai T, Kikuchi K, Imai K, Fahrudin M. Handmade somatic cell cloning and its related studies in farm animals. J Mamm Ova Res 2007; 24: 99–106 [Google Scholar]
- 21.Maedomari N, Kikuchi K, Nagai T, Fahrudin M, Kaneko H, Noguchi J, Nakai M, Ozawa M, Somfai T, Nguyen LV, Ito J, Kashiwazaki N. Nuclear replacement of in vitro-matured porcine oocytes by a serial centrifugation and fusion method. Reprod Domest Anim 2010; 45: 659–665 [DOI] [PubMed] [Google Scholar]
- 22.Viet Linh N, Kikuchi K, Nakai M, Noguchi J, Kaneko H, Dang-Nguyen TQ, Maedomari N, Nguyen BX, Nagai T, Manabe N. Improvement of porcine oocytes with low developmental ability after fusion of cytoplasmic fragments prepared by serial centrifugation. J Reprod Dev 2011; 57: 620–626 [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi K, Onishi A, Kashiwazaki N, Iwamoto M, Noguchi J, Kaneko H, Akita T, Nagai T. Successful piglet production after transfer of blastocysts produced by a modified in vitro system. Biol Reprod 2002; 66: 1033–1041 [DOI] [PubMed] [Google Scholar]
- 24.Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil Suppl 1993; 48: 61–73 [PubMed] [Google Scholar]
- 25.Nakai M, Kashiwazaki N, Takizawa A, Maedomari N, Ozawa M, Noguchi J, Kaneko H, Shino M, Kikuchi K. Morphologic changes in boar sperm nuclei with reduced disulfide bonds in electrostimulated porcine oocytes. Reproduction 2006; 131: 603–611 [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi K, Nagai T, Kashiwazaki N, Ikeda H, Noguchi J, Shimada A, Soloy E, Kaneko H. Cryopreservation and ensuing in vitro fertilization ability of boar spermatozoa from epididymides stored at 4 degrees C. Theriogenology 1998; 50: 615–623 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki K, Asano A, Eriksson B, Niwa K, Nagai T, Rodriguez-Martinez H. Capacitation status and in vitro fertility of boar spermatozoa: effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int J Androl 2002; 25: 84–93 [DOI] [PubMed] [Google Scholar]
- 28.Vajta G, Peura TT, Holm P, Paldi A, Greve T, Trounson AO, Callesen H. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev 2000; 55: 256–264 [DOI] [PubMed] [Google Scholar]
- 29.Cran DG. The distribution of organelles in mammalian oocytes following centrifugation prior to injection of foreign DNA. Gamete Res 1987; 18: 67–76 [DOI] [PubMed] [Google Scholar]
- 30.Tatham BG, Sathananthan AH, Dharmawardena V, Munesinghe DY, Lewis I, Trounson AO. Centrifugation of bovine oocytes for nuclear micromanipulation and sperm microinjection. Hum Reprod 1996; 11: 1499–1503 [DOI] [PubMed] [Google Scholar]
- 31.Basu SS, Blair IA. Rotenone-mediated changes in intracellular coenzyme A thioester levels: implications for mitochondrial dysfunction. Chem Res Toxicol 2011; 24: 1630–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somfai T, Inaba Y, Watanabe S, Geshi M, Nagai T. Follicular fluid supplementation during in vitro maturation promotes sperm penetration in bovine oocytes by enhancing cumulus expansion and increasing mitochondrial activity in oocytes. Reprod Fertil Dev 2012; 24: 743–752 [DOI] [PubMed] [Google Scholar]
- 33.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011; 11: 797–813 [DOI] [PubMed] [Google Scholar]
- 34.Van Blerkom J, Davis P. Mitochondrial signaling and fertilization. Mol Hum Reprod 2007; 13: 759–770 [DOI] [PubMed] [Google Scholar]
- 35.Dumollard R, Duchen M, Sardet C. Calcium signals and mitochondria at fertilisation. Semin Cell Dev Biol 2006; 17: 314–323 [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-triphosphate/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol 1993; 158: 62–78 [DOI] [PubMed] [Google Scholar]
- 37.Swann K. Soluble sperm factors and Ca2+ release in eggs at fertilization. Rev Reprod 1996; 1: 33–39 [DOI] [PubMed] [Google Scholar]
- 38.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 1999; 211: 157–176 [DOI] [PubMed] [Google Scholar]
- 39.Takeda K, Tasai M, Iwamoto M, Onishi A, Tagami T, Nirasawa K, Hanada H, Pinkert CA. Microinjection of cytoplasm or mitochondria derived from somatic cells affects parthenogenetic development of murine oocytes. Biol Reprod 2005; 72: 1397–1404 [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, Tasai M, Akagi S, Matsukawa K, Takahashi S, Iwamoto M, Srirattana K, Onishi A, Tagami T, Nirasawa K, Hanada H, Pinkert CA. Microinjection of serum-starved mitochondria derived from somatic cells affects parthenogenetic development of bovine and murine oocytes. Mitochondrion 2010; 10: 137–142 [DOI] [PubMed] [Google Scholar]
- 41.Tarín JJ, Trounson AO. Effects of stimulation or inhibition of lipid peroxidation on freezing-thawing of mouse embryos. Biol Reprod 1993; 49: 1362–1368 [DOI] [PubMed] [Google Scholar]
- 42.Nagashima H, Kashiwazaki N, Ashman RJ, Grupen CG, Nottle MB. Cryopreservation of porcine embryos. Nature 1995; 374: 416 [DOI] [PubMed] [Google Scholar]
- 43.Nagashima H, Hiruma K, Saito H, Tomii R, Ueno S, Nakayama N, Matsunari H, Kurome M. Production of live piglets following cryopreservation of embryos derived from in vitro-matured oocytes. Biol Reprod 2007; 76: 900–905 [DOI] [PubMed] [Google Scholar]
- 44.Li R, Murphy CN, Spate L, Wax D, Isom C, Rieke A, Walters EM, Samuel M, Prather RS. Production of piglets after cryopreservation of embryos using a centrifugation-based method for delipation without micromanipulation. Biol Reprod 2009; 80: 563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]