Abstract
The Japanese quail (Coturnix japonica) is a valuable bird as both an experimental animal, for a wide range of scientific disciplines, and an agricultural animal, for the production of eggs and meat. Cryopreservation of PGCs would be a feasible strategy for the conservation of both male and female fertility cells in Japanese quail. However, the effects of freeze-thaw treatment on viability, migration ability and germline transmission ability of quail PGCs still remain unclear. In the present study, male and female PGCs were isolated from the blood of 2-day-old embryos, which were cooled by slow freezing and then cryopreserved at –196 C for 77–185 days, respectively. The average recovery rate of PGCs after freeze-thawing was 47.0%. The viability of PGCs in the frozen group was significantly lower than that of the control group (P<0.05) (85.5% vs. 95.1%). Both fresh and Frozen-thawed PGCs that were intravascularly transplanted into recipient embryos migrated toward and were incorporated into recipient gonads, although the number of PGCs settled in the gonads was 48.5% lower in the frozen group than in the unfrozen control group (P<0.05). Genetic cross analysis revealed that one female and two male recipients produced live progeny derived from the frozen-thawed PGCs. The frequency of donor-derived offspring was slightly lower than that of unfrozen controls, but the difference was not significant (4.0 vs. 14.0%). These results revealed that freeze-thaw treatment causes a decrease in viability, migration ability and germline transmission ability of PGCs in quail.
Keywords: Cryopreservation, Germline chimera, Japanese quail, Primordial germ cells
The Japanese quail (Coturnix japonica) is a species of quail found in East Asia. It is a migratory species that breeds in Manchuria, southeastern Siberia, northern Japan, and the Korean Peninsula, and winters in the southern regions of Japan and China. The wild Japanese quail is classified as vulnerable (i.e., a species facing a high risk of extinction in the wild) by the Japanese Ministry of Environment Red List. At present, domesticated Japanese quails for industry are maintained as live birds. However, infectious disease poses a constant threat to live birds. Indeed, seven quail farms in the Aichi Prefecture, Japan, were found to be infected with a low pathogenic avian influenza virus (H7N6) in February and March 2009. All quails on the seven infected farms were destroyed, including the breeding quails. This corresponded to ~27% of the quails raised in Japan at the time and led to a delay in the supply of hatching eggs and chicks [1]. Cryobanking provides an effective conservation measure for use in conjunction with the preservation of natural populations. However, techniques for freezing ova and embryos, although used widely for some mammalian species, are not available for birds because of their large size and yolk. Semen can be cryopreserved from some poultry, such as the chicken, duck, goose and turkey [2, 3], but semen cryopreservation has not been successful for the Japanese quail [4]. Recent studies have demonstrated that ovarian and testicular tissues can be cryopreserved and subsequently recovered following transplantation into the Japanese quail [5, 6]. Cryopreservation of ovaries will be beneficial for the preservation of female reproductive potential in the Japanese quail. However, the use of testes for genetic conservation appears to be restricted by the complicated process involved in the production of offspring using surgical insemination and its low fertility. Transplantation of primordial germ cells (PGCs), which are the embryonic precursors of gametes, may allow for the cryopreservation of female and male fertility in avian species [7].
Unlike other species, in avian embryos, PGCs are transported via blood circulation to the developing gonadal region [8, 9]. This unique accessibility of avian PGCs during early development provides an opportunity to collect and transplant PGCs. Germline chimeric chickens have been produced by the transfer of PGCs collected from the germinal crescent region [10], blood [11,12,13,14], and gonads [15, 16], and these chickens were able to produce live offspring. In addition to chickens, techniques for the production of germline chimeras using PGCs have also been established for the Japanese quail [17, 18]. It has been demonstrated that chicken PGCs can be cryopreserved using simple protocols, while still maintaining their commitment to the germline [19,20,21]. Chang et al. [22] were the first to produce germline chimeric quails by the transfer of frozen-thawed gonadal cell suspensions of 5-day-old embryos that had been in culture for 12–16 h prior to cryopreservation, indicating that gonadal cells, presumably germ cells, maintained their commitment to the germline after freeze-thawing. However, the frequency of donor-derived offspring in their study was very low (0~2.5%). Therefore, practical cryobanking is required to improve the frequency of donor-derived progeny from germline chimeric quails. Additionally, the effect of freeze-thaw treatment on quail PGCs still remains unclear. Isolation of quail PGCs is one of the approaches to solve such issues. In the present study, we therefore clarified the effects of freeze-thaw treatment on viability, migration ability and germline transmission ability of isolated quail PGCs.
Materials and Methods
Animal care and use
Fertilized eggs from wild-type (WT) Japanese quails, maintained at the NARO Institute of Livestock and Grassland Science (NILGS), and the recessive dotted white (DW) mutant of the Japanese quail, generously provided by Dr Shinichi Ito (Faculty of Applied Biological Sciences, Gifu University, Gifu, Japan), were obtained by natural mating. All animal care and use in this study was conducted in accordance with the animal experimentation guidelines issued by the Animal Care and Use Committee of NILGS.
Experimental design
Blood was retrieved from the dorsal aorta of 2-day-old DW embryos, and PGCs were isolated by density gradient centrifugation. In the frozen group, DW PGCs were cryopreserved in liquid nitrogen for at least 77 days. Recovery and viability of DW PGCs were analyzed after thawing. Frozen-thawed or fresh DW PGCs were subsequently transferred to the dorsal aorta of 2-day-old WT embryos. Recipient embryos were incubated until they reached stage 28 or until hatching. Gonadal migration of frozen-thawed PGCs was evaluated in recipient embryos. The germline transmission of frozen-thawed PGCs was demonstrated by genetic cross analysis.
Collection of donor PGCs
Fertilized DW eggs were incubated for 40–42 h to obtain stage 14–16 [23] embryos at 39 C and relative humidity of 50–60% with 90° tilting once an hour in a forced-air incubator (P-008B Biotype; Showa Furanki, Saitama, Japan). Blood (1–3 μl) was drawn from the dorsal aorta and peripheral vein of the embryos using a fine glass micropipette under a microscope (MS5; Leica Microsystems, Tokyo, Japan). Each collected blood sample was suspended in 100 μl Ca2+- and Mg2+-free phosphate-buffered saline [PBS(–)]. After sexing each sample, male and female blood samples were pooled separately in the PBS(–). Male and female PGCs were purified from embryonic blood using Nycodenz density gradient centrifugation [24], in which 10% fetal bovine serum (FBS; Gibco/Invitrogen, Grand Island, NY, USA) in PBS(–) was used as the buffer instead of KAv-1 medium [25]. To assess gonadal migration of the donor PGCs, some isolated cells were labeled with PKH26 (Zynaxis, Malvern, PA, USA), a fluorescent lipophilic carbocyanine dye, according to the protocol of Yamamoto et al. [26]. Avian PGCs are easily distinguished from blood cells by their large size and the presence of a considerable number of refractive granules in the cytoplasm, as observed under a phase contrast microscope [27]. PGCs were picked up through a fine grass micropipette from the isolated cell suspension under a phase contrast microscope (IX71; Olympus, Tokyo, Japan).
Freezing and thawing of donor PGCs
In the frozen group, PGCs were suspended in 200 μl Cell Banker 1 (Juji Field, Tokyo, Japan) in a 1.2-ml cryotube (Nalgene, Rochester, NY, USA) and then cryopreserved. In the unfrozen control group, PGCs were used immediately for viability testing and transfer. Cryotubes were placed in a freezing container (Nalgene Cryo 1°C Freezing Container; Nalgene) and stored at –80 C overnight. Then, the cryotubes were cryopreserved in liquid nitrogen (–196 C) for 77–185 days. For thawing, the cryotubes were taken out of the liquid nitrogen and immediately placed in water at 39 C until the ice had melted. After thawing, DW PGCs were washed with 1 ml of 10% FBS in PBS(–) and then centrifuged at 200 × g for 4 min at room temperature to remove the cryomedium. The supernatant was removed, and approximately 20 μl of the cell suspension was placed in a plastic dish. Recovered cells from each vial, which were defined as morphologically normal PGCs after freeze thawing, were counted under a phase contrast microscope. After counting, PGCs were used for viability testing and subsequent transplantation.
Viability assay of donor PGCs
Viability assays were performed for the frozen and control groups before germline chimera production. In the frozen group, recovered DW PGCs were pooled by sex to avoid variation in viability among the vials, and a sample was used for viability testing. Ten microliters of a 0.4% trypan blue solution (w/v) was added to 20 μl of the PGC suspension, and the mixture was incubated for 2 min at room temperature. The viability of PGCs in both groups was then determined by trypan blue exclusion [28].
Gonadal migration of donor PGCs
WT embryos were incubated for 38–40 h, until they reached stage 14, under the same conditions as described above. A window (approx. 0.5 cm in diameter) was opened at the sharp end of each eggshell (after making a small hole at the blunt end). Approximately 1 μl of blood was collected from the dorsal aorta of WT embryos. Each collected blood sample was diluted to 100 μl with PBS(–) and used for sex determination. After sexing, 30 DW PGCs labeled with PKH26 from the frozen groups or control groups were microinjected through the dorsal aorta into the bloodstream of a recipient WT embryo of the same sex. Manipulated embryos were cultured further, and whole gonads were collected at stage 28. The number of PGCs labeled with PKH26 in the gonads was counted under a fluorescence microscope (DFC480-Note OY; Leica Microsystems).
Germline chimera production
Recipient WT embryos were prepared using the same procedures as described above. One hundred DW PGCs were picked up from the cell suspensions of the frozen or control group. DW PGCs were then transplanted into the dorsal aorta of recipient embryos of the same sex. Manipulated embryos were incubated until hatching under the conditions described above.
Sexing
Tissues obtained from donor DW embryos were digested in 70 μl of buffer [10 mM Tris-HCl (pH 7.5), 5 mM EDTA, 0.5% Tween 20, and 50 μg/ml proteinase K] by vigorously vortexing for 20 min at room temperature. Blood samples from recipient WT embryos were digested in 10 μl of the same buffer using the same method. Molecular sexing was conducted by detecting the conserved regions of the CHD-W and CHD-Z genes using the primers 2550 F (5′-GTTACTGATTCGTCTACGAGA-3′) and 2718 R (5′-ATTGAAATGATCCAGTGCTTG-3′). The polymerase chain reaction (PCR) conditions were 94 C for 1 min, followed by 52 cycles of 94 C for 5 sec and 65 C for 10 sec. PCR products were electrophoresed on a 1.5% agarose gel. DNA was visualized by ethidium bromide staining.
Genetic cross testing
Donor PGCs were obtained from DW Japanese quails displaying some pigmentation of the WT plumage on a white background, which is controlled by the third allele (sdw) of the s locus with sdw/sdw [29]. Recipient WT Japanese quails had brown feathers (s+/s+). Manipulated recipient Japanese quails derived from PGC transfers that survived to sexual maturity were mated with DW (sdw/sdw) Japanese quails of the opposite sex, and then the feather color of their offspring was examined. White offspring with spots (sdw/sdw) were derived from DW donor PGCs, whereas brown offspring (s+/ sdw) were derived from recipient WT PGCs.
Production of DW progeny by mating germline chimeras
To produce DW offspring using the sperm and eggs derived from donor PGCs, male and female germline chimeras were mated, and the feather color of their offspring was examined.
Statistical analysis
All data are presented as means ± standard error (SE). Survival and hatching rates of the embryos were analyzed using χ2 tests. Differences in cell viability, the number of PGCs labeled with PKH-26 within recipient gonads and the proportions of donor-derived offspring were compared between the frozen and control groups using unpaired Student’s t-tests. Differences were considered significant at P<0.05.
Results
Recovery and viability rates of PGCs after freeze-thawing
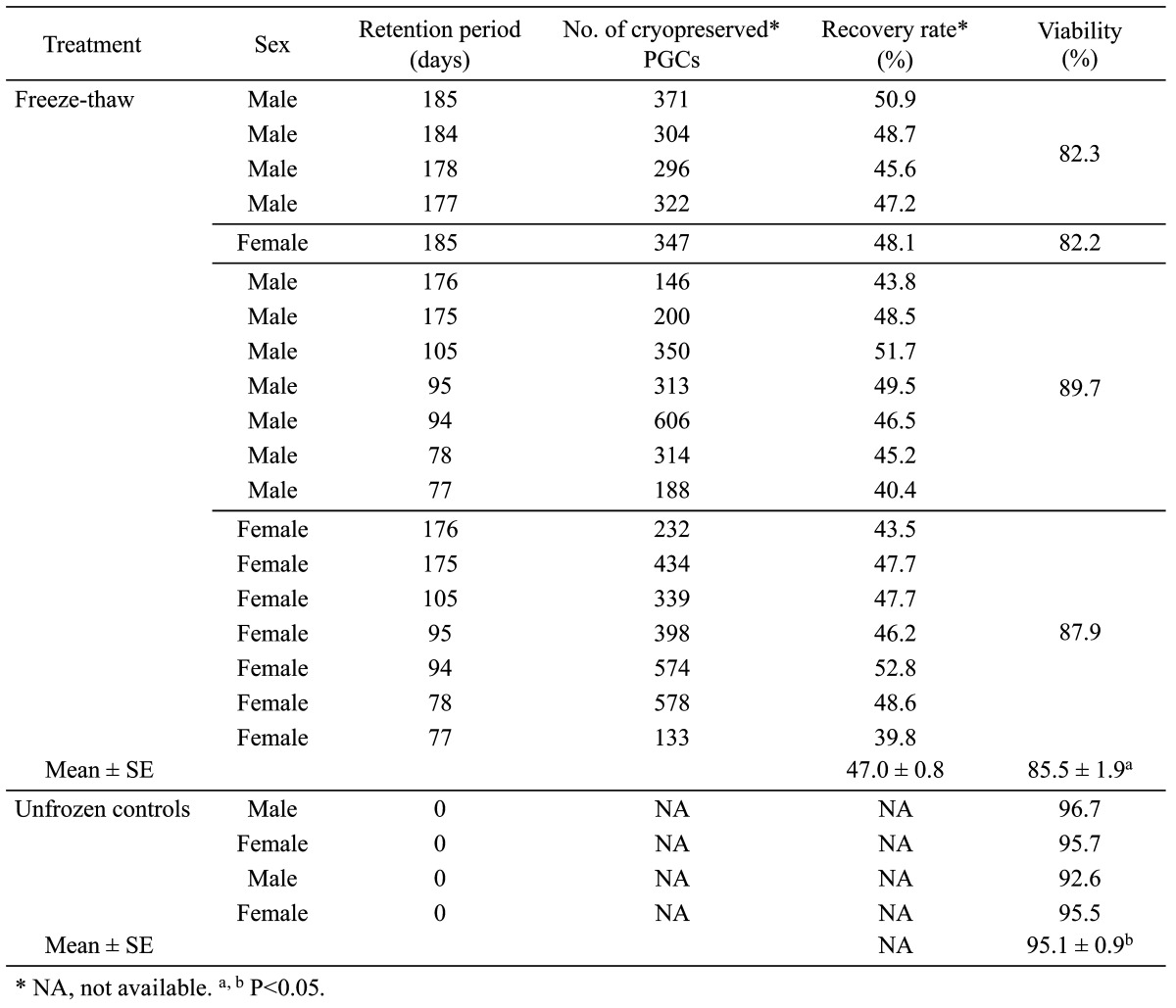
The average percentage of recovery and viability of DW PGCs after freeze-thawing is shown in Table 1. The average rate of viable PGCs, as measured by trypan blue exclusion, was significantly lower in the frozen group than that in the unfrozen control group (85.5% vs. 95.1%; P<0.05).
Table 1. Percentage of recovered and viable DW PGCs after freeze-thawing.
Settlement of donor PGCs in the gonads of recipient embryos
To determine whether PGCs could settle in recipient gonads after freeze-thawing, 30 fluorescently labeled PGCs from DW embryos were transplanted intravascularly into recipient WT embryos. Gonadal migration of donor PGCs from both the frozen and control groups was observed in all manipulated recipient embryos (Table 2). However, in stage 28 gonads, the number of PGCs transplanted from the frozen group was significantly lower than that of the PGCs transplanted from the control group (P<0.05).
Table 2. Number of DW PGCs settled in the gonads of WT embryos with or without freeze-thawing.
| Donor PGC treatment/ Chimera ID | Chimera sex | No. of donor PGCs |
| Freeze-thaw | ||
| FME-1 | Male | 8 |
| FME-2 | Male | 5 |
| FME-3 | Male | 14 |
| FME-4 | Male | 12 |
| FME-5 | Male | 4 |
| FME-6 | Male | 9 |
| FME-7 | Male | 11 |
| FME-8 | Male | 5 |
| FME-9 | Male | 19 |
| FME-10 | Male | 14 |
| FFE-1 | Female | 10 |
| FFE-2 | Female | 8 |
| FFE-3 | Female | 3 |
| FFE-4 | Female | 17 |
| FFE-5 | Female | 13 |
| FFE-6 | Female | 6 |
| Mean ± SE | 9.9 ± 1.2a | |
| Unfrozen controls | ||
| UME-1 | Male | 21 |
| UME-2 | Male | 22 |
| UME-3 | Male | 16 |
| UME-4 | Male | 17 |
| UME-5 | Male | 22 |
| UME-6 | Male | 16 |
| UME-7 | Male | 21 |
| UME-8 | Male | 15 |
| UME-9 | Male | 17 |
| UME-10 | Male | 19 |
| UME-11 | Male | 25 |
| UME-12 | Male | 22 |
| UFE-1 | Female | 20 |
| UFE-2 | Female | 18 |
| UFE-3 | Female | 26 |
| UFE-5 | Female | 25 |
| UFE-6 | Female | 24 |
| Mean ± SE | 20.4 ± 0.8b | |
a, b P<0.05.
Germline transmission of frozen-thawed PGCs after transplantation
To demonstrate whether PGCs could differentiate into functional gametes in recipient gonads after freeze-thawing, 100 PGCs from DW embryos were transplanted intravascularly into recipient WT embryos of the same sex. The survival and hatching rates of recipient embryos in windowed eggs following transfer of frozen-thawed or fresh PGCs are shown in Table 3. In the frozen group, among the 27 manipulated embryos (14 males and 13 females), 19 (10 males and nine females) hatched, and 14 (seven males and seven females) survived to sexual maturity. In the unfrozen control group, among the 18 manipulated embryos (nine males and nine females), 14 (eight males and six females) hatched, and 10 (five males and five females) reached sexual maturity. Genetic cross testing showed that three (two males and one female) of the 14 (21.4%) recipients that reach sexual maturity and eight (five males and three females) of the 10 (80%) recipients that reach sexual maturity were stable germline chimeric quails in the frozen group and unfrozen control group, respectively (Table 4 and Fig. 1). The average proportion of donor-derived offspring in the frozen group (4.0 ± 3.7%) was slightly lower compared with that in the unfrozen control group (14.0 ± 4.6%), but the difference was not significant.
Table 3. Survival and hatching rates of WT Japanese quail embryos in windowed eggs following injection of DW PGCs with or without freeze-thawing into the embryonic bloodstream.
| Donor PGC treatment | No. of embryos manipulated | Survival of embryos on incubation day (%) | Hatched | ||||
| 3 | 6 | 9 | 12 | 15 | (%) | ||
| Freeze-thaw | 27 | 100 | 92.6 | 92.6 | 92.6 | 88.9 | 70.4 |
| Unfrozen controls | 18 | 100 | 94.4 | 94.4 | 94.4 | 94.4 | 77.8 |
Table 4. Genetic cross testing of germline chimeras produced after transfer of DW PGCs with or without freeze-thawing into WT embryos.
| Donor PGC treatment/ Chimera ID | Chimera sex | No. of spotted white progeny (donor-derived progeny) | No. of brown progeny(recipient-derived progeny) | Proportion of donor-derived progeny (%) |
| Freeze-thaw | ||||
| FMQ-1 | Male | 2 | 204 | 1.0 |
| FMQ-10 | Male | 0 | 146 | 0 |
| FMQ-12 | Male | 4 | 155 | 2.5 |
| FMQ-18 | Male | 0 | 128 | 0 |
| FMQ-19 | Male | 0 | 113 | 0 |
| FMQ-20 | Male | 0 | 6 | 0 |
| FMQ-26 | Male | 0 | 3 | 0 |
| FFQ-2 | Female | 23 | 21 | 52.3 |
| FFQ-7 | Female | 0 | 95 | 0 |
| FFQ-8 | Female | 0 | 67 | 0 |
| FFQ-14 | Female | 0 | 69 | 0 |
| FFQ-15 | Female | 0 | 51 | 0 |
| FFQ-21 | Female | 0 | 32 | 0 |
| FFQ-25 | Female | 0 | 82 | 0 |
| Mean ± SE | 4.0 ± 3.7 | |||
| Unfrozen controls | ||||
| UMQ-3 | Male | 69 | 80 | 46.3 |
| UMQ-7 | Male | 18 | 99 | 15.4 |
| UMQ-10 | Male | 34 | 141 | 19.4 |
| UMQ-13 | Male | 36 | 98 | 26.9 |
| UMQ-18 | Male | 2 | 86 | 2.3 |
| UFQ-1 | Female | 9 | 44 | 17.0 |
| UFQ-2 | Female | 3 | 76 | 3.9 |
| UFQ-11 | Female | 8 | 80 | 9.1 |
| UFQ-15 | Female | 0 | 61 | 0 |
| UFQ-17 | Female | 0 | 89 | 0 |
| Mean ± SE | 14.0 ± 4.6 | |||
Fig. 1.
Phenotypes of offspring from a female germline chimeric quail (FFQ2). White offspring with spots were derived from donor DW PGCs. Brown offspring were derived from recipient WT PGCs.
Production of donor PGC-derived Japanese quails by mating germline chimeras
In the unfrozen control group, three male germline chimeras (UMQ-3, UMQ-7 and UMQ-13) were mated with two female germline chimeras (UFQ-1 and UFQ-11). Sixty-four quails were obtained from these combinations of germline chimeras; nine were white with brown spots (DW) derived from donor DW PGCs (Table 5). Unfortunately, such breeding experiments could not be performed for the frozen group because male and female chimeric quails did not survive during genetic cross testing (FMQ-12 and FFQ-2). One surviving male chimeric Japanese quail in the frozen group (FMQ-1) was crossed with a female chimeric Japanese quail in the unfrozen control group (UFQ-2), but no donor-derived offspring were obtained from this combination.
Table 5. Number of donor-derived offspring by mating germline chimeric Japanese quails produced by transfer of DW PGCs into WT recipient embryos.
| Male chimera ID | Female chimera ID | Phenotypes of offspring* | Proportion of derived progeny (%) | |
| Spotted white | Brown | |||
| UMQ-3 | UFQ-1 | 1 | 0 | 100 |
| UMQ-3 | UFQ-11 | 3 | 4 | 42.9 |
| UMQ-7 | UFQ-11 | 0 | 3 | 0 |
| UMQ-13 | UFQ-1 | 5 | 48 | 9.4 |
| FMQ-1 | UFQ-2 | 0 | 26 | 0 |
* Spotted white offspring are DW × DW; brown offspring are DW × WT or WT × WT.
Discussion
The present study succeeded in producing functional gametes derived from frozen PGCs by transplantation in the Japanese quail. The freezing procedure used for chicken PGCs [21, 30] was applied to Japanese quail PGCs. PGCs isolated from the blood of 2-day-old embryos were suspended in a commercial cryomedium (Cell Banker 1), slowly frozen and then cryopreserved in liquid nitrogen for 77–185 days. Frozen-thawed donor PGCs transferred to recipient embryos were successfully incorporated into the gonads and gave rise to functional gametes. These results demonstrate that the cryopreservation of PGCs can be applied to the cryobanking of the Japanese quail.
Slow freezing, in which cells are usually frozen in a programmable freezer (or a simplified freezing container, which was used in the present study) at controlled slow cooling rates, has been widely applied for the cryopreservation of various cell types and simple cell aggregations. To date, the main method of cryopreserving chicken PGCs has been the slow-freezing process. Dimethyl sulfoxide-containing cryomedia, including commercial products, are available for the storage of chicken PGCs in liquid nitrogen, which maintains their ability to transmit to the next generation [19,20,21]. In the present study, a protocol that was recently described for the cryopreservation of chicken PGCs was used to cryopreserve Japanese quail PGCs. However, the Japanese quail PGCs cryopreserved in this study had a lower recovery rate (47.0%) than the chicken PGCs in our previous studies (54.3%) [30], possibly because the Japanese quail PGCs are more sensitive to cryogenic damage than the chicken PGCs. The current understanding is that the major cause of lethal injury during cryopreservation is the formation of more than a trace amount of ice within a cell. The mechanisms by which intracellular ice damages the cells have not been resolved. It is assumed that some of the Japanese quail PGCs cryopreserved in this study might have been lysed by cryogenic damage and then washed out during centrifugation, which would have affected the recovery rate. Two approaches are available for improving cell recovery and survival: (1) the cooling of cells at the highest rate possible without causing intracellular ice formation [31] and (2) the addition of low molecular weight compounds to the cryomedium [32]. Further refinements are required, such as to the rates of cooling and warming and the type and concentration of the cryoprotective agent, to develop a practical approach for genetic conservation of the Japanese quail.
In contrast to slow freezing, the vitrification method can theoretically preserve cells both intracellularly and extracellularly by ice-free solidification. In the vitrification method, ice formation is avoided by suspending the cells in very high concentrations of cryoprotective agents, including those that permeate the cell, and then cooling the cells at very high rates. As a result, the water in the system is converted from a liquid to a glass without ice formation. Although it has been reported that the recovery and survival rates of vitrified chicken PGCs are lower than those of slow-frozen PGCs [33], the vitrification method improves the recovery and survival of avian PGCs after optimization of the protocol.
Japanese quail PGCs cryopreserved in this study had slightly lower cell viability and decreased efficiencies for gonadal migration and the production of donor-derived offspring when compared with the unfrozen controls. The number of donor PGCs that settled in the gonads of recipient embryos at 3 days post transplantation decreased to 48.5% of that in the unfrozen control group after freeze-thawing. Frozen-thawed PGCs showed a decline in their ability to colonize recipient gonads, even when accounting for their low viability. This low colonization rate was probably caused by a decrease in the migratory ability and/or post-migratory proliferation activity of Japanese quail PGCs due to cryogenic damage. These occurrences might lead to a reduction in the germline transmission efficiency of frozen-thawed PGCs in chimeric quails. The combination of donor and recipient breeds or lines used for chimera production is important factors affecting germline chimerism. An important factor affecting germline chimerism is the combination of donor and recipient breeds, and the lines used for chimera production in chickens [12, 21]. One of the reasons for this is the differential ability of PGCs to colonize recipient gonads between chicken breeds after transplantation [30]. A similar tendency was observed for Japanese quail PGCs. WT PGCs had a higher colonization ability than DW PGCs (data not shown). For successful production of germline chimeras in Japanese quail, further study is needed to demonstrate whether the germline transmission efficiency of donor PGCs is affected by the combination of donor and recipient lines.
Both male and female germline chimeric quails generated in the present study produced offspring originating from transplanted donor PGCs for a prolonged period of time by natural mating. Once germline chimeric quails are generated by the transfer of frozen-thawed PGCs, there will be an opportunity to obtain donor-derived offspring, given that they produce gametes. In the present study, we could not perform trials to generate viable offspring using eggs and spermatozoa derived from frozen-thawed PGCs because of the poor survival of the germline chimeras in the frozen group. Nevertheless, the generation of viable Japanese quails from frozen-thawed PGCs will be achieved because this study showed that frozen-thawed PGCs gave rise to functional gametes after transplantation, and viable donor-derived progeny were obtained by mating germline chimeras in the unfrozen control group.
The sex and fitness of donor PGCs and recipient PGCs is important because the frequency of donor-derived offspring from germline chimeric chickens was significantly higher for the same-sex combinations of donor PGCs and recipient embryos when compared with different-sex combinations [34, 35]. Hence, male and female PGCs were isolated, cryopreserved and transplanted into same-sex recipient embryos in the present study to increase the frequency of donor-derived offspring and clarify their origin. In future practice, an improvement in efficiency is required to obtain donor-derived progeny from frozen-thawed PGCs. Two approaches are available to enhance germline transmission: (1) increasing the number of PGCs for transplantation and (2) decreasing the number of endogenous PGCs in recipient embryos. The number of donor PGCs incorporated into recipient gonads gradually increases with an increase in the number of transferred PGCs [36], and germline chimerism would thus be expected to increase using this methodology. However, this methodology is not appropriate when the number of donor PGCs is limited, such as in the case of regeneration of living birds from cryopreserved PGCs after extinction. The recent establishment of culture conditions for extended propagation of chicken PGCs without losing their germline transmission ability [37] has provided important breakthroughs for the genetic conservation of chickens. By establishing such a cultivation method for quails, PGCs can be expanded in vitro prior to cryopreservation. In contrast, several attempts have been made to eliminate endogenous PGCs using radiation or drugs. Exposure of chicken embryos to X-rays reduces the number of endogenous PGCs [38,39,40] and increases germline transmission of transferred PGCs in germline chimeras [14]. Germline transmission of donor PGCs would be increased in chimeric quails because X-ray irradiation has the same effects on removing endogenous PGCs in early quail embryos [41]. Endogenous PGCs can be removed following the application of busulfan, an alkylating agent with cytotoxic effects on germ cells in chickens [10, 42, 43] and quails [44]. We have developed an efficient method for the delivery of busulfan to developing chicken embryos using a sustained-release emulsion [45]. A subsequent study demonstrated that early administration of busulfan to recipient embryos allows for a reduction in the sterilizing effect of the residual busulfan on donor PGCs [46]. Successful production of high-grade consistent germline chimeric chickens has been achieved through the transfer of donor PGCs into partially sterilized recipient embryos [13]. By applying this method to germline chimera production in the Japanese quail, a large increase in the number of progeny can be obtained from cryopreserved PGCs.
In conclusion, Japanese quail PGCs could be cryopreserved using a simple slow-freezing method and then recovered by transplantation, but the viability, migration ability and germline transmission ability were decreased after the freeze-thaw treatment. Cryopreservation of PGCs is a feasible strategy for conservation of both male and female germplasm from Japanese quails, though further refinement in all aspects of freeze-thawing is needed. Cryobanking of the Japanese quail will be made more reliable by preservation of ovarian tissues in addition to PGCs in liquid nitrogen.
Acknowledgments
This work was funded by a grant from “Genebank Project” of the National Institute of Agrobiological Sciences (NIAS) to TT. The authors wish to thank the staff of the Poultry Management Section of the NILGS for maintaining the birds and providing the fertilized eggs.
References
- 1.Sugiura K, Fushimi K, Takehisa T, Miwa M, Saito T, Uchida Y, Onodera T. An outbreak of H7N6 low pathogenic avian influenza in quails in Japan. Vet Ital 2009; 45: 481–489 [PubMed] [Google Scholar]
- 2.Lake PE, Stewart JM. Preservation of fowl semen in liquid nitrogen – an improved method. Br Poult Sci 1978; 19: 187–194 [DOI] [PubMed] [Google Scholar]
- 3.Hammerstedt RH, Graham JK. Cryopreservation of poultry sperm: the enigma of glycerol. Cryobiology 1992; 29: 26–38 [DOI] [PubMed] [Google Scholar]
- 4.Fulton JE, Delany ME. Genetics. Poultry genetic resources – operation rescue needed. Science 2003; 300: 1667–1668 [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Song Y, Cheng KM, Silversides FG. Production of donor-derived offspring from cryopreserved ovarian tissue in Japanese quail (Coturnix japonica). Biol Reprod 2010; 83: 15–19 [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Cheng KM, Silversides FG. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in Japanese quail (Coturnix japonica). Biol Reprod 2013; 88: 124 [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y, Kagami H, Tagami T. Development, differentiation and manipulation of chicken germ cells. Dev Growth Differ 2013; 55: 20–40 [DOI] [PubMed] [Google Scholar]
- 8.Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 2000; 127: 2741–2750 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Yamamoto Y, Usui F, Mushika T, Ono T, Setioko AR, Takeda K, Nirasawa K, Kagami H, Tagami T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci 2007; 86: 2182–2193 [DOI] [PubMed] [Google Scholar]
- 10.Vick L, Luke G, Simkiss K. Germ-line chimaeras can produce both strains of fowl with high efficiency after partial sterilization. J Reprod Fertil 1993; 98: 637–641 [DOI] [PubMed] [Google Scholar]
- 11.Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ line chimera by transfer of primordial germ cells in the domestic chicken (Gallus domesticus). Theriogenology 1993; 40: 509–519 [DOI] [PubMed] [Google Scholar]
- 12.Naito M, Tajima A, Yasuda Y, Kuwana T. Production of germline chimeric chickens, with high transmission rate of donor-derived gametes, produced by transfer of primordial germ cells. Mol Reprod Dev 1994; 39: 153–161 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Usui F, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Germline replacement by transfer of primordial germ cells into partially sterilized embryos in the chicken. Biol Reprod 2010; 83: 130–137 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Usui F, Miyahara D, Mori T, Ono T, Kagami H, Takeda K, Nirasawa K, Tagami T. X-irradiation removes endogenous primordial germ cells (PGCs) and increases germline transmission of donor PGCs in chimeric chickens. J Reprod Dev 2012; 58: 432–437 [DOI] [PubMed] [Google Scholar]
- 15.Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germline chimeras by transfer of cryopreserved gonadal primordial germ cells (gPGCs) in chicken. J Exp Zool 1998; 280: 265–267 [PubMed] [Google Scholar]
- 16.Park TS, Jeong DK, Kim JN, Song KH, Hong YH, Lim JM, Han JY. Improved germline transmission in chicken chimeras produced by transplantation of gonadal primordial germ cells into recipient embryos. Biol Reprod 2003; 68: 1657–1662 [DOI] [PubMed] [Google Scholar]
- 17.Ono T, Matsumoto T, Arisawa Y. Production of donor-derived offspring by transfer of primordial germ cells in Japanese quail. Exp Anim 1998; 47: 215–219 [DOI] [PubMed] [Google Scholar]
- 18.Kim MA, Park TS, Kim JN, Park HJ, Lee YM, Ono T, Lim JM, Han JY. Production of quail (Coturnix japonica) germline chimeras by transfer of gonadal primordial germ cells into recipient embryos. Theriogenology 2005; 63: 774–782 [DOI] [PubMed] [Google Scholar]
- 19.Naito M, Tajima A, Tagami T, Yasuda Y, Kuwana T. Preservation of chick primordial germ cells in liquid nitrogen and subsequent production of viable offspring. J Reprod Fertil 1994; 102: 321–325 [DOI] [PubMed] [Google Scholar]
- 20.Kuwana T, Kawashima T, Naito M, Yamashita H, Matsuzaki M, Takano T. Conservation of a threatened indigenous fowl (Kureko dori) using the germline chimeras transplanted from primordial germ cells. J Poult Sci 2006; 43: 60–66 [Google Scholar]
- 21.Nakamura Y, Usui F, Miyahara D, Mori T, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Efficient system for preservation and regeneration of genetic resources in chicken: concurrent storage of primordial germ cells and live animals from early embryos of a rare indigenous fowl (Gifujidori). Reprod Fertil Dev 2010; 22: 1237–1246 [DOI] [PubMed] [Google Scholar]
- 22.Chang IK, Naito M, Kuwana T, Mizutani M, Sakurai M. Production of germline chimeric quail by transfer of gonadal primordial germ cells preserved in liquid nitrogen. Jpn Poult Sci 1998; 35: 321–328 [Google Scholar]
- 23.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: 49–92 [PubMed] [Google Scholar]
- 24.Zhao DF, Kuwana T. Purification of avian circulating primordial germ cells by Nycodenz density gradient centrifugation. Br Poult Sci 2003; 44: 30–35 [DOI] [PubMed] [Google Scholar]
- 25.Kuwana T, Hashimoto K, Nakanishi A, Yasuda Y, Tajima A, Naito M. Long-term culture of avian embryonic cells in vitro. Int J Dev Biol 1996; 40: 1061–1064 [PubMed] [Google Scholar]
- 26.Yamamoto Y, Usui F, Nakamura Y, Ito Y, Tagami T, Nirasawa K, Matsubara Y, Ono T, Kagami H. A novel method to isolate primordial germ cells and its use for the generation of germline chimeras in chicken. Biol Reprod 2007; 77: 115–119 [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto T, Ukeshima A, Kiyofuji R. The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat Rec 1976; 185: 139–145 [DOI] [PubMed] [Google Scholar]
- 28.Freshney RI . Culture of Animal Cells, 2nd ed. New York: Alan R Liss; 1987: 245–256. [Google Scholar]
- 29.Miwa M, Inoue-Murayama M, Aoki H, Kunisada T, Hiragaki T, Mizutani M, Ito S. Endothelin receptor B2 (EDNRB2) is associated with the panda plumage colour mutation in Japanese quail. Anim Genet 2007; 38: 103–108 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Usui F, Miyahara D, Mori T, Watanabe H, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Viability and functionality of primordial germ cells after freeze-thaw in chickens. J Poult Sci 2011; 48: 57–63 [Google Scholar]
- 31.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol 1984; 247: C125–C142 [DOI] [PubMed] [Google Scholar]
- 32.McGann LE. Differing actions of penetrating and nonpenetrating cryoprotective agents. Cryobiology 1978; 15: 382–390 [DOI] [PubMed] [Google Scholar]
- 33.Kohara Y, Kanai Y, Tajima A. Cryopreservation of gonadal germ cells (GGCs) from the domestic chicken using vitrification. J Poult Sci 2008; 45: 57–61 [Google Scholar]
- 34.Naito M, Matsubara Y, Harumi T, Tagami T, Kagami H, Sakurai M, Kuwana T. Differentiation of donor primordial germ cells into functional gametes in the gonads of mixed-sex germline chimaeric chickens produced by transfer of primordial germ cells isolated from embryonic blood. J Reprod Fertil 1999; 117: 291–298 [DOI] [PubMed] [Google Scholar]
- 35.Tagami T, Kagami H, Matsubara Y, Harumi T, Naito M, Takeda K, Hanada H, Nirasawa K. Differentiation of female primordial germ cells in the male testes of chicken (Gallus gallus domesticus). Mol Reprod Dev 2007; 74: 68–75 [DOI] [PubMed] [Google Scholar]
- 36.Kim JN, Park TS, Park SH, Park KJ, Kim TM, Lee SK, Lim JM, Han JY. Migration and proliferation of intact and genetically modified primordial germ cells and the generation of a transgenic chicken. Biol Reprod 2010; 82: 257–262 [DOI] [PubMed] [Google Scholar]
- 37.van de Lavoir MC, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, Bradshaw R, Kerchner A, Hooi LT, Gessaro TM, Swanberg SE, Delany ME, Etches RJ. Germline transmission of genetically modified primordial germ cells. Nature 2006; 441: 766–769 [DOI] [PubMed] [Google Scholar]
- 38.Lim JM, Kwon HM, Kim DK, Kim JN, Park TS, Ono T, Han JY. Selective decrease of chick embryonic primordial germ cells in vivo and in vitro by soft X-ray irradiation. Anim Reprod Sci 2006; 95: 67–74 [DOI] [PubMed] [Google Scholar]
- 39.Atsumi Y, Tagami T, Kagami H, Ono T. Restriction of germline proliferation by soft X-ray irradiation of chicken embryos and its application to chimera production. J Poult Sci 2008; 45: 292–297 [Google Scholar]
- 40.Atsumi Y, Yazawa S, Usui F, Nakamura Y, Yamamoto Y, Tagami T, Hiramatsu K, Kagami H, Ono T. Depletion of primordial germ cells (PGCs) by X-irradiation to extraembryonic region of chicken embryos and expression of xenotransplanted quail PGCs. J Poult Sci 2009; 46: 136–143 [Google Scholar]
- 41.Li HC, Kagami H, Matsui K, Ono T. Restriction of proliferation of primordial germ cells by the irradiation of Japanese quail embryos with soft X-rays. Comp Biochem Physiol A Mol Integr Physiol 2001; 130: 133–140 [DOI] [PubMed] [Google Scholar]
- 42.Aige-Gil V, Simkiss K. Sterilisation of avian embryos with busulphan. Res Vet Sci 1991; 50: 139–144 [DOI] [PubMed] [Google Scholar]
- 43.Song Y, D’Costa S, Pardue SL, Petitte JN. Production of germline chimeric chickens following the administration of a busulfan emulsion. Mol Reprod Dev 2005; 70: 438–444 [DOI] [PubMed] [Google Scholar]
- 44.Hallett JS, Wentworth BC. The effects of busulfan on gonadal differentiation and development in Japanese quail (Coturnix coturnix japonica). Poult Sci 1991; 70: 1619–1623 [DOI] [PubMed] [Google Scholar]
- 45.Nakamura Y, Yamamoto Y, Usui F, Atsumi Y, Ito Y, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Increased proportion of donor primordial germ cells in chimeric gonads by sterilisation of recipient embryos using busulfan sustained-release emulsion in chicken. Reprod Fertil Dev 2008; 20: 900–907 [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Usui F, Atsumi Y, Otomo A, Teshima A, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Effects of busulfan sustained-release emulsion on depletion and repopulation of primordial germ cells in early chicken embryos. J Poult Sci 2009; 46: 127–135 [Google Scholar]



