Abstract
The aim of the present study was to determine if the estradiol-induced luteinizing hormone (LH) surge is influenced by the constant exposure to TAK-683, an investigational metastin/kisspeptin analog, that had been established to depress the pulsatile gonadotropin-releasing hormone (GnRH) and LH secretion in goats. Ovariectomized goats subcutaneously received TAK-683 (TAK-683 group, n=6) or vehicle (control group, n=6) constantly via subcutaneous implantation of an osmotic pump. Five days after the start of the treatment, estradiol was infused intravenously in both groups to evaluate the effects on the LH surge. Blood samples were collected at 6-min intervals for 4 h prior to the initiation of either the TAK-683 treatment or the estradiol infusion, to determine the profiles of pulsatile LH secretion. They were also collected at 2-h intervals from –4 h to 32 h after the start of estradiol infusion for analysis of LH surges. The frequency and mean concentrations of LH pulses in the TAK-683 group were remarkably suppressed 5 days after the start of TAK-683 treatment compared with those of the control group (P<0.05). On the other hand, a clear LH surge was observed in all animals of both groups. There were no significant differences in the LH concentrations for surge peak and the peak time of the LH surge between the TAK-683 and control groups. These findings suggest that the effects of continuous exposure to kisspeptin or its analog on the mechanism(s) that regulates the pulsatile and surge mode secretion of GnRH/LH are different in goats.
Keywords: Estradiol, Goats, Kisspeptin analog, LH surge
Kisspeptin (also known as metastin) was first discovered as a ligand for G protein-coupled receptor 54 (GPR54) in 2001 [1]. The majority of research on kisspeptin to date has focused on its regulatory role in reproductive function. A number of studies have reported that exogenous administration of kisspeptin or kisspeptin-10 (kp-10), the C-terminal amidated 10-amino-acid sequence necessary for GPR54 activation, induces a rise of peripheral luteinizing hormone (LH) concentration in many mammalian species [2,3,4]. Recent studies showed that kp-10 directly stimulated gonadotropin-releasing hormone (GnRH) neurosecretion into the hypophyseal portal circulation accompanied by increases in the peripheral concentrations of LH in sheep [5] and goats [6]. In rodents, kisspeptin-induced LH release is blocked by pretreatment with the GnRH antagonist [7, 8]. These lines of evidence suggest that kisspeptin influences LH secretion by regulating hypothalamic GnRH secretion, which gives rise to the modulation of reproductive function.
TAK-683 is an investigational metastin/kisspeptin analog evaluated by Takeda Pharmaceutical Company Limited, Osaka, Japan [9,10,11]. Our previous study demonstrated that a bolus injection of TAK-683 stimulates GnRH secretion into the hypophyseal portal circulation and peripheral LH secretion in castrated goats [10]. Moreover, remarkable suppression of the testicular size and the peripheral LH secretion was observed in male rats when TAK-683 or TAK-448, another investigational metastin/kisspeptin analog, was chronically administered [9, 12]. Several studies have shown that chronic or repeated administration of human kisspeptin-54 or kp-10 results in a reduction of the pituitary and/or gonadal function together with inhibition of the peripheral LH levels in rats [13], monkeys [14, 15] and women [16]. Our recent study demonstrated in castrated goats that the suppressive action of chronic TAK-683 treatment on LH was attributable to complete suppression of pulsatile GnRH secretion [10]. These studies indicate that chronic administration of kisspeptin or its analog suppresses pulsatile GnRH/LH secretion after an initial stimulatory action.
An important endocrine event of the estrous cycle is a large continuous increase in GnRH release, namely, the GnRH surge. The GnRH surge is induced by a high peripheral level of estradiol from the preovulatory follicle, and causes an LH surge for ovulation. The relationship of kisspeptin signaling with the GnRH/LH surge has been demonstrated. Similarly to the hypothalamic location of GnRH neurons, kisspeptin neurons have been identified in the hypothalamic anteroventral periventricular area (AVPV) and preoptic area (POA) in mammals [17]. POA infusion of the anti-rat kisspeptin monoclonal antibody blocks the estrogen-induced [18] and the proestrous LH surges [19] in rats. Intracerebroventricular infusion of kisspeptin antagonist reduced the magnitude of the estradiol-stimulated LH surge in ewes during the anestrous season [5]. Increased cFos and Kiss-1 mRNA expressions in the AVPV or POA at the time of the LH surge have been observed in ovariectomized rats [18, 20] and mice [21], and in sheep [22, 23] treated with exogenous estradiol. While the importance of hypothalamic kisspeptin on surge mode secretion of GnRH/LH has been shown in many species, the effect of long-term exposure to kisspeptin or its analog on an estradiol-induced GnRH/LH surge is still unclear. The present study aimed to determine if the estradiol-induced LH surge is also influenced by the chronic administration of TAK-683, which has been established to suppress the pulsatile GnRH and LH secretion in goats [10].
Materials and Methods
Eight long-term (>10 months) ovariectomized Shiba goats were used, and 4 of these animals were assigned to both groups. There was at least 5 months between treatment of goats with TAK-683 in the treatment group and reassignment of the goats to the control group. The goats were fed a standard pelleted diet and dry hay or hay cubes with ad libitum access to water and supplemental minerals. They were kept individually in cages temporarily when they were subjected to treatment and frequent blood sampling. All procedures were approved by the Committee for the Care and Use of Experimental Animals at the National Institute of Agrobiological Sciences (#22-67).
The chemical structure of TAK-683 was described previously [24], and chronic treatment with TAK-683 (50 nmol/kg BW/week) for 5 days was confirmed to cause severe suppression of pulsatile GnRH and LH secretion in our previous study [10]. In the present study, TAK-683 was subcutaneously administered at a concentration ten times higher than in the previous study to induce more profound suppression of pulsatile LH secretion. The animals were divided into two groups, and the TAK-683 group (n=6) constantly received TAK-683 at a rate of 500 nmol/kg BW/week via an osmotic pump (ALZET model 2ML1, DURECT, Cupertino, CA, USA) until the end of the experiment. The control group (n=6) received vehicle (50% DMSO). The osmotic pump was subcutaneously implanted under brief anesthesia using ketamine chloride, and the flow rate for the administration was 10 μl/h. Five days after the start of TAK-683 or vehicle treatment, estradiol (Sigma Chemical, St. Louis, MO, USA) dissolved in 0.3% ethanol saline (0.6 μg/ml) was infused with a peristaltic mini pump into the jugular vein for 16 h at a rate of 6 μg/h through one of the catheters (18 gauge, Medicut; Nippon Sherwood Medical Industries, Tokyo, Japan) fitted bilaterally into the jugular vein in both groups, as described previously to evaluate the effects on the LH surge [25].
Blood samples (1 ml) were collected via a jugular catheter into heparinized tubes at 6-min intervals for 4 h prior to the initiation of either the TAK-683 (or vehicle) treatment (Day 0) or the estradiol infusion (Day 5), to determine the profiles of pulsatile LH secretion. They were also collected at 2-h (2 ml) and 6-h (4 ml) intervals from –4 h to 32 h after the onset of estradiol infusion for analyses of the LH surge and of the plasma estradiol concentration, respectively. Blood samples were immediately stored on ice and centrifuged at 3,000 rpm for 20 min, and the plasma was kept at –30 C until assayed for plasma LH and estradiol concentrations.
Plasma LH concentrations were measured in duplicate by a specific radioimmunoassay (RIA) [26] using rabbit anti-ovine LH serum (YM #18) [27] and expressed in terms of ovine LH standard (NIDDK-oLH-I-4). The sensitivity of the assay was 9.5 pg/tube, and the intra- and inter-assay coefficients of variation were 5.1% and 6.5%, respectively. Plasma concentrations of estradiol were assayed by a previously described method [28]. The sensitivity of the assays was 0.1 pg/tube, and the intra- and inter-assay coefficients of variation were 5.7 and 12.0%, respectively.
Data are expressed as means ± SD and statistical differences were determined by Student’s t-test or ANOVA. A confidence level of P<0.05 was considered to be statistically significant. For the identification of LH pulses, the cluster analysis program developed by Veldhuis and Johnson [29] was used. The nadir and peak clusters for LH pulse detection were 2/2 points, and the t statistics for significant increase and decrease were 2/2. The LH surge was defined as the point when a sustained rise (for at least two consecutive points of blood sampling) in the plasma LH concentration exceeded twice the average baseline level during the pretreatment period before the estradiol infusion, as described previously [25].
Results
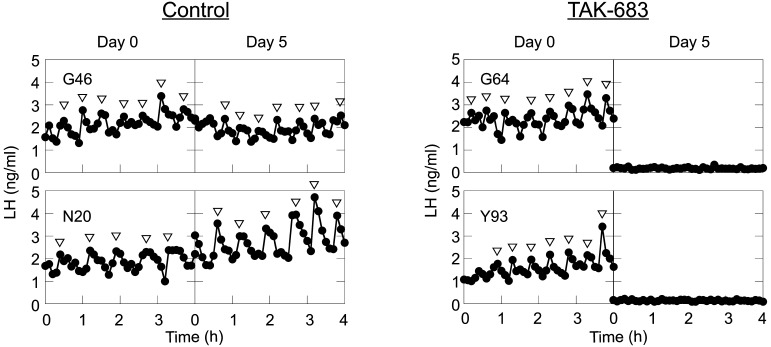
Representative patterns of pulsatile LH secretion before (Day 0) and after (Day 5) TAK-683 treatment in the control and TAK-683 groups are shown in Fig. 1. The effects of chronic treatment of TAK-683 on LH pulses are summarized in Table 1. There was no significant difference in the profiles of the pulsatile LH secretion on Day 0 between the TAK-683 and control groups, whereas continuous exposure to TAK-683 for 5 days remarkably suppressed both the pulse frequency and amplitude of LH secretion. The frequency and mean concentrations of LH pulses in the TAK-683 group were significantly decreased on Day 5 compared with those of the control group.
Fig. 1.
Representative profiles of pulsatile LH secretion on Day 0 and Day 5 in the control (left) and TAK-683 (right) groups. Arrowheads represent LH pulses identified by cluster analysis.
Table 1. The effects of chronic treatment with TAK-683 on LH pulses and the estradiol-induced LH surge.
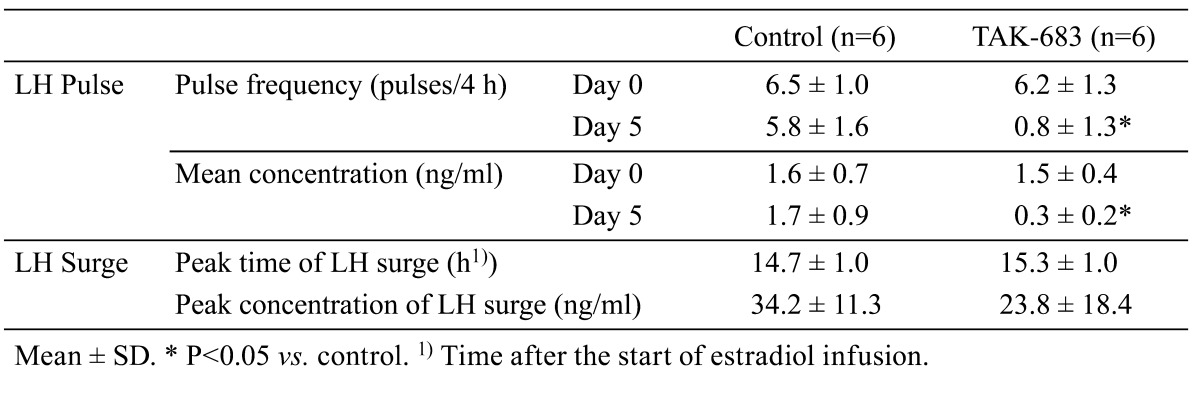
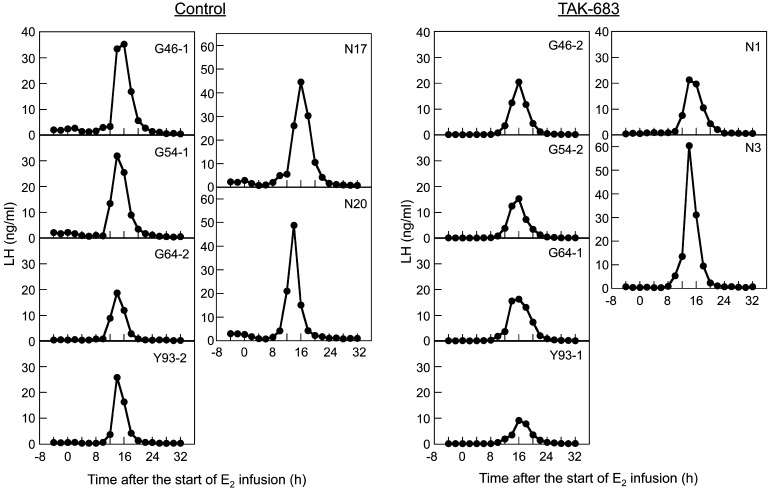
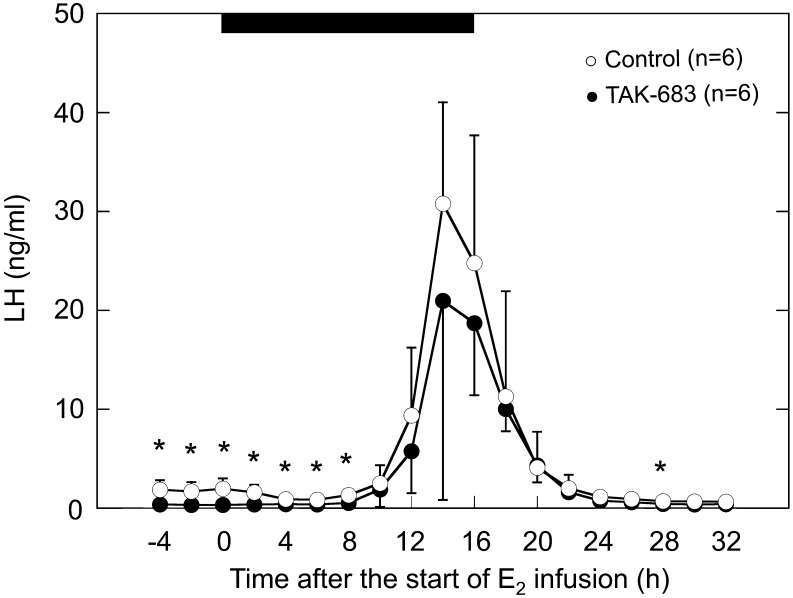
The mean plasma concentrations of estradiol during estradiol infusion ranged from 21.7 to 67.0 pg/ml; there was no significant difference between the two groups (data not shown). The changes in the LH concentration after estradiol infusion are shown in Fig. 2 and Fig. 3. Clear sustained rises in LH concentrations detected as an LH surge were observed in all animals of both groups (Fig. 2). LH concentrations in the TAK-683 group were significantly lower than those of the control group from –4 to 8 h after the start of estradiol infusion (Fig. 3). However, no significant difference in the LH concentration during the LH surge (the period from 12 to 20 h after the start of estradiol infusion) was detected between the two groups. The profiles of the estradiol-induced LH surge in both groups are summarized in Table 1. There was no significant difference in the peak time and peak concentration of the LH surge between the two groups.
Fig. 2.
Effects of the continuous administration of TAK-683 for 5 days on the estradiol-induced LH surge in all goats of the control (left 6 panels) and TAK-683 (right 6 panels) groups. A clear LH surge was observed in all animals of both groups.
Fig. 3.
Changes in the plasma concentrations of LH after the start of estradiol infusion in the TAK-683 (closed circles) and vehicle (open circles) groups. The horizontal black bar indicates the period of estradiol infusion. Mean ± SD. * P<0.05 vs. control.
Discussion
The present study reconfirmed in ovariectomized goats that pulsatile LH secretion was remarkably suppressed by continuous exposure to TAK-683. Under this condition, a large sustained rise in LH secretion was observed after the start of estradiol infusion. The timing of its peak and the peak level of LH concentrations were accordance with the previous findings reported in ovariectomized goats given estradiol [25, 30]. The present findings indicate that the LH surge was induced by estradiol treatment in all animals under the chronic administration of TAK-683 that remarkably suppressed the pulsatile LH secretion.
Several studies have clearly demonstrated that hypothalamic input of estradiol is necessary for induction of the LH surge [31, 32]. GnRH secretion is substantially increased during the LH surge in estradiol-treated ovariectomized goats [33] and ewes [34, 35]. Our previous study demonstrated in castrated goats that the suppressive effect of chronic treatment of TAK-683 on the pulsatile LH secretion was due to complete suppression of pulsatile GnRH secretion without an influence on the responsiveness of pituitary gonadotrophs to a GnRH analog [10]. Taken together, the present results suggest that the occurrence of an estradiol-induced GnRH surge is not interfered with by the continuous action of TAK-683 negatively influencing pulsatile GnRH secretion.
The pulsatile and surge mode secretion of GnRH is considered to be regulated by two independent hypothalamic neural generators in females [36]. The present results suggest that the effects of continuous exposure to TAK-683 on these mechanisms are different. A different reaction of the estradiol-induced LH surge compared with that of pulsatile LH secretion has been reported under several physiological conditions. In ovariectomized lactating rats, the suckling stimulus strongly suppressed pulsatile LH secretion, whereas it did not prevent the occurrence of the LH surge after estradiol treatment [37]. On the other hand, it has been clinically reported in female goat that a subnormal level of progesterone in the peripheral circulation blocks the estradiol-induced LH surge without a suppressive influence on pulsatile LH secretion [25]. The present results are similar to the former phenomenon; it is likely that the hypothalamic generator for the GnRH surge functions in the case of the continuous stimulation of kisspeptin or its analogs.
In this case, what role does kisspeptin have in the estradiol-induced GnRH/LH surge? One possible interpretation is that kisspeptin action is not involved in the induction of the GnRH surge. However, a large number of studies do not support this hypothesis. Immunoneutralization of kisspeptin in the POA [18] and central infusion of kisspeptin antagonist [5] suppressed the estradiol-induced LH surge. Expression of kiss1 mRNA and cFos expression increased concomitantly with the preovulatory LH surge [18, 20], suggesting that the kisspeptin neurons participate in the induction of the GnRH surge. It seems to be plausible that a synergistic action of estradiol and endogenous kisspeptin (and/or TAK-683) can drive the GnRH surge-generating system to release a large amount of GnRH from GnRH-producing neurons that have lost the ability for pulsatile GnRH release in response to chronic administration of TAK-683.
Two hypotheses concerning the mechanism regulating the suppressive effects of long-term treatment of kisspeptin or TAK-683 on the pulsatile GnRH/LH secretion have been drawn from the several studies. Firstly, the involvement of the desensitization of GPR54 on the GnRH neurons after continuous or repeated kisspeptin treatment was proposed in previous studies on rats [12, 13] and rhesus monkeys [14]. This is similar to the fact that chronic administration of GnRH agonists suppresses the LH secretion due to the desensitization to GnRH on gonadotrophs of the pituitary after initial agonistic stimulation [38]. If this is the case, a possible explanation for the present findings is that GPR54 and its intracellular signal transduction relating to the GnRH surge were selectively prevented from undergoing desensitization. A previous study showed that kisspeptin cells are located in the POA and arcuate nucleus (ARC) in sheep [39]. The POA kisspeptin cells provide substantial input to GnRH cells in the POA [39], whereas the site of action of ARC kisspeptin cells is suggested to be on the GnRH axon terminal located in the hypothalamic median eminence [40, 41], which is a circumventricular organ lacking a blood-brain barrier [42]. Recent studies have suggested that pulsatile GnRH secretion is generated by the pulsatile release of kisspeptin at the ARC adjacent to the median eminence [3, 43, 44]. In contrast to the median eminence, the blood-brain barrier of the POA appears to restrict the entry of large molecules, for example, kp-10 or TAK-683, into the nervous system. The specific anatomical component such as the blood-brain barrier might play a role in prevention of the desensitization of GPR54 located in the POA resulting from continuous exposure to TAK-683 circulating in the bloodstream. Another possibility is that the estradiol-induced GnRH surge might be modulated by endogenous kisspeptin through a different pathway from GPR54. Although the presence of a kisspeptin receptor(s) besides GPR54 in the regulation of GnRH secretion has not been clearly identified, in a recent study supporting this hypothesis, kp-10 exhibited potent binding and activation of GPR147 and GPR74 using a binding inhibition assay in vitro [45].
Secondly, Matsui et al. [9, 12] hypothesized that depression of LH pulses after the chronic treatment with kisspeptin analogs is associated with the severe attenuation of GnRH storage due to the continuous release of GnRH by receiving continuous stimulatory signals. In their study, a single injection of kisspeptin analogs after chronic administration of the analog clearly induced cFos expression in the majority of GnRH neurons without inducing LH release, and GPR54 mRNA levels were not downregulated after chronic administration in male rats. In this case, the present results imply that estradiol under the continuous activation of GPR54 might induce the initiation of supplemental GnRH production to stock GnRH molecules for the surge on the GnRH neurons. Then, the hypothalamic GnRH content to be released for induction of the LH surge might be restored by their actions during the period between the start of estradiol infusion and the onset of the LH surge.
In conclusion, the present study revealed that, while continuous exposure to TAK-683 strongly suppresses pulsatile LH secretion, it does not suppress the occurrence of the LH surge in ovariectomized goats given estradiol in the current experimental settings. These findings suggest that the effects of continuous exposure to metastin/kisspeptin or its analog on the reaction of the mechanism(s) that regulates the pulsatile and surge mode secretion of GnRH/LH are different. They also suggest that GnRH surge generator activity is not influenced by the chronic administration of TAK-683 that suppresses the pulsatile GnRH secretion in goats.
Acknowledgments
We thank Drs Y Mori (The University of Tokyo) and AF Parlow (Harbor-UCLA Medical Center), and the National Hormone & Peptide Program for providing reagents used in the LH RIA, and Drs T Ohtaki, T Watanabe, H Matsumoto, and K Ishikawa (Takeda Pharmaceutical Company) for valuable discussions.
References
- 1.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene kiss-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411: 613–617 [DOI] [PubMed] [Google Scholar]
- 2.Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides 2009; 30: 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821 [DOI] [PubMed] [Google Scholar]
- 4.Plant TM, Ramaswamy S. Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (macaca mulatta). Peptides 2009; 30: 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 2011; 152: 1001–1012 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka T, Ohkura S, Wakabayashi H, Okamura H. Effect of peripherally administered kisspeptin-10 on GnRH neurosecretion into the hypophyseal portal circulation in ovariectomized goat does. Small Rumin Res 2012; 105: 273–276 [Google Scholar]
- 7.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073–4077 [DOI] [PubMed] [Google Scholar]
- 8.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 2004; 320: 383–388 [DOI] [PubMed] [Google Scholar]
- 9.Matsui H, Takatsu Y, Tanaka A, Asami T, Nishizawa N, Kiba A, Kumano S, Suzuki A, Kusaka M, Ohtaki T. Potent and efficient testosterone suppression by chronic administration of novel metastin analogues, TAK-448 and TAK-683, in male rats. European J Cancer suppl 2010; 8: 251 [Google Scholar]
- 10.Ohkura S, Tanaka T, Kuroiwa T, Wakabayashi Y, Ohtaki T, Kusaka M, Okamura H. Effects of investigational metastin/kisspeptin analog, TAK-683, on luteinizing hormone secretion in peripheral plasma, and gonadotropin-releasing hormone secretion in the pituitary portal circulation in goats. In: ENDO 2011; Boston, USA. Abstract P2-273.
- 11.Scott G, Ahmad I, Howard K, MacLean D, Oliva C, Warrington S, Wilbraham D, Worthington P. Double-blind, randomized, placebo-controlled study of safety, tolerability, pharmacokinetics and pharmacodynamics of TAK-683, an investigational metastin analogue in healthy men. Br J Clin Pharmacol 2013; 75: 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui H, Tanaka A, Yokoyama K, Takatsu Y, Ishikawa K, Asami T, Nishizawa N, Suzuki A, Kumano S, Terada M, Kusaka M, Kitada C, Ohtaki T. Chronic administration of the metastin/kisspeptin analog kiss1-305 or the investigational agent TAK-448 suppresses hypothalamic pituitary gonadal function and depletes plasma testosterone in adult male rats. Endocrinology 2012; 153: 5297–5308 [DOI] [PubMed] [Google Scholar]
- 13.Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, Cooke JH, Jethwa PH, Todd JF, Ghatei MA, Bloom SR. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab 2006; 291: E1074–E1082 [DOI] [PubMed] [Google Scholar]
- 14.Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (macaca mulatta). Endocrinology 2007; 148: 3364–3370 [DOI] [PubMed] [Google Scholar]
- 15.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (macaca mulatta): a finding with therapeutic implications. Endocrinology 2006; 147: 2122–2126 [DOI] [PubMed] [Google Scholar]
- 16.Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 2009; 94: 4315–4323 [DOI] [PubMed] [Google Scholar]
- 17.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res 2010; 1364: 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378 [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436 [DOI] [PubMed] [Google Scholar]
- 20.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 2006; 26: 6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of kiss1 neurons: Implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 2009; 150: 3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology 2011; 152: 214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology 2012; 153: 5406–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida N, Nishizawa N, Matsui H, Moriya Y, Kitada C, Asami T, Matsumoto H. Development and validation of sensitive sandwich elisas for two investigational nonapeptide metastin receptor agonists, TAK-448 and TAK-683. J Pharm Biomed Anal 2012; 70: 369–377 [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Tanaka T, Kamomae H. Different effects of subnormal levels of progesterone on the pulsatile and surge mode secretion of luteinizing hormone in ovariectomized goats. Biol Reprod 2003; 69: 141–145 [DOI] [PubMed] [Google Scholar]
- 26.Ohkura S, Ichimaru T, Itoh F, Matsuyama S, Okamura H. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology 2004; 145: 3239–3246 [DOI] [PubMed] [Google Scholar]
- 27.Mori Y, Kano Y. Changes in plasma concentrations of LH, progesterone and oestradiol in relation to the occurrence of luteolysis, oestrus and time of ovulation in the Shiba goat (Capra hircus). J Reprod Fertil 1984; 72: 223–230 [DOI] [PubMed] [Google Scholar]
- 28.Taya K, Watanabe G, Sasamoto S. Radioimmunoassay for progesterone, testosterone and estradiol-17β using 125I-iodohistamine radioligands. Jpn J Anim Reprod 1985; 31: 186–197 (In Japanese) [Google Scholar]
- 29.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 1986; 250: E486–E493 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T, Mori Y, Hoshino K. Hypothalamic GnRH pulse generator activity during the estradiol-induced LH surge in ovariectomized goats. Neuroendocrinology 1992; 56: 641–645 [DOI] [PubMed] [Google Scholar]
- 31.Gregg DW, Nett TM. Direct effects of estradiol-17β on the number of gonadotropin-releasing hormone receptors in the ovine pituitary. Biol Reprod 1989; 40: 288–293 [DOI] [PubMed] [Google Scholar]
- 32.Jackson GL, Kuehl D, McDowell K, Zaleski A. Effect of hypothalamic deafferentation on secretion of luteinizing hormone in the ewe. Biol Reprod 1978; 18: 808–819 [DOI] [PubMed] [Google Scholar]
- 33.Manabe Y, Yamaguchi M, Tanaka T, Mori Y. Estradiol-induced GnRH surge in ovariectomized goats. J Reprod Dev 1993; 39: 91–96 [Google Scholar]
- 34.Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol 1989; 123: 375–382 [DOI] [PubMed] [Google Scholar]
- 35.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 1990; 127: 1375–1384 [DOI] [PubMed] [Google Scholar]
- 36.Nishihara M, Takeuchi Y, Tanaka T, Mori Y. Electrophysiological correlates of pulsatile and surge gonadotrophin secretion. Rev Reprod 1999; 4: 110–116 [DOI] [PubMed] [Google Scholar]
- 37.Tsukamura H, Maeda KI, Yokoyama A. Effect of the suckling stimulus on daily LH surges induced by chronic oestrogen treatment in ovariectomized lactating rats. J Endocrinol 1988; 118: 311–316 [DOI] [PubMed] [Google Scholar]
- 38.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 1980; 36: 53–88 [DOI] [PubMed] [Google Scholar]
- 39.Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 2009; 150: 5488–5497 [DOI] [PubMed] [Google Scholar]
- 40.d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 2008; 149: 3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 2008; 149: 4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol 2010; 518: 943–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata K, Wakabayashi Y, Sakamoto K, Tanaka T, Takeuchi Y, Mori Y, Okamura H. Effects of brief exposure of male pheromone on multiple-unit activity at close proximity to kisspeptin neurons in the goat arcuate nucleus. J Reprod Dev 2011; 57: 197–202 [DOI] [PubMed] [Google Scholar]
- 44.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oishi S, Misu R, Tomita K, Setsuda S, Masuda R, Ohno H, Naniwa Y, Ieda N, Inoue N, Ohkura S, Uenoyama Y, Tsukamura H, Maeda K-I, Hirasawa A, Tsujimoto G, Fujii N. Activation of neuropeptide FF receptors by kisspeptin receptor ligands. ACS Med Chem Lett 2011; 2: 53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]