Abstract
This study was conducted to clarify the feasibility of newly developed vitrification techniques for porcine embryos using the micro volume air cooling (MVAC) method without direct contact with liquid nitrogen (LN2). Expanded blastocysts were vitrified in a solution containing 6 M ethylene glycol, 0.6 M trehalose and 2% (wt/vol) polyethylene glycol in 10% HEPES-buffered PZM-5. The blastocysts were collected from gilts and vitrified using the new device (MVAC) or a Cryotop (CT). Blastocysts were stored in LN2 for at least 1 month. After warming, cryoprotective agents were removed using a single step. Survival of the embryos was assessed by in vitro culture (Experiment 1) and by embryo transfer to recipients (Experiment 2). In Experiment 1, the embryos vitrified by the MVAC or CT and fresh embryos without vitrification (Control) were used. The survival rates of embryos in the MVAC, CT and Control groups were 88.9% (32/36), 91.7% (33/36) and 100% (34/34), respectively, after 48 h culture, and the hatching rates of embryos after 48 h incubation were 69.4% (25/36), 63.9% (23/36) and 94.1% (32/34), respectively. In Experiment 2, 64 vitrified embryos were transferred to 5 recipient gilts, and 8 healthy piglets were produced from 3 recipients in the MVAC group. Similarly, 66 vitrified embryos were transferred to 5 recipient gilts, and 9 healthy piglets were produced from 2 recipients in the CT group. These results indicated that porcine expanded blastocysts can be cryopreserved using the MVAC method without potential pathogen contamination from LN2.
Keywords: Cryopreservation, MVAC method, Porcine embryo, Vitrification
Cryopreservation of mammalian embryos allows the storage and transport of valuable genetic materials at a lower cost and under less stressful conditions compared with transport of live animals. The technology is also advantageous for minimizing the risk of disease transmission. However, it is well known that porcine embryos are highly sensitive to chilling, which makes their cryopreservation difficult [1, 2]. Therefore, cryopreservation of porcine embryos has not yet been put to practical use in the pig industry.
Recently, piglets have been produced from vitrified embryos (morulae to expanded blastocysts) by several methods such as open pulled straw (OPS) vitrification [3, 4, 5, 6, 7], Cryotop vitrification [8], metal mesh vitrification (MMV) [9] and microdroplet vitrification, which we previously reported [10]. However, there is a risk of uncontrollable contamination with pathological microorganisms from liquid nitrogen (LN2), since embryos are usually exposed to LN2 in these methods [11, 12].
Therefore, we developed a device for the micro volume air cooling (MVAC) method to avoid direct contact with LN2 during vitrification of porcine embryos. In the present study, we examined the efficiency of the MVAC method for cryopreservation of porcine expanded blastocysts and compared it with that of the Cryotop, one of the conventional vitrification methods.
Materials and Methods
Donors and embryo recovery
All animals used in the present study were maintained and fed in accordance with the manuals issued by the Animal Care and Use Committee of the National Livestock Breeding Center (NLBC). Embryos were collected from 17 donor pigs (7 Landrace and 10 Duroc gilts) >8 months old. These donors were then administered 0.562 mg of sodium cloprostenol (Planate, Schering-Plough Animal Health, Tokyo, Japan) twice with a 24-h interval during the 20–40 d gestation period. During the second treatment, 1500 IU of eCG (Serotropin; Asuka Pharmaceutical, Tokyo, Japan) was also administered, and 500 IU of hCG (Puvelogen; Novartis Animal Health, Tokyo, Japan) was given 72 h after the eCG [13, 14]. Semen was collected from a Landrace and a Duroc boar and diluted with Modena solution [15]. Donors showing estrus after the hCG treatment were artificially inseminated (AI) by a conventional method. On day 6 (day 0=the day of hCG treatment), both uterine horns were flushed (surgically or after slaughter) with M2 solution [16] to collect expanded blastocysts.
Preparation of media
The vitrification medium (ETP) contained 6 M ethylene glycol (EG; Wako Pure Chemical Industries, Osaka, Japan ), 0.6 M trehalose (Nacalai Tesque, Kyoto, Japan) and 2% (w/v) polyethylene glycol (PEG #6000 MW=7300 to 9000, Nacalai Tesque). Two types of equilibration media were prepared: EM1, containing 1.8 M EG, and EM2, containing 1.8 M EG, 0.3 M trehalose and 1% (w/v) PEG. The basic solution for vitrification and equilibration was 20 mM HEPES-buffered PZM-5 defined medium, which does not contain any animal-derived materials such as serum or bovine serum albumin (BSA) (H-PZM-5 [17, 18]). The warming and dilution medium (WDM) contained 1.8 M EG and 0.3 M trehalose in H-PZM-5.
Preparation of a novel cryopreservation device for the MVAC method
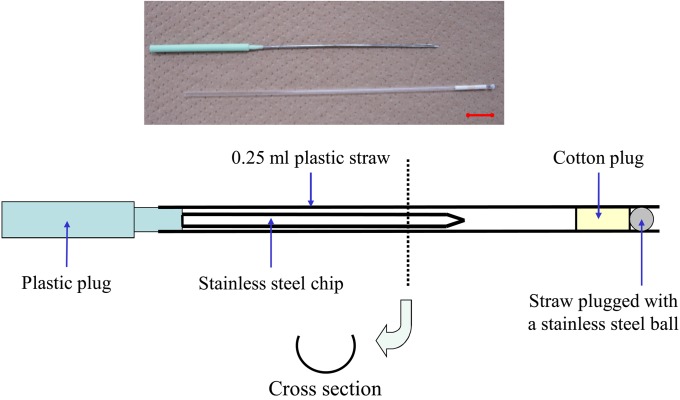
We manufactured a new device in which embryos can be vitrified on a stainless steel chip inserted into a 0.25-ml straw without direct contact with LN2 (Fig. 1). We manually crafted the device with a 19-G needle (Nipro, Osaka, Japan) and a plastic plug (Marker Rod, Cryo Bio System, IMV Technologies, L’Aigle, France). First half of the needle (approx. 4.5-cm long) was removed using a microgrinder, resulting in a U-shaped groove. The plastic connector for attachment to a syringe was removed, and the processed groove was bonded to a plastic plug.
Fig. 1.
A new device for the MVAC vitrification method. The device is made with a 19-G needle and a plastic plug. Half (one side) of the needle is removed, forming a U-shaped groove. The plastic connector is then removed, and the processed groove is bonded to a plastic plug. Bar=1 cm.
Vitrification, warming and dilution of cryoprotectants
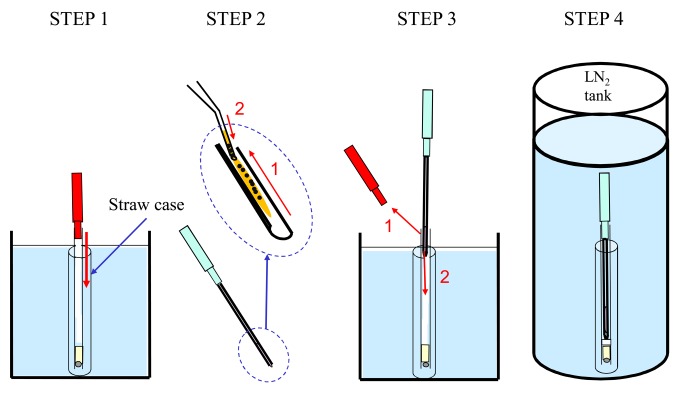
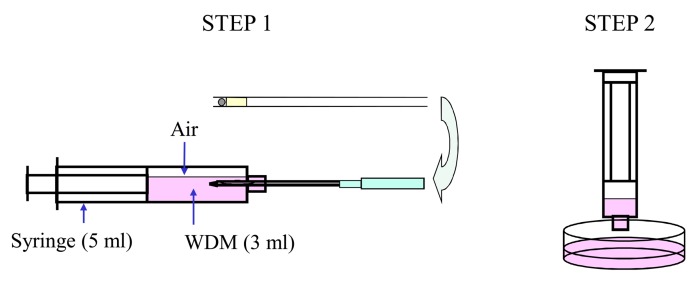
MVAC method: A straw (0.25 ml) was sealed with a plastic plug and stainless ball so that LN2 would not penetrate the interior and was precooled in LN2. Briefly, porcine embryos were equilibrated in EM1 followed by EM2 for 5 min each. After equilibration, the embryos were washed once with ETP and immediately transferred into ETP using a fine-tipped Pasteur pipette. Four to 15 embryos in approximately 1 μl vitrification medium were then placed on the inner surface of a stainless steel chip. After the end of the straw was pulled up from the surface of the LN2, the plastic plug was removed. The new device with embryos was inserted into the straw, and the straw was preserved in LN2. The process, beginning with embryo exposure to the ETP and ending with immersion in LN2, was completed within 1 min. All media used for the vitrification procedure were maintained at 38 C (Fig. 2). Storage in LN2 was carried out for at least 1 month. To warm the embryos and dilute the ETP, the device was removed from the straw and inserted into a syringe containing 3 ml WDM at 38 C for 5 min (Fig. 3).
Fig. 2.
The MVAC vitrification method. STEP 1: A 0.25-ml plastic straw that had been precooled in LN2 is sealed by a plastic plug (shown in red) so that LN2 does not enter the straw. STEP 2: After equilibration, a group of 4 to 15 embryos in approximately 1 μl of a vitrification medium is placed on the internal surface of the stainless steel chip. STEP 3: The top of the strap is raised above the surface of the LN2, and then the plastic plug (red color) is removed. LN2 does not enter the plastic straw. Next, the MVAC device is inserted entirely into the 0.25-ml straw. STEP 4: The straw into which the MVAC device with the embryos was inserted is stored in LN2; the embryos can be cryopreserved without direct contact with LN2.
Fig. 3.
Warming and dilution method after MVAC vitrification. STEP 1: The device is removed from the straw and inserted into the syringe. The syringe contains 3 ml of WDM, which was pre-warmed at 38 C. The embryos in the device are warmed and diluted with WDM for 5 min. STEP 2: The WDM containing embryos in the syringe is pushed out onto a plate, and the embryos can be collected under a stereo microscope.
Cryotop method
Vitrification of embryos using the Cryotop method was performed as described by Ushijima et al. [8] with slight modification. After an equilibration step and washing once in ETP as performed for the MVAC method, 4 to 6 embryos were loaded onto the tip of a Cryotop® device (Kitazato BioPharma, Fujinomiya, Japan) and immediately plunged into LN2. The process, beginning with embryo exposure to ETP and ending with plunging into LN2, was completed in 1 min. The EM1, EM2 and ETP solutions were maintained at 38 C. Storage in LN2 was performed in the same manner as mentioned above. The vitrified embryos attached to the Cryotop tip were directly warmed in 3 ml of the WDM in a 35-mm plastic dish and diluted for 5 min at 38 C.
Recipients and embryo transfer
Ten gilts (Landrace × Large White crossbreed) >8 months old were employed as embryo recipients. Estrus synchronization in the recipients was performed similarly to that in the donors, without the AI service. The embryos were surgically transferred into day 5 recipients as mentioned elsewhere [19].
Experiment 1: In vitro survival
To examine post-warming in vitro survival after vitrification, day 6 embryos recovered from 7 gilts were randomly allocated at each collection into the two vitrification groups (MVAC and Cryotop methods: MVAC and CT) along with a control group using fresh embryos. The vitrified embryos were warmed and diluted to remove cryoprotectants after 7–63 d of storage in LN2. These embryos were incubated in PZM-5 supplemented with 10% fetal bovine serum (FBS: Cat #10099-141, Lot #A0275242, Gibco, Invitrogen, Carlsbad, CA, USA). Embryo culture in vitro was conducted in an 80 µl drop of the above-mentioned medium covered with paraffin oil (Paraffin Liquid, 261-14, Nacalai Tesque) in a 35-mm plastic dish (TC DISH 35 × 10, Nunc, Thermo Fisher Scientific, Waltham, NA, USA) in a humidified atmosphere with 5% O2, 5% CO2 and 90% N2 at 39 C. The survival and hatching rates after 48 h incubation were examined in the 7 replicated trials.
Experiment 2: In vivo survival
To examine the post-warming in vivo survival after vitrification, day 6 embryos recovered from 10 gilts were randomly allocated at each collection into the two vitrification groups (MVAC and CT). The 66 expanded blastocysts vitrified by CT were warmed and surgically transferred into 5 recipient gilts (12 to 15 embryos were transferred into each recipient). The 64 expanded blastocysts vitrified by MVAC were warmed and surgically transferred into 5 recipient gilts (8 to 15 embryos were transferred into each recipient).
Statistical analysis
Data from Experiments 1 and 2 were subjected to one-way analysis of variance (ANOVA) with the General Linear Model procedures followed by Tukey’s test using Statistical Analysis System V. 9.3 (SAS Institute, Cary, NC, USA). Percentage data were arcsine-transformed before the analysis. P-values <0.05 were considered to be significant in both experiments.
Results
Experiment 1
Thirty-six, 36 and 34 embryos were randomly allocated into the CT, MVAC and Control groups, respectively. As shown in Table 1, there were no differences between the MVAC, CT, and Control groups in survival rates after 48 h. Regarding the development rates to hatching blastocysts after 48 h incubation, the MVAC group did not differ from the CT group, but the MVAC and CT groups showed significantly lower hatching rates (P<0.01) than the Control group.
Table 1. Survival and hatching rates after in vitro culture of vitrified embryos.
| Source of embryos | No. of embryos | Survival rate after48 h (%)a | Hatching blastocysts after 48 h (%)a |
| MVAC | 36 | 32 (88.9 ± 3.9) | 25 (69.4 ± 7.3) b |
| CT | 36 | 33 (91.7 ± 3.6) | 23 (63.9 ± 5.0) b |
| Control | 34 | 34 (100.0 ± 0.0) | 32 (94.1 ± 3.7) c |
a Means ± SEM. Seven replicated trials were performed for each embryo source. b,c Values with different subscripts within each column differ significantly (P<0.01). MVAC, micro volume air cooling using the new device (Fig. 1); CT, vitrified with Cryotop; Control, fresh embryos without vitrification.
Experiment 2
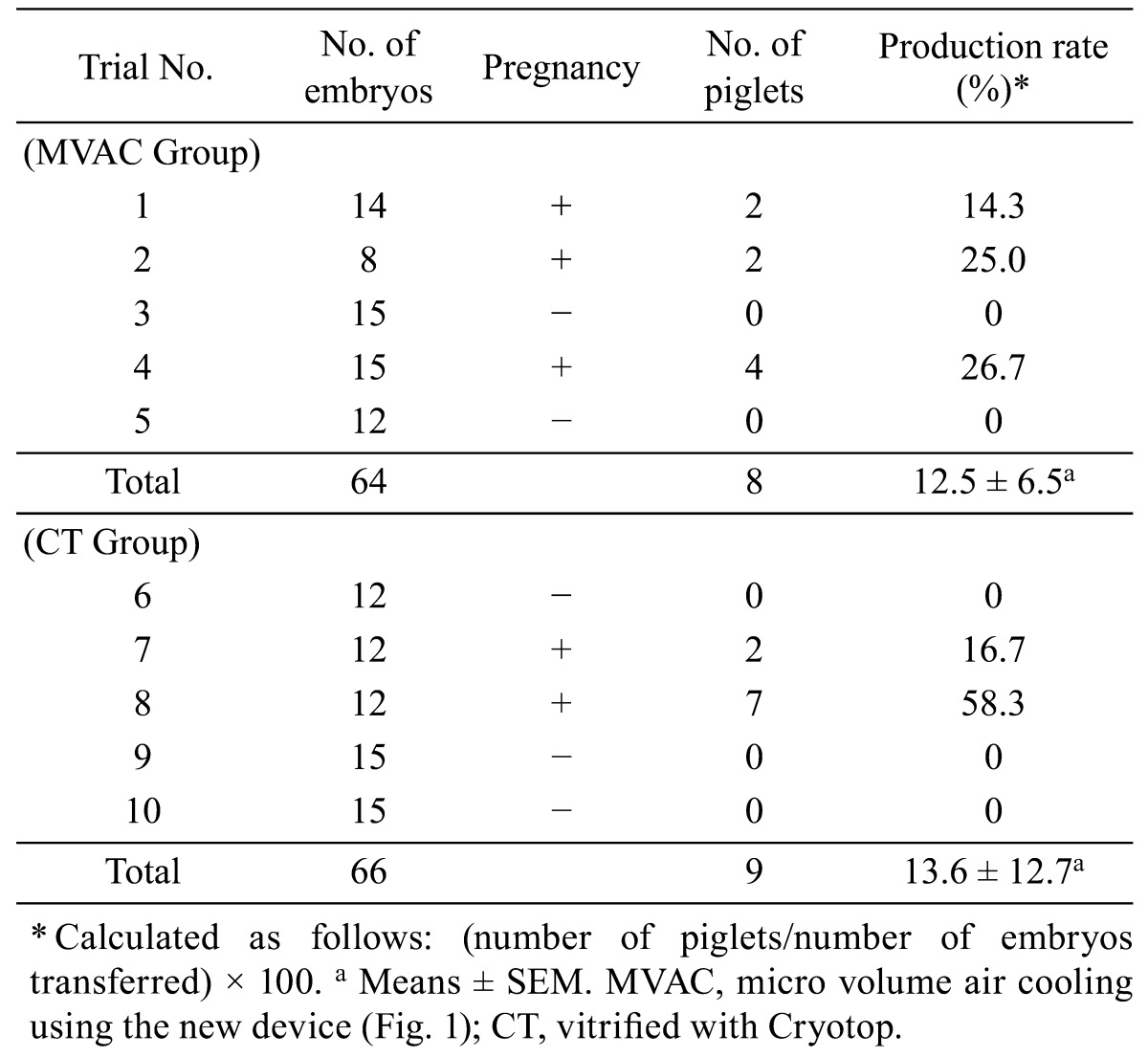
Two of the 5 recipients in the CT group became pregnant and farrowed 9 normal, live piglets. Three of the 5 recipients in the MVAC group became pregnant and delivered 8 normal, live piglets (Table 2). There was no significant difference between the MVAC and CT groups in piglet productivity (number of piglets per number of embryos transferred).
Table 2. Results of transfer of vitrified embryos and piglet production.
Discussion
The present study indicates that the MVAC vitrification method is equivalent to the Cryotop method for cryopreservation of porcine expanded blastocysts produced in vivo. With the adoption of the MVAC method, 32 out of 36 expanded blastocysts survived vitrification in in vitro culture. Furthermore, normal living piglets were obtained from transfer of the embryos vitrified using the MVAC method.
Porcine embryos have been successfully cryopreserved using ultrarapid vitrification methods such as the OPS [3, 4, 5, 6, 7], Cryotop [8] and MMV [9] methods. These methods require direct plunging of embryos into LN2 to secure a rapid cooling rate. In the present study, the viability of embryos cooled by the MVAC method was compared with that of embryos vitrified by the Cryotop method (Experiments 1 and 2). In terms of the in vitro survival of vitrified embryos, the MVAC method was equivalent to the Cryotop method (88.9% vs. 91.7%, respectively). The MVAC method was also equivalent to the Cryotop method in piglet production rate of vitrified embryos after embryo transfer (12.5% vs. 13.6%, respectively). Fujino et al. [9] reported a piglet production rate of 18.5% (37/200) when 200 expanding blastocysts were vitrified and transferred to 10 recipients (20 embryos per recipient). In the present study, 8 to 15 embryos from each donor were collected as a batch, and a single batch of embryos was transferred to a recipient. This method enables correct identification of the parents of piglets produced by embryo transfer. Two piglets were obtained from a recipient after transfer of 8 vitrified embryos (trial No. 2 in the MVAC group in Table 2). As far as we know, this may be the first report on successful production of piglets from transfer of fewer than 10 cryopreserved embryos. In this regard, the MVAC method may be an effective vitrification procedure for cryopreservation of porcine expanded blastocysts when embryo numbers are limited. The cooling rate of the MVAC method was slower than that of the Cryotop method of direct plunging into LN2. The results of measurements with a highly sensitive electronic recorder (Chino, Tokyo, Japan) of the cooling rate from 35 to −198 C by the MVAC method (860 C/min) showed a rate that was approximately 20 times slower than that obtained using the Cryotop method (−17,250 C/min). The results obtained in the present study were contrary to the common view that porcine embryos vitrified in a small volume have better viability when plunged directly into LN2. It has been reported that porcine embryos experience lethal cooling damage below 15 C [1], suggesting that the cooling rate should be increased for better survival. However, in the microdroplet method that we previously reported [10], vitrification of porcine embryos was completed at approximately −155 C using LN2 vapor. Using the microdroplet method, piglets were obtained from the transfer of vitrified embryos from the compacted morula to early blastocyst stages [10]. The cooling rate of the microdroplet method (360 C/min from 35 to −155 C) is also slower than that of the Cryotop method, which requires direct plunging into LN2. It was proven that the cooling rate enabling vitrification of the porcine expanded blastocyst has a flexible range.
Bielanski et al. reported identification of 32 bacterial species and 1 fungal species from randomly drawn liquid nitrogen, frozen semen and embryo samples stored in 8 commercial and 8 research facilities in LN2 tanks. Further, Stenotrophomonas maltophilia was detected from the outside wall of straws stored for 1 month in a LN2 tank [12]. These reports show that sterile LN2 should be used for embryo vitrification by the Cryotop and OPS methods. On the other hand, the MVAC method can prevent embryos from being contaminated by microorganisms because it does not allow direct contact with LN2. Consequently, the possibility of contamination of the vitrification medium containing the embryo by microorganisms in the LN2 tank is reduced to almost zero as long as the embryos are handled carefully, which is the major advantage of the MVAC method. This idea is now supported by other researchers. Recently, Beebe et al. [20] reported that vitrification of porcine blastocysts obtained in vivo and vitrified without any contact with LN2 using the Cryologic Vitrification Method enabled the farrowing of piglets. Further, Men et al. [21] succeeded in producing piglets from delipidated embryos produced in vitro using 0.25 ml straws. These methods are carried out using embryos that are encapsulated separately during containment and cooling. However, the MVAC method can be carried out by simultaneously cooling and encapsulation of the embryo. Thus, vitrification work using the MVAC method is simple compared with the other methods described above.
The basal solution for the equilibration, vitrification and warming and dilution media was HEPES-buffered PZM-5, which does not contain any substances of animal origin, such as serum and BSA [19]. The cryoprotectants and macromolecular substances were the same as those that we reported previously [10]. To date, the cryopreservation medium used for porcine embryos has contained fetal bovine serum and/or BSA. Such substances of animal origin may contain pathogens and other unknown factors that affect the validity and/or variability of results. Previous reports have suggested that the production lot itself may affect results, as pathogens might exist in the serum used for medium supplementation [22, 23, 24]. On this basis, Sanchez-Osorio et al. [25] reported that vitrification of porcine blastocysts and morulae using chemically defined media that did not contain any serum resulted in high survival rates during in vitro culture after warming (67.9% to 99.5%). However, the vitrified embryos were not transferred to recipients in their experiment. Accordingly, the present study may be the first successful case in which piglets derived from vitrified embryos were produced using cryopreservation media without serum or BSA.
In conclusion, the results of the present study demonstrate that porcine expanded blastocysts can be cryopreserved in a pathogen-free manner by the MVAC method using chemically defined synthetic media.
Acknowledgments
The authors are grateful to Dr T Kojima for critical reading and editing of this manuscript. The authors would like to thank Mr M Souma, Mr T Ariga and Mr T Mori for their skillful technical assistance. This research was supported in part by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry from Bio-oriented Technology Research Advancement Institution (BRAIN).
References
- 1.Polge C, Wilmut I, Rowson LEA. The low temperature preservation of cow, sheep, and pig embryos. Cryobiology 1974; 11: 560 [Google Scholar]
- 2.Pollard JW, Leibo SP. Chilling sensitivity of mammalian embryos. Theriogenology 1994; 41: 101–106 [Google Scholar]
- 3.Beebe LFS, Cameron RDA, Blackshaw AW, Higgins A, Nottle MB. Piglets born from centrifuged and vitrified early and peri-hatching blastocysts. Theriogenology 2002; 57: 2155–2165 [DOI] [PubMed] [Google Scholar]
- 4.Berthelot F, Martinat-Botte F, Perreau C, Terqui M. Birth of piglets after OPS vitrification and transfer of compacted morula stage embryos with intact zona pellucida. Reprod Nutr Dev 2001; 41: 267–272 [DOI] [PubMed] [Google Scholar]
- 5.Berthelot F, Martinat-Botte F, Locatelli A, Perreau C, Terqui M. Piglets born after vitrification of embryos using the open pulled straw method. Cryobiology 2000; 41: 116–124 [DOI] [PubMed] [Google Scholar]
- 6.Cuello C, Gil MA, Parrilla I, Tornel J, Vázquez JM, Roca J, Berthelot F, Martinat-Botté F, Martínez EA. In vitro development following one-step dilution of OPS-vitrified porcine blastocysts. Theriogenology 2004; 62: 1144–1152 [DOI] [PubMed] [Google Scholar]
- 7.Beebe LFS, Cameron RD, Blackshaw AW, Keates HL. Changes to porcine blastocyst vitrification methods and improved litter size after transfer. Theriogenology 2005; 64: 879–890 [DOI] [PubMed] [Google Scholar]
- 8.Ushijima H, Yoshioka H, Eraki R, Takahashi K, Kuwayama M, Nakane T, Nagashima H. Improved survival of vitrified in vivo-derived porcine embryos. J Reprod Dev 2004; 50: 481–486 [DOI] [PubMed] [Google Scholar]
- 9.Fujino Y, Kojima T, Nakamura Y, Kobayashi H, Kikuchi K, Funahashi H. Metal mesh vitrification (MMV) method for cryopreservation of porcine embryos. Theriogenology 2008; 70: 809–817 [DOI] [PubMed] [Google Scholar]
- 10.Misumi K, Suzuki M, Sato S, Saito N. Successful production of piglets derived from vitrified morulae and early blastocysts using a microdroplet method. Theriogenology 2003; 60: 253–260 [DOI] [PubMed] [Google Scholar]
- 11.Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallance C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology 2000; 40: 110–116 [DOI] [PubMed] [Google Scholar]
- 12.Bielanski A, Bergeron H, Lau PCK, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology 2003; 46: 146–152 [DOI] [PubMed] [Google Scholar]
- 13.Guthrie HD, Polge C. Treatment of pregnant gilts with a prostaglandin analogue, Cloprostenol, to control oestrus and fertility. J Reprod Fertil 1978; 52: 271–273 [DOI] [PubMed] [Google Scholar]
- 14.Love RJ, Grey RG. Early abortion of gilts as a way of synchronizing oestrus and improving litter size. Aust Vet J 1993; 70: 452 [DOI] [PubMed] [Google Scholar]
- 15.Weitze KF. Long-term storage of extended boar semen. Reprod Domest Anim Belfast 1991; 1: 231–253 [Google Scholar]
- 16.Quinn P, Barros C, Whittingham G. Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa. J Reprod Fertil 1982; 66: 161–168 [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka K, Suzuki C, Itoh S, Kikuchi K, Iwamura S, Rodriguez-Martinez H. Production of piglets derived from in vitro produced blastocysts fertilized and cultured in chemically defined media: effects of theophylline, adenosine and cysteine during in vitro fertilization. Biol Reprod 2003; 69: 2092–2099 [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka K, Suzuki C, Onishi A. Defined system for in vitro production of porcine embryos using a single basic medium. J Reprod Dev 2008; 54: 208–213 [DOI] [PubMed] [Google Scholar]
- 19.Hancock JL, Hovell GJR. Egg transfer in the sow. J Reprod Fertil 1962; 4: 195–201 [Google Scholar]
- 20.Beebe LFS, Bouwman EG, Mcllfatrick SM, Nottle MB. Piglets produced from in vivo blastocysts vitrified using the Cryologic Vitrification Method (solid surface vitrification) and a sealed storage container. Theriogenology 2011; 75: 1453–1458 [DOI] [PubMed] [Google Scholar]
- 21.Men H, Zhao C, Si W, Murphy CN, Spate L, Liu Y, Walters EM, Samuel MS, Prather RS, Critser JK. Birth of piglets from in vitro-produced, zona-intact porcine embryos vitrified in a closed system. Theriogenology 2011; 76: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thibier M, Nibart M. Disease control and embryo imports. Theriogenology 1987; 27: 37–47 [Google Scholar]
- 23.Wrathall AE. Embryo transfer and disease transmission in livestock: a review of recent research. Theriogenology 1995; 43: 81–88 [Google Scholar]
- 24.Guerin B, Nibart M, Marquant-Le Guienne B, Humblot P. Sanitary risks related to embryo transfer in domestic species. Theriogenology 1997; 47: 33–42 [Google Scholar]
- 25.Sanchez-Osorio J, Cuello C, Gil MA, Parrilla I, Maside C, Almiñana C, Lucas X, Roca J, Vazquez JM, Martinez EA. Vitrification and warming of in vivo-derived porcine embryos in a chemically defined medium. Theriogenology 2010; 73: 300–308 [DOI] [PubMed] [Google Scholar]