Abstract
Memory and naive CD8+ T cells exhibit distinct trafficking patterns. Specifically, memory but not naive CD8+ T cells are recruited to inflamed tissues in an antigen-independent manner. However, the molecular mechanisms that regulate memory CD8+ T cell trafficking are largely unknown. Here, using murine models of infection and T cell transfer, we found that memory but not naive CD8+ T cells dynamically regulate expression of core 2 O-glycans, which interact with P- and E-selectins to modulate trafficking to inflamed tissues. Following infection, antigen-specific effector CD8+ T cells strongly expressed core 2 O-glycans, but this glycosylation pattern was lost by most memory CD8+ T cells. After unrelated infection or inflammatory challenge, memory CD8+ T cells synthesized core 2 O-glycans independently of antigen restimulation. The presence of core 2 O-glycans subsequently directed these cells to inflamed tissue. Memory and naive CD8+ T cells exhibited the opposite pattern of epigenetic modifications at the Gcnt1 locus, which encodes the enzyme that initiates core 2 O-glycan synthesis. The open chromatin configuration in memory CD8+ T cells permitted de novo generation of core 2 O-glycans in a TCR-independent, but IL-15–dependent, manner. Thus, IL-15 stimulation promotes antigen-experienced memory CD8+ T cells to generate core 2 O-glycans, which subsequently localize them to inflamed tissues. These findings suggest that CD8+ memory T cell trafficking potentially can be manipulated to improve host defense and immunotherapy.
Introduction
Mature but antigen-naive CD8+ T cells use the peripheral blood to reach various secondary lymphoid tissues throughout the body in search of foreign antigen. Once properly activated by dendritic cells displaying antigenic peptide, costimulation, and inflammatory cytokines, naive CD8+ T cells undergo robust proliferation (1, 2). Although many of these newly formed daughter cells are short-lived, a substantial percentage will survive the contraction phase and persist for long periods of time as memory cells, capable of providing protection from pathogen reinfection (3–5). In fact, memory CD8+ T cell populations are able to confer host protection against infection in a number of different experimental models (6–9).
Along with the numerical increase of antigen-specific CD8+ T cells that occurs following memory formation, several functional differences enhance the protective capacity of memory CD8+ T cells compared with that of naive CD8+ T cells, such as the ability to rapidly produce cytokines and immediately kill infected cells following antigenic recognition (10–13). Furthermore, memory CD8+ T cells are able to populate peripheral tissues, such as the skin, lung, and gut, thereby providing a first line of defense against pathogen reinfection (14–18). In addition, recent studies have demonstrated that memory CD8+ T cells are rapidly recruited to inflamed tissues following infection in an antigen-independent fashion (19–21). Importantly, these recruited cells are also highly cytolytic and are able to provide immediate protection against pathogens expressing cognate antigen (22, 23). Although antigen-independent recruitment of memory CD8+ T cells to the lung airways has been shown to be dependent upon the CCR5 chemokine receptor (24), the molecular mechanisms controlling the recruitment of memory CD8+ T cells to inflamed tissues prior to chemokine recognition on endothelium remain ill defined.
The C-type lectin proteins of the selectin family are known to be critical regulators of immune cell homing, during both the steady state and following inflammation. L-selectin is expressed by many leukocytes and is critical for lymph node homing of naive CD8+ T cells and a subset of memory CD8+ T cells during the steady state (9, 25). In contrast, P- and E-selectin are expressed by inflamed endothelium and assist in the tissue recruitment of leukocytes that express the corresponding ligands (26). Extensive studies in neutrophils have revealed that posttranslational glycosylation plays an essential role in generating functional P- and E-selectin ligands. Specifically, core 2 O-glycans decorated with the tetrasaccharide sialyl Lewis X are critical for mediating the calcium-dependent interaction between selectins and their corresponding ligands (27, 28). Furthermore, a variety of molecules can serve as functional P- and E-selectin ligands, including P-selectin glycoprotein ligand-1 (PSGL-1), E-selectin ligand-1 (ESL-1), CD44, and perhaps more yet unidentified glycoproteins (29–31). Thus, the generation of functional ligands for P- and/or E-selectin on CD8+ T cells likely requires both the expression and appropriate glycosylation of a number of different cell surface proteins.
Studies examining the formation of functional P- and E-selectin ligands on T cells have, for the most part, been restricted to in vitro models of T cell activation (32). In general, the findings from these studies suggest that TCR stimulation is required to promote the generation of core 2 O-glycans, resulting in the formation of functional P- and E-selectin ligands on T cells. In contrast, neutrophils constitutively express P- and E-selectin ligands (30, 31, 33), demonstrating there are both fundamental and biological differences among various leukocyte populations in the regulation of the genes that ultimately dictate whether a cell will bind to a given selectin. Furthermore, the regulation of core 2 O-glycan expression on T cells responding to infection in vivo remains largely undefined. Enhanced localization of memory CD8+ T cells to various target tissues could increase the overall efficacy of vaccines for pathogens or malignancies and could be used to enhance T cell adoptive immunotherapy approaches. Therefore, we investigated the molecular mechanisms that regulate expression of core 2 O-glycans on CD8+ T cells and determined whether this contributes to the tissue-specific trafficking of memory CD8+ T cells.
Results
Antigen-specific CD8+ T cells transiently express core 2 O-glycans following viral infection.
Interactions between P- and E-selectin expressed on inflamed endothelium and the corresponding ligands expressed on immune cells coordinate the “rolling and tethering” events that are important initiators of cellular extravasation into tissues (26). Because core 2 O-linked glycosylation is critical for the formation of functional selectin ligands, we conducted a longitudinal analysis of core 2 O-glycan expression on antigen-specific CD8+ T cells following viral infection by transferring a physiologic number of naive Thy1.1+ P14 TCR-tg CD8+ T cells (specific for the lymphocytic choriomeningitis virus [LCMV] epitope GP33–41) into Thy1.2+ B6 recipients, followed by infection with LCMV Armstrong. To detect changes in expression of core 2 O-glycans, we used the monoclonal antibody 1B11, which identifies a modified isoform of CD43 that is generated following core 2 O-linked glycosylation of the protein (34–36). Naive CD8+ T cells exhibited no reactivity with the 1B11 antibody, suggesting that they do not express core 2 O-glycans on their cell surface. However, following infection with LCMV, antigen-specific CD8+ T cells strongly expressed cell surface core 2 O-glycans during the effector phase of the response (days 6–8), but 1B11 reactivity was rapidly lost and core 2 O-glycan expression was found only on a small percentage of memory CD8+ T cells (days 13–150) (Figure 1A, top row). In contrast, expression of the core 2 O-linked glycosylated isoform of CD43 on neutrophils remained stable at all time points following the viral infection (Figure 1A, bottom row). Thus, these data demonstrate that unlike neutrophils, which constitutively express core 2 O-glycans during both the steady state and during viral infection, expression of core 2 O-glycans on antigen-specific CD8+ T cells is dynamically upregulated on effector populations, but the majority of memory CD8+ T cells do not express core 2 O-glycans during homeostatic conditions.
Figure 1. CD8+ T cells transiently express core 2 O-glycans and bind P- and E-selectin following viral infection.
(A) Naive Thy1.1 P14 CD8+ T cells were transferred into naive Thy1.2 B6 mice and infected with LCMV Armstrong. Expression of core 2 O-linked glycosylated CD43 (1B11) was analyzed on P14 CD8+ T cells and neutrophils (Ly6c+, Ly6g+) from the blood at the indicated time points after infection (unfilled histogram; gray histograms represent isotype control staining. (B) P-selectin and E-selectin binding and expression of core 2 O-linked glycosylated CD43 and PSGL-1 on naive P14 CD8+ T cells or on P14 CD8+ T cells from blood on days 7 or 70 after infection with LCMV (unfilled histogram; gray histograms shown control). (C) Naive and effector (day 6 after infection) P14 CD8+ T cells generated following LCMV infection were purified and lysed. The ability of VCAM-1 (control), P-selectin, or E-selectin to precipitate PSGL-1 from either naive or effector cell lysate was determined by immunoblot (Pulldown). WCE, whole cell extracts. (D) Effector cell lysates and PSGL-1 precipitations were performed as in C. The addition of EDTA was included to determine whether the interaction was Ca2+ dependent. Data are representative of 2 or more independent experiments.
Because we observed transient expression of core 2 O-glycans on antigen-specific CD8+ T cells following LCMV infection, we next wanted to determine whether the ability of CD8+ T cells to interact with either P- or E-selectin also occurred transiently during the course of CD8+ T cell activation. We analyzed the ability of antigen-specific CD8+ T cells to bind P- and E-selectin following LCMV infection using P- and E-selectin IgG-Fc chimeric proteins. Consistent with the observation that naive CD8+ T cells do not express core 2 O-glycans, these cells also could not bind to P- or E-selectin (Figure 1B). However, on day 7 after infection, the majority of antigen-specific CD8+ T cells efficiently bound to P- and E-selectin, but only a small fraction of the CD8+ T cells could bind to these proteins on day 70 (Figure 1B). Therefore, these data demonstrate that antigen-specific effector CD8+ T cells collectively express core 2 O-glycans and bind to P- and E-selectin, but both core 2 O-glycan expression and the ability to bind P- and E-selectin is lost by the majority of memory CD8+ T cells.
PSGL-1 is a homodimeric cell surface protein that can serve as a ligand for all 3 members of the selectin family (29). Surface protein expression of PSGL-1 was readily detectable on naive CD8+ T cells, even though the cells did not express core 2 O-glycans or bind to P- or E-selectin (Figure 1B). Interestingly, surface expression of PSGL-1 did not change on antigen-specific CD8+ T cells following infection with LCMV, even though these cells efficiently bound to both P- and E-selectin (Figure 1B). Indeed, both P- and E-selectin-Fc fusion proteins readily precipitated PSGL-1 from antigen-specific effector CD8+ T cells following LCMV infection but not from naive CD8+ T cells, suggesting that the ability for PSGL-1 to become both a functional P- and E-selectin ligand on CD8+ T cells is regulated exclusively through posttranslational modification (Figure 1C). In fact, following infection, most of the PSGL-1 from antigen-specific CD8+ T cells was found to be in a slower migrating form (37), following separation by SDS-PAGE, which corresponds to the glycosylated isoform of PSGL-1 (Figure 1C). Furthermore, chelation of Ca2+ inhibited the ability of both P- and E-selectin-Fc fusion proteins to precipitate PSGL-1, demonstrating specific interactions with sialyl Lewis X structures (Figure 1D). Overall, these data demonstrate that PSGL-1 becomes glycosylated on antigen-specific CD8+ T cells following viral infection and binds to both P- and E-selectin.
Memory CD8+ T cells that localize to inflamed tissues express core 2 O-glycans.
It has been demonstrated that the initial recruitment of memory CD8+ T cells to the lung occurs in an antigen-independent fashion following viral infection (19, 24). However, it is unknown whether a specific population of memory CD8+ T cells is preferentially recruited to inflamed tissues. To address this, we transferred memory P14 CD8+ T cells (>60 days after infection) into naive hosts that were subsequently infected with the unrelated lung pathogen, respiratory syncytial virus (RSV), which does not express the epitope recognized by P14 CD8+ T cells. Indeed, on day 3 after infection with RSV, memory P14 CD8+ T cells could be found at significantly increased numbers in the lungs (Figure 2, A and B). In addition, the percentage of memory P14 CD8+ T cells expressing core 2 O-glycans also increased in the lungs following RSV infection (Figure 2, C and D). To determine whether this finding could be generalized, we next infected mice with Listeria monocytogenes, a bacterial pathogen that infects the liver. In agreement with our lung infection model, localization of memory P14 CD8+ T cells into the L. monocytogenes–infected liver was enhanced on day 3 after infection, and these cells also expressed the core 2 O-linked glycosylated isoform of CD43 (Figure 2, E–H). Thus, both viral and bacterial infections of tissues result in antigen-independent recruitment of memory CD8+ T cells that express core 2 O-glycans.
Figure 2. Memory CD8+ T cells that localize to inflamed tissues following an unrelated infection express core 2 O-glycans.
(A) 1 × 106 purified memory Thy1.1 P14 CD8+ T cells (>60 days after infection) were transferred into naive B6 (Thy1.2) mice and infected with RSV. On day 3 after infection, localization of memory P14 CD8+ T cells into the lung was analyzed by flow cytometry. (B) Quantification of total memory P14 CD8+ T cells in the lung from A. (C) Expression of core 2 O-linked glycosylated CD43 (1B11) on memory P14 CD8+ T cells from A. Numbers indicate percentages of memory CD8+ T cells that express core 2 O-linked glycosylated CD43 from mice that received no infection (shaded histogram; bottom number) or RSV (unfilled histogram; top number). (D) Quantification of expression shown in C. Studies shown in E, F, G, and H are the same as those in A, B, C, and D, respectively, except mice were infected with virulent L. monocytogenes (LM), and cells were analyzed in the liver. Data are representative of 3 or more independent experiments, and statistical analysis used the Student’s t test.
We next asked whether cognate antigen would influence the initial recruitment of memory CD8+ T cells to infected tissues. To test this, memory P14 CD8+ T cells were generated in mice with LCMV infections, as described in the legend for Figure 1. At greater than 60 days after infection, mice with similar frequencies of circulating memory P14 CD8+ T cells were infected on the ears with vaccinia virus (VacV) expressing the cognate antigen for P14 CD8+ T cells (VacV-GP33) or an irrelevant antigen (VacV-OVA). Indeed, on day 3 after infection, there were approximately 100-fold more memory P14 CD8+ T cells in the infected ears compared with those in the contralateral uninfected ears, and recruitment was independent of cognate antigen recognition (Figure 3, A and B). In agreement with both RSV and L. monocytogenes infections, memory P14 CD8+ T cells that localized to VacV-infected ears expressed core 2 O-linked glycosylated CD43, and this also did not require antigen specificity (Figure 3C). Therefore, these data demonstrate that the initial recruitment of memory CD8+ T cells to infected tissues does not require recognition of cognate antigen.
Figure 3. Memory CD8+ T cell recruitment to VacV-infected skin is independent of cognate antigen recognition.
(A) LCMV-immunized mice (>60 days after infection) containing memory Thy1.1+ P14 CD8+ T cells were infected on the left ear with VacV-GP33 or VacV-OVA. Accumulation of memory P14 CD8+ T cells was analyzed in the uninfected ear and infected ear on day 3 after infection. Numbers in parentheses indicate total number of memory P14 CD8+ T cells in the ear. (B) Cumulative data from A. (C) Expression of core 2 O-linked glycosylated CD43 from A. Statistical analysis used the Student’s t test.
Previous studies have demonstrated that recruitment of memory CD8+ T cells to the lung will also occur in response to intranasal challenge with TLR agonists (22, 24). Therefore, we next determined whether memory CD8+ T cells expressing core 2 O-glycans would also be recruited following challenge with a TLR agonist, which will elicit inflammation in the absence of protein antigens. Indeed, intranasal administration of the TLR9 agonist, CpG, resulted in a progressive increase in core 2 O-linked glycosylated CD43 expression on memory P14 CD8+ T cells that accumulated in the lungs (Figure 4, A–C). Importantly, accumulation of memory CD8+ T cells in the lungs following CpG challenge was also accompanied by decreased representation of this cell population in both the blood and spleen (Figure 4D), suggesting that memory CD8+ T cells are recruited out of the circulation and into the inflamed lung. The increased number of memory CD8+ T cells in the inflamed lungs was not due to proliferation, as these cells did not incorporate BrdU following CpG challenge (Figure 4E). Similar results were found when mice were challenged with the TLR4 agonist LPS (Figure 4, F–H). Collectively, these data suggest that, in response to localized inflammatory stimuli, memory CD8+ T cells are recruited out of the circulation and into inflamed tissues and these cells express core 2 O-glycans.
Figure 4. Intranasal challenge with TLR agonists recruits memory CD8+ T cells that express core 2 O-glycans.
(A) 1 × 106 purified memory Thy1.1 P14 CD8+ T cells were transferred into naive B6 (Thy1.2) mice. One day later, mice were challenged intranasally with CpG. At 12, 36, and 60 hours following CpG challenge, expression of core 2 O-linked glycosylated CD43 (1B11) was analyzed on memory P14 CD8+ T cells in the lung. (B) Quantification of expression shown in A. (C) Quantification of total memory P14 CD8+ T cells in the lung 60 hours following challenge from A. (D) Same as in A, with the exception that at 60 hours following CpG challenge, the frequency of Thy1.1 memory P14 CD8+ T cells was analyzed in the blood, spleen, and lung. (E) Same as in A, with the exception that proliferation of memory P14 CD8+ T cells in the lung was determined by BrdU incorporation. (F) Same as in A, with the exception that mice were challenged with LPS. (G) Quantification of total memory P14 CD8+ T cells from F. (H) Expression of core 2 O-linked glycosylated CD43 on P14 CD8+ T cells in the lungs from mice that received saline (shaded histogram; bottom number) or LPS (unfilled histogram; top number). Numbers indicate percentages of memory CD8+ T cells that express glycosylated CD43. Data are representative of 3 or more independent experiments, and statistical analysis used the Student’s t test.
P- and E-selectin interactions regulate memory CD8+ T cell recruitment to inflamed tissue.
Because our data suggested that memory CD8+ T cells expressing core 2 O-glycans were preferentially recruited to inflamed tissues, we next determined whether memory CD8+ T cells also bound to P- and/or E-selectin. Indeed, following CpG treatment, memory CD8+ T cells expressing core 2 O-linked glycosylated CD43 that were recruited to the lungs also bound both P- and E-selectin (Figure 5A). Interestingly, not all 1B11+ memory CD8+ T cells bound E-selectin, suggesting expression of core 2 O-glycans alone may not be sufficient for the generation of functional E-selectin ligands. Furthermore, i.v. administration of blocking antibodies against both P- and E-selectin or PSGL-1 inhibited memory CD8+ T cell recruitment to the lungs following CpG challenge (Figure 5, B and C). These data suggest that expression of functional ligands for P- and E-selectin on memory CD8+ T cells is critical for their antigen-independent recruitment to inflamed tissues.
Figure 5. P- and E-selectin interactions are required for memory CD8+ T cell recruitment to inflamed tissues.
(A) 1 × 106 purified memory P14 CD8+ T cells (Thy1.1) were transferred into naive B6 mice (Thy1.2) that were then challenged intranasally with either saline or CpG. Three days after challenge, memory P14 CD8+ T cells in the lung were analyzed for coexpression of core 2 O-linked glycosylated CD43 (1B11) and the ability to bind to either P- or E-selectin. (B and C) 1 × 106 memory P14 CD8+ T cells were transferred into naive B6 mice followed by intranasal challenge with either saline or CpG. On day 3 after challenge, total memory P14 CD8+ T cells in the lungs were quantified in mice that received i.v. administration of an isotype control antibody or blocking antibodies against either (B) P- and E-selectin or (C) PSGL-1. (D) Mice containing memory P14 CD8+ T cells were generated as in Figure 3 and infected on the left ear with VacV-GP33. Total P14 CD8+ T cells in the ears were quantified on day 3 after infection in mice receiving an isotype control or P- and E-selectin–blocking antibodies. (E) Same as in D, with the exception that viral burden of the ear was determined on day 4 after infection. Data are representative of 2 or more independent experiments, and statistical analysis used 1-way ANOVA with a Bonferroni post-test for multiple comparisons.
We next asked whether the initial antigen-independent recruitment of memory CD8+ T cells to infected tissue was required for providing protective immunity against pathogens expressing cognate antigens. As shown in Figure 3, recruitment of memory CD8+ T cells to VacV-infected skin was independent of cognate antigen. However, recruitment does require interactions with P- and E-selectin, as blocking antibodies against these proteins prevented memory P14 CD8+ T cell recruitment to the VacV-GP33–infected ears (Figure 5D). In addition, when LCMV-immune mice were infected with VacV-GP33, the GP33-specific memory CD8+ T cells rapidly decreased viral burden in the skin, and this was dependent on their recruitment, as treatment of immune mice with blocking antibodies against P- and E-selectin resulted in viral loads that were similar to those in VacV-GP33–infected naive animals (Figure 5E). Collectively, these data demonstrate that the protective immunity provided by circulating antigen-specific memory CD8+ T cells requires antigen-independent P- and E-selectin–mediated recruitment.
Inflammatory signals are sufficient to generate core 2 O-glycans on circulating memory CD8+ T cells.
The observation that most memory CD8+ T cells express core 2 O-glycans in the lung following inflammatory challenge suggested that these cells were preferentially recruited. However, the previous experiments were not able to differentiate whether this was solely due to the recruitment of the small percentage of memory cells that expressed core 2 O-glycans or whether inflammatory cues were also sufficient to generate new core 2 O-glycans on memory CD8+ T cells. To test this, memory P14 CD8+ T cells that did not express core 2 O-linked glycosylated CD43 (or bound P- or E-selectin; J.C. Nolz and J.T. Harty, data not shown) were purified (Figure 6A) and transferred into naive hosts. Following CpG treatment, a high percentage of the transferred 1B11lo memory CD8+ T cells found in the inflamed lung now expressed core 2 O-linked glycosylated CD43, whereas most of the cells in control-treated mice did not (Figure 6B). Thus, these data demonstrate that generation of core 2 O-glycans is not a static process that only occurs following antigen recognition by CD8+ T cells but that it can be dynamically regulated by a TCR-independent mechanism in memory CD8+ T cells following inflammatory challenge.
Figure 6. Inflammation is sufficient to generate core 2 O-glycans on circulating memory CD8+ T cells.
(A) Pre- and post-sort analysis following depletion of memory P14 CD8+ T cells isolated from the spleen that expressed core 2 O-linked glycosylated CD43 (1B11). (B) 1 × 106 1B11lo cells (Thy1.1) from A were transferred into naive B6 mice (Thy1.2) that were then challenged with either saline or CpG. Three days after challenge, memory P14 CD8+ T cells in the lungs were analyzed for the expression of core 2 O-linked glycosylated CD43 (1B11). (C and D) 1 × 106 purified memory P14 CD8+ T cells (Thy1.1) were transferred into naive B6 mice (Thy1.2) followed by intranasal challenge with either saline or CpG. One group of mice received pertussis toxin (Ptx) following CpG challenge. On day 3 after challenge, the number of memory P14 CD8+ T cells in the (C) lung and (D) blood was determined. (E) Expression of core 2 O-linked glycosylated CD43 on memory P14 CD8+ T cells in the lung and blood from C and D. Data are representative of 2 independent experiments, and statistical analysis used 1-way ANOVA with a Bonferroni post-test for multiple comparisons.
Because we observed de novo generation of core 2 O-glycans on memory CD8+ T cells following exposure to inflammation, we next determined whether this occurred prior to their localization to inflamed tissue. To test this, we used pertussis toxin to inhibit G protein–coupled chemokine receptor signaling in vivo. Indeed, treatment of mice with pertussis toxin following intranasal administration of CpG resulted in impaired trafficking to the inflamed lungs, and memory CD8+ T cells instead accumulated in the blood (Figure 6, C and D). Importantly, the majority of memory CD8+ T cells that were now “trapped” in the peripheral blood were found to express core 2 O-linked glycosylated CD43 (Figure 6E), demonstrating that de novo expression of core 2 O-glycans occurs on circulating memory CD8+ T cells prior to tissue localization. Overall, these data demonstrate that inflammatory signals are sufficient to drive expression of core 2 O-glycans on memory CD8+ T cells that are in circulation.
Memory and naive CD8+ T cells exhibit the opposite pattern of epigenetic modifications at the Gcnt1 gene locus.
To understand the molecular mechanisms regulating de novo expression of core 2 O-glycans, we first determined whether recruitment to inflamed tissues and the corresponding increase in core 2 O-linked glycosylated CD43 expression occurred specifically on memory CD8+ T cells. Equal numbers of Thy1-disparate memory and naive P14 CD8+ T cells were transferred into the same WT recipient, followed by intranasal challenge with CpG. Three days later, the total number of memory CD8+ T cells in the lungs was substantially higher, whereas the number of recovered naive CD8+ T cells did not change (Figure 7A). In addition, increased expression of core 2 O-linked glycosylated CD43 also only occurred on the memory CD8+ T cell population (Figure 7B). Importantly, no changes in either localization or expression of core 2 O-glycans occurred when WT memory CD8+ T cells were transferred into CpG-treated Tlr9–/– hosts (Figure 7), suggesting that TLR agonists do not act directly on memory CD8+ T cells. These data demonstrate that the localized inflammatory response generated following TLR stimulation acts specifically on circulating memory CD8+ T cells, resulting in de novo expression of core 2 O-glycans. Furthermore, these data suggest the intriguing notion that prior antigen encounter programs genetic changes in memory CD8+ T cells that control their recruitment to inflamed tissues.
Figure 7. Localization to inflamed tissue and generation of core 2 O-glycans is restricted to antigen-experienced CD8+ T cells.
(A) Equal numbers (1 × 106) of memory (Thy1.1/1.2) and naive (Thy1.1/1.1) P14 CD8+ T cells were transferred into WT or Tlr9–/– naive B6 (Thy1.2/1.2) mice and then challenged intranasally with either saline or CpG. Three days after challenge, total numbers of both naive and memory P14 CD8+ T cells were quantified in the lungs using Thy1.1 and Thy1.2 expression to discriminate between the 2 P14 CD8+ T cell populations. (B) Same as in A, with the exception that expression of core 2 O-linked glycosylated CD43 (1B11) was analyzed on both naive and memory P14 CD8+ T cells in the lungs of WT and Tlr9–/– mice. Statistical analysis used the Student’s t test.
Formation of core 2 O-glycans and generation of the functional sialyl Lewis X first requires the core 1 O-glycan structure as a substrate (Figure 8A), which can be detected with the lectin jacalin (38). In contrast to the transient expression of core 2 O-glycans, core 1 O-glycan expression remains unchanged on CD8+ T cells following LCMV infection and on the ensuing memory population (Figure 8B). The interaction between jacalin and core 1 O-glycans on CD8+ T cells was specific, as binding can be inhibited with saturating amounts of galactose. This prompted us to analyze changes in expression of core 2 β1,6-N-acetylglucosaminyltransferases (C2GlcNAcT) in CD8+ T cells, which catalyze formation of the core 2 O-glycan structure (Figure 8A). Both the mouse and human genomes contain 3 C2GlcNAcT genes, Gcnt1, Gcnt3, and Gcnt4 (Gcnt2 does not exhibit core 2 activity) (39, 40). However, following LCMV infection, only expression of Gcnt1 (both mRNA and protein) increased in antigen-specific CD8+ T cells (Figure 8, C and D). Importantly, Gcnt1 expression was not readily detectable in memory CD8+ T cells compared with that in the effector population, consistent with the observation that the majority of memory CD8+ T cells did not express core 2 O-glycans during the steady state. Thus, these data demonstrate that Gcnt1 expression in antigen-specific CD8+ T cells is transiently upregulated following viral infection and TCR ligation but is not strongly expressed by memory CD8+ T cells during the steady state.
Figure 8. Regulation of Gcnt1 expression in antigen-specific CD8+ T cells following infection.
(A) The core 1 O-glycan structure originating from a serine or threonine amino acid (S/T) is formed by the addition of galactose in a β1-3 linkage to N-acetylgalactosamine. Generation of the core 2 structure occurs following activity of one of three core 2 β1,6-N-acetylglucosaminyltransferases (C2GlcNAcT) on the core 1 structure. Subsequent activity by other glycotransferases ultimately results in core 2 O-glycan extension and generation of the sialyl Lewis X. (B) Core 1 O-glycan expression (binding of the lectin jacalin) of P14 CD8+ T cells following LCMV infection. (C) Quantification of Gcnt1, Gcnt3, and Gcnt4 mRNA in naive, effector (day 5 after infection), and memory (day 75 after infection) P14 CD8+ T cells following LCMV infection. Changes in gene expression were determined using Hprt as a reference gene. (D) Naive, effector, and memory P14 CD8+ T cells were purified, and Gcnt1 expression was determined by immunoblot. (E) Using transcript variant-specific 5′ primers, only expression of Gcnt1 transcript variant 1 could be detected in antigen-specific CD8+ T cells following LCMV infection. (F) Enrichment of trimethylated lysine 27 (H3K27me3) and trimethylated lysine 4 (H3K4me3) histone H3 modifications at the Gcnt1 promoter region in naive and memory CD8+ T cell populations. Values on the x axis indicate the median position of the amplified region of DNA relative to the transcription start site of Gcnt1 variant 1. Data are representative of 2 or more independent experiments.
Covalent modifications of histones are critical regulators of gene expression (41). Because our data suggested that memory but not naive CD8+ T cells could express core 2 O-glycans in response to inflammation, we next determined whether antigen encounter in vivo resulted in differential epigenetic modifications at the Gcnt1 locus. Three transcript variants of Gcnt1, each with a unique transcription start site but a single, common translated exon, have been identified in B6 mice. However, in antigen-specific effector CD8+ T cells, only one transcript variant could be detected following LCMV infection (Figure 8E). Thus, we analyzed histone H3 modifications in the 5′ promoter region of this Gcnt1 transcription start site. Indeed, in naive CD8+ T cells, this region of DNA was heavily enriched with histone H3 trimethylated lysine 27 (H3K27me3), a histone modification associated with inactive genes (Figure 8F). Furthermore, in naive CD8+ T cells, there was no enrichment of histone H3 trimethylated lysine 4 (H3K4me3) at the Gcnt1 promoter, which is associated with genes that can be actively transcribed. In contrast, memory CD8+ T cells exhibited the opposite pattern of histone H3 modifications, with an enrichment of H3K4me3 and decreased H3K27me3 at the Gcnt1 promoter (Figure 8F). Therefore, these data demonstrate that, although memory CD8+ T cells do not express high levels of Gcnt1 protein during the steady state, the Gcnt1 locus exhibits an epigenetic pattern consistent with genes that can be actively transcribed.
IL-15 regulates core 2 O-glycan and Gcnt1 expression in memory CD8+ T cells.
Previous reports have demonstrated that a variety of immune cells (i.e., dendritic cells, macrophages, inflammatory monocytes) express IL-15 following an inflammatory challenge, and this contributes to the “early activation” of memory CD8+ T cells, independent of antigen recognition (42, 43). In agreement with this, following respiratory challenge with CpG, increased levels of IL-15 could be detected in both the sera and spleens (Figure 9, A and B). Furthermore, we found that IL-15 was a potent stimulator of core 2 O-glycan expression on memory CD8+ T cells in vitro (Figure 9C), resulting in binding to both P- and E-selectin (Figure 9D). Importantly, in agreement with our data demonstrating epigenetic differences at the Gcnt1 locus, IL-15 did not cause expression of core 2 O-glycans on naive CD8+ T cells (Figure 9C). Furthermore, addition of the STAT5 inhibitor, N′-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide, blocked IL-15–dependent expression of core 2 O-glycans on memory CD8+ T cells in a dose-dependent fashion (Figure 9E). Finally, stimulation with IL-15 also increased Gcnt1 protein expression in memory CD8+ T cells, resulting in the generation of glycosylated isoforms of PSGL-1, as indicated by slower mobility following separation by SDS-PAGE (Figure 9F). Collectively, these data show that, in memory CD8+ T cells, Gcnt1 protein expression and generation of core 2 O-glycans can be regulated by IL-15 in a STAT5-dependent, but TCR-independent, manner.
Figure 9. IL-15 regulates expression of core 2 O-glycans and Gcnt1 in memory CD8+ T cells.
(A and B) Naive B6 mice were challenged intranasally with CpG, and IL-15 levels were measured in the (A) sera and (B) spleens. LOD, limit of detection. (C) Naive and memory P14 CD8+ T cells were purified and cultured in vitro for 3 days with the indicated concentrations of IL-15. Expression of core 2 O-linked glycosylated CD43 (1B11) was then determined. (D) Memory P14 CD8+ T cells were purified and cultured in vitro for 3 days in media alone (control) or with 50 ng/ml IL-15. The ability for the cells to bind to either P- or E-selectin was then determined using P- and E-selectin chimeric proteins. (E) Memory P14 CD8+ T cells were purified and cultured in vitro with 50 ng/ml IL-15 and the indicated concentrations of the STAT5 inhibitor N′-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide for 3 days. Expression of core 2 O-linked glycosylated CD43 (1B11) was then determined. (F) Memory CD8+ T cells were purified and cultured in vitro for 3 days with the indicated concentrations of IL-15. Expression of Gcnt1 and glycosylation of PSGL-1 from whole cell extracts was then determined by immunoblot.
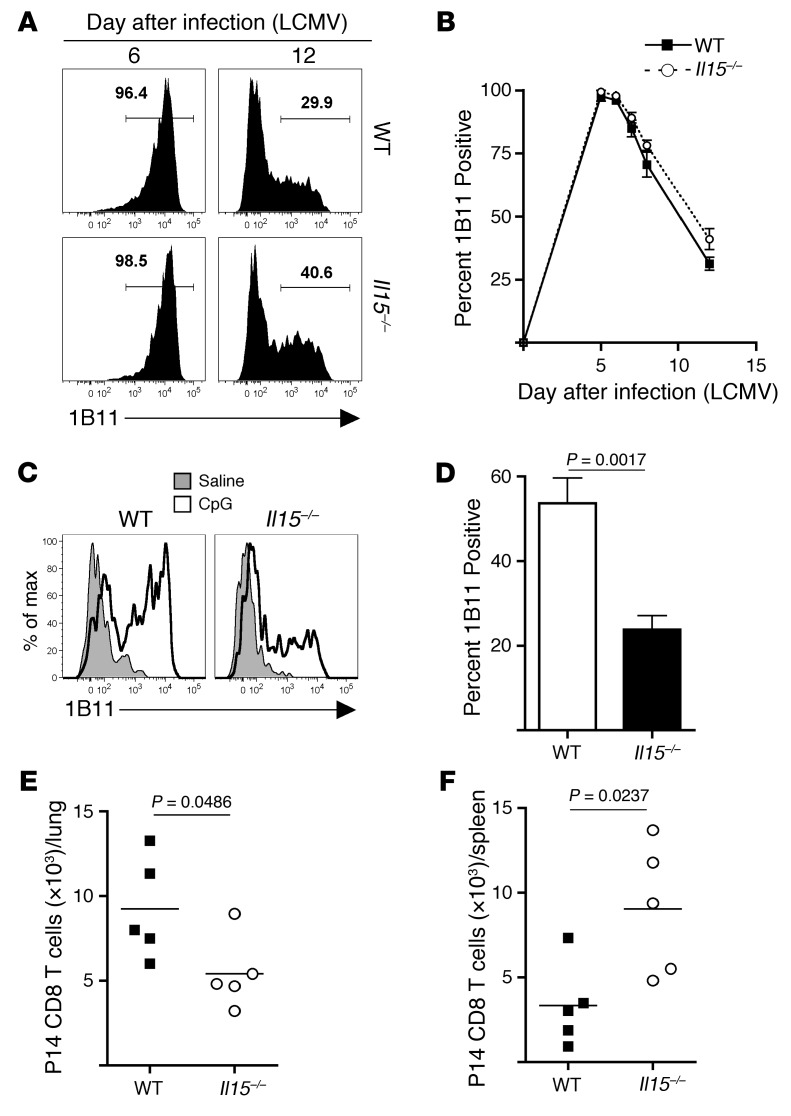
Because we found that IL-15 could dramatically increase expression of both Gcnt1 and core 2 O-glycans on memory CD8+ T cells, we next tested whether IL-15 signaling was also required in vivo. First, naive P14 CD8+ T cells were transferred into either WT or Il15–/– recipients that were then infected with LCMV. Expression of core 2 O-glycans on antigen-specific effector CD8+ T cells was similar in both WT and Il15–/– recipients following viral infection (Figure 10, A and B), suggesting that TCR stimulation may be sufficient for the generation of selectin ligands on effector CD8+ T cells. Thus, this result prompted us to specifically analyze whether de novo changes in core 2 O-glycan expression on memory CD8+ T cells required IL-15. 1B11-depleted WT memory P14 CD8+ T cells were purified and transferred into either WT or Il15–/– recipients, followed by intranasal challenge with CpG. Indeed, de novo expression of core 2 O-glycans on memory CD8+ T cells in Il15–/– recipients was impaired compared with that on those cells transferred into WT recipients (Figure 10, C and D). This resulted in less recruitment of memory P14 CD8+ T cells to the inflamed lungs of Il15–/– recipients and increased representation in the spleens (Figure 10, E and F). Therefore, these data demonstrate that IL-15 regulates core 2 O-glycan expression on memory CD8+ T cells during inflammatory challenges but is not required for core 2 O-glycan synthesis on effector CD8+ T cells generated following antigenic stimulation.
Figure 10. IL-15 is required for de novo generation of core 2 O-glycans on memory CD8+ T cells in response to inflammation.
(A) Naive P14 CD8+ T cells were transferred into WT or Il15–/– mice and infected with LCMV. Core 2 O-glycosylated CD43 expression was analyzed on days 6 and 12 after infection. (B) Quantification of data from A over time. (C) 1B11lo WT memory P14 CD8+ T cells were purified by FACS, and 5 × 105 cells were transferred into either WT or Il15–/– recipients that were subsequently challenged intranasally with CpG. On day 3 after challenge, de novo expression of core 2 O-linked glycosylated CD43 (1B11) was analyzed on cells in the lung. (D) Quantification of 1B11 expression on memory P14 CD8+ T cells in the lung. (E and F) Quantification of memory P14 CD8+ T cells in the (E) lung and (F) spleen. Data are representative of 2 or more independent experiments, and statistical analysis used the Student’s t test.
IL-15 stimulates P- and E-selectin binding of antigen-experienced human CD8+ T cells.
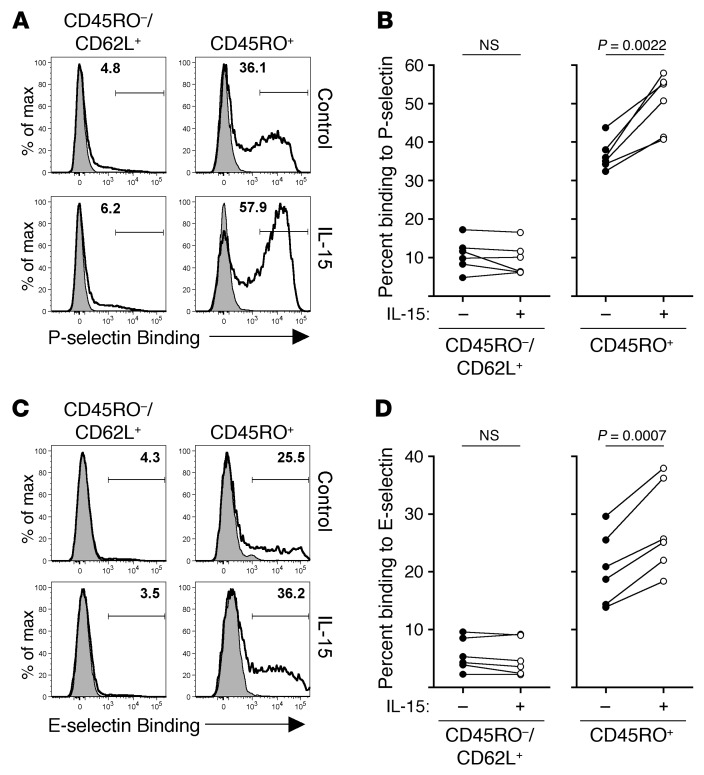
To extend the clinical relevance of our study, we next tested whether IL-15 would stimulate human CD8+ T cells to bind P- and/or E-selectin. Purified human CD8+ T cells from normal, healthy donors were cultured in vitro for 3 days with or without IL-15. Binding to P-selectin was detectable primarily in the “antigen-experienced” CD8+ T cell population (CD45RO+) and was further increased following IL-15 stimulation (Figure 11, A and B). In agreement with our previous data, binding to P-selectin was not detected readily in “naive” CD8+ T cells (CD45RO–, CD62L+) and was not induced following culture with IL-15 (Figure 11, A and B). Similar results were obtained when analyzing binding to E-selectin (Figure 11, C and D). Thus, these data demonstrate that the ability to bind to P- and E-selectin is largely restricted to previously activated CD45RO+ human CD8+ T cells and is enhanced by IL-15 stimulation.
Figure 11. P- and E-selectin binding within human CD8+ T cell populations.
(A) Human CD8+ T cells were purified and cultured for 3 days with or without 250 ng/ml IL-15. The ability to bind to P-selectin was then analyzed on the indicated CD8+ T cell populations. (B) Cumulative data of 6 individual donors as shown in A. (C and D) Same as in A and B, with the exception that data were analyzed for binding to E-selectin. Statistical analysis used the paired Student’s t test.
Discussion
Antigen-independent, rapid recruitment of memory T cells has recently emerged as a critical feature of cell-mediated protective immunity (20, 22, 44). However, the molecular mechanisms regulating this process remained largely unknown. Our results demonstrate that memory CD8+ T cells rapidly generate core 2 O-glycans and bind to both P- and E-selectin in response to inflammation. Importantly, by directly comparing memory and naive CD8+ T cell populations, we demonstrate that the mechanisms that dictate antigen-independent CD8+ T cell recruitment to inflamed tissues are restricted to those cells that have previously been activated by antigen. Specifically, we show that the Gcnt1 gene locus, which is critical for generation of core 2 O-glycans, undergoes chromatin remodeling and remains “open” in memory CD8+ T cells. Furthermore, we show that, in response to an inflammatory challenge, IL-15 signaling drives Gcnt1 expression, resulting in memory CD8+ T cells that strongly express core 2 O-glycans. Finally, we show that, in human CD8+ T cell populations, binding to P- and E-selectin is found almost exclusively in the antigen-experienced CD45RO+ population and binding can be further enhanced with IL-15 stimulation in vitro. Overall, these data demonstrate that appropriate activation of naive CD8+ T cells during infection drives permanent genetic changes, which allow the resulting memory population to quickly respond to inflammatory cues and synthesize core 2 O-glycans. Memory CD8+ T cells can then bind to P- and E-selectin, expressed specifically on inflamed tissues, and, if cognate antigen is present, provide host protection against reinfection.
Although recent studies suggest that there are specialized subsets of memory CD8+ T cells that may populate certain peripheral tissues for extended periods of time, most memory CD8+ T cells are able to circulate freely among the blood, spleen, and lymphoid compartments (45). Therefore, it would seem advantageous for the host to rapidly recruit all memory CD8+ T cells to sites of localized inflammation, with the expectation that a certain percentage of these cells will recognize a previously encountered pathogen and be able to immediately control reinfection. Furthermore, memory CD8+ T cells are capable of undergoing localized proliferation in nonlymphoid tissues following the recognition of cognate antigen (46). Thus, the nonspecific recruitment of memory cells to inflamed tissues results in accelerated pathogen recognition that does not depend on expansion of newly primed naive CD8+ T cells or memory CD8+ T cells following their activation (or reactivation) in the draining lymph node. Overall, these studies would suggest that the antigen-independent mobility of the memory CD8+ T cell compartment is critical for early control of previously encountered pathogens.
Expression of Gcnt1 has been shown to be critical for the formation of functional ligands for P- and E-selectin when CD8+ T cells are activated by TCR signals in vitro (32, 47–50). In these experimental systems, the addition of cytokines, such as IL-12 or IL-2, enhanced the TCR-dependent expression of Gcnt1. However, a more recent study suggested that IL-2 and IL-12 are dispensable for the formation of functional P-selectin ligands when CD8+ T cells respond to antigen in vivo (51). Thus, previous data suggest that TCR ligation is necessary but also sufficient to generate functional P- and E-selectin ligands on CD8+ T cells. In agreement with those studies, our data clearly show that ligation of the TCR is required for the generation of core 2 O-glycans on previously naive CD8+ T cells. However, in contrast to these studies, we now demonstrate that core 2 O-glycan expression can be regulated in memory CD8+ T cells in a TCR-independent, IL-15–dependent fashion, which is critical for appropriately localizing memory CD8+ T cell populations to inflamed tissue. In fact, a recent study also demonstrates that IL-15 increases expression of granzyme B in memory CD8+ T cells (42). Thus, this suggests that IL-15 is a critical cytokine that regulates both early activation and mobilization of memory CD8+ T cells, allowing for enhanced cytolytic activity and specific localization to inflamed tissues that ultimately results in optimal protective immunity.
In conclusion, our data demonstrate that memory CD8+ T cells rapidly generate core 2 O-glycans following exposure to inflammation. Furthermore, we demonstrate that memory CD8+ T cells are programmed to rapidly generate core 2 O-glycans, whereas naive CD8+ T cells are not. Thus, memory CD8+ T cells are able to efficiently localize to inflamed tissues, like cells of the innate immune system, such as neutrophils. However, in contrast to neutrophils, which constitutively express selectin ligands, memory CD8+ T cells only generate functional selectin ligands during episodes of inflammation. The reason for this altered expression of core 2 O-glycans and functional selectin ligands among different leukocyte populations can only be speculated upon, but it may be that it is beneficial to the host that neutrophils localize to newly infected tissue more rapidly than memory lymphocytes. Furthermore, the half-life of neutrophils is incredibly short (<10 hours) (52) compared with the life-long persistence of memory CD8+ T cell populations. Thus, the time it takes for memory CD8+ T cells to express core 2 O-glycans in response to inflammation is actually longer than the life span of most neutrophils. Overall, our data suggest that memory CD8+ T cells could potentially be manipulated both in vivo (for vaccinations) or ex vivo (for various immunotherapies) to localize more efficiently to inflamed tissues and provide enhanced protection against disease.
Methods
Mice and pathogens.
C57BL/6 mice were obtained from the National Cancer Institute and used for experiments at 6 to 10 weeks of age. Il15–/– mice were purchased from Taconic. P14 TCR-tg (Thy1.1+ and Thy1.1+/1.2+) mice have been previously described (53) and maintained by sibling × sibling mating. Tlr9–/– B6 mice were provided by Joel Kline (University of Iowa). LCMV Armstrong (2 × 105 PFUs) was injected i.p. Virulent L. monocytogenes (strain 10403s) was injected i.v. at a dose of 5 × 104 CFUs. For infection with RSV (strain A2, provided by Steve Varga, University of Iowa), mice were anesthetized with isoflurane and 3 × 106 PFUs were administered intranasally. VacV (54) infections of the skin were performed by inoculating 5 × 106 PFUs in 5 μl saline on the center of the ventral side of the ear pinna and poking 25 times with a 27-gauge needle. Viral titers were quantified with standard plaque assaying on VERO cells as previously described (9).
TLR agonist administration and antibody treatment.
For administration of TLR agonists, 50 nmoles CpG ODN 1826 (Integrative DNA Technology) or 5 μg LPS (Sigma-Aldrich) was diluted in sterile saline and administered intranasally to anesthetized mice. Pertussis toxin (1 μg) from Bordetella pertussis (Calbiochem) was diluted in sterile saline and injected i.p. on days 1 and 2 following CpG administration. Antibodies against P-selectin (clone RB40.34, 200 μg), E-selectin (clone 9A9, 200 μg), and PSGL-1 (clone 4RA10, 300 μg) or control rat IgG were administered i.v. 2 consecutive days before and 2 consecutive days after CpG administration or VacV infection.
BrdU incorporation.
Mice were injected i.p. with 2 mg BrdU (Sigma-Aldrich) prior to intranasal challenge with CpG and subsequently given drinking water containing 0.8 mg/ml BrdU. Proliferation of memory P14 CD8+ T cells in the lung was assessed using the FITC BrdU Flow Kit (BD Pharmingen) according to the manufacturer’s protocols.
Generation and purification of memory CD8+ T cells.
To generate memory P14 CD8+ T cells, 1 × 104 naive P14 CD8+ T cells (Thy1.1) were transferred into naive B6 mice (Thy1.2) and subsequently infected with LCMV. At 60 days or more after infection, single cell suspensions of total splenocytes from mice containing Thy1-disparate primary memory P14 CD8+ T cells were stained with PE-anti-Thy1.1 antibody (Clone OX-7, BD Pharmingen) in PBS containing 5% FCS and subsequently purified with magnetic bead sorting using standard AutoMacs protocols. For depletion of 1B11hi memory P14 CD8+ T cells, total splenocytes were also stained with FITC-anti-CD43 clone 1B11 antibody (Biolegend). Following AutoMacs purification, 1B11lo memory P14 CD8+ T cells were sorted using a Becton Dickinson ARIA II cell sorter.
Cell isolation from tissue.
Perfusion of lungs and liver was performed by gently pushing 10 ml PBS through the right heart ventricle. Organ lobes or ears were then cut into approximately 0.25-cm2 pieces and allowed to incubate for 1 to 2 hours at 37°C with 1 to 2 ml HBSS containing CaCl2 and MgCl2 supplemented with 125 U/ml collagenase (Invitrogen) at 37°C. Whole organ suspensions were then generated by gently forcing the tissue through a mesh screen. Leukocytes were then purified with 35% Percoll (GE Healthcare) in HBSS.
P- and E-selectin binding and antibodies.
Detection of functional ligands for P- and E-selectin was performed using mouse P-selectin or E-selectin human IgG-Fc chimeric proteins (R&D Systems) at a concentration of 1 μg/ml and 5 μg/ml, respectively, diluted in FACS buffer containing Ca2+ and Mg2+. Cells were then washed, and bound chimeric proteins were detected with PE-anti-human IgG-Fc (BD Pharmingen). The following antibodies were used with the indicated specificity and the appropriate combinations of fluorochromes: CD8α (clone 53-6.7, eBioscience), Thy1.1 (clone OX-7, BD Pharmingen), BrdU (clone PRB-1, eBioscience), glycosylated CD43 (clone 1B11, BioLegend), PSGL-1 (CD162) (clone 2PH1, BD Pharmingen), human CD8α (clone HIT8α, Biolegend), human CD62L (clone DREG-56, Biolegend), human CD45RO (clone UCHL1, Biolegend), and appropriate isotype controls. Fluorescein-labeled jacalin (Vector Labs) was inhibited with 1 M galactose (Sigma-Aldrich). Cells were analyzed on a FACSCanto or LSRFortessa flow cytometer (BD Biosciences).
Immunoprecipitation and immunoblotting.
Thy1.1+ P14 CD8+ T cells were purified and lysed in selectin binding buffer (50 mM HEPES [pH 7.9], 1% NP-40, 125 mM NaCl, 2.5 mM CaCl2, plus protease inhibitors). Equal amounts of protein were allowed to rotate for 2 to 3 hours on Protein-G Sepharose (Sigma-Aldrich) that had previously been incubated for >1 hour with 2 μg VCAM-1 (control), P-selectin, or E-selectin human IgG-Fc chimeric proteins (R&D Systems). Beads were washed twice, and bound proteins were eluted with 25 mM EDTA, separated by SDS-PAGE, and transferred. Immunoblotting was performed with rat anti–PSGL-1 antibody (clone 4RA10, BD Pharmingen). The rabbit anti-Gcnt1 antibody was from Abcam. HRP secondary antibodies were from Santa Cruz Biotechnology Inc.
In vitro stimulation.
Mouse memory P14 CD8+ T cells were purified as described above and cultured in FCS supplemented RPMI for 3 days. Human CD8+ T cells were purified prior to stimulation with the RosetteSep CD8+ T cell enrichment cocktail (Stemcell Technologies). The indicated concentrations of recombinant IL-15 (Peprotech) were added daily. The STAT5 inhibitor N′-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide (Calbiochem) was dissolved in DMSO at a concentration of 50 mM and stored at –80°C prior to use.
ELISA.
IL-15 protein was quantified using the Mouse IL-15R/IL-15 Complex ELISA Ready-SET-Go Kit from eBioscience according to the manufacture’s protocol. Spleens were homogenized in 1 ml RIPA buffer (50 mM Tris [pH 7.4], 1% NP-40, 0.1% SDS, 150 mM NaCl, 0.5% sodium deoxycholate, 1 mM EDTA, and protease inhibitors) prior to analysis.
Quantitative RT-PCR.
RNA was isolated from CD8+ T cell populations using the RNeasy Kit (Qiagen) according to the manufacturer’s protocol. Quantitative RT-PCR was performed using the iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad), and data were collected using an Applied Biosystems 7000 Real-Time Thermalcycler. Relative changes in gene expression were calculated using the ΔΔCt method, in which expression of the gene of interest was normalized to expression of hypoxanthine-guanine phosphoribosyltransferase (Hprt). Primer pairs for detection of Gcnt1 variant 1 were 5′-GAGCGCGCCAGTATCCGCTT-3′ and 5′-TGGCCCTCAGGAGCCTGTCA-3′.
Chromatin immunoprecipitation.
Detection of histone modifications at the Gcnt1 gene was performed using the Agarose ChIP Kit (Pierce) according to the manufacturer’s protocol. Briefly, naive and memory P14 CD8+ T cells were purified from spleens of mice and fixed with fresh 1% formaldehyde for 10 minutes at room temperature. Fixing was terminated with the addition of a saturating amount of glycine. Cells were then thoroughly washed with PBS and lysed with membrane extraction buffer. The isolated cell nuclei were then digested with micrococcal nuclease (40 U/ml) for 15 minutes at 37°C. Nuclei were then lysed with nuclear extraction buffer, and nuclear lysates were incubated with isotype control, anti-trimethylated lysine 4 histone H3 (clone C42D8), or anti-trimethlyated lysine 27 histone H3 (clone C36B11) antibodies (Cell Signaling) overnight at 4°C. Bound protein/DNA complexes were then purified with Protein A/G Plus Agarose and isolated following the manufacturer’s protocol. Enrichment of DNA (fold increase) was determined by quantitative PCR (2–ΔCt), where ΔCt = Ct (H3 modification-specific antibody) – Ct (isotype control antibody). The following primer pairs were used, where numbers in parentheses represent mean locations relative to transcription start site of Gcnt1 (NC_000085.6 chromosome 19, reference GRCm38.p1 C57BL/6J; transcription start site 17,356,667): (–3,160) 5′-CAAACACAGCTTGTCTGATTCAGGC-3′, 5′-CAGCTTGACTAGCTCATAGCCAGC-3′; (–2,730) 5′-GACTGAATTATAGATAAGGGCAGTT-3′, 5′-GGAATTGAGGCTTATTTCAGCAGGC-3′; (–1,727) 5′-CAGGTGATTATGAGCTGCCATGTG-3′, 5′-GTAATGGAAACCAGGGACTTAGG-3′; (–779) 5′-GCAATCTTCCAAGAGTCAACCC-3′, 5′-GGCTGAGCTGTCTTGGAAGCAC-3′; and (–372) 5′-GCGAAAGAGGAACCAGCTTTCAAG-3′, 5′-GCCAACTGTTGTCCTGTCTGATCT-3′.
Statistics.
All error bars indicate mean ± SD. Statistical analyses used either the Student’s t test or 1-way ANOVA with Bonferroni post-test of multiple comparisons as indicated in the figure legends. P values of less than 0.05 were considered significant.
Study approval.
All animal experiments followed protocols approved by the Institutional Animal Care and Use Committee of the University of Iowa. Human blood leukocytes from leukocyte reduction cones were acquired from anonymous donors that had consented for blood donation at the DeGowin Blood Center at the University of Iowa. Consent forms were approved by the University of Iowa’s Institutional Review Board.
Acknowledgments
The authors would like to thank members of the Harty lab for helpful discussion and Stanley Perlman and Vladimir Badovinac for critical review of the manuscript. We also thank Klaus Ley for providing antibodies and the University of Iowa Flow Cytometry and DNA Core Facilities. This work was supported by NIH grants AI42767, AI085515, AI095178, AI96850, and AI100527 to J.T. Harty and NIH immunology training grant T32-AI07260 to J.C. Nolz.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2014;124(3):1013–1026. doi:10.1172/JCI72039.
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8(2):107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 3.Nolz JC, Harty JT. Strategies and implications for prime-boost vaccination to generate memory CD8 T cells. Adv Exp Med Biol. 2012;780:69–83. doi: 10.1007/978-1-4419-5632-3_7. [DOI] [PubMed] [Google Scholar]
- 4.Butler NS, Nolz JC, Harty JT. Immunologic considerations for generating memory CD8 T cells through vaccination. Cell Microbiol. 2011;13(7):925–933. doi: 10.1111/j.1462-5822.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt NW, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105(37):14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/S0092-8674(02)01139-X. [DOI] [PubMed] [Google Scholar]
- 8.Pham NL, et al. Exploiting cross-priming to generate protective CD8 T-cell immunity rapidly. Proc Natl Acad Sci U S A. 2010;107(27):12198–12203. doi: 10.1073/pnas.1004661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34(5):781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho BK, Wang C, Sugawa S, Eisen HN, Chen J. Functional differences between memory and naive CD8 T cells. Proc Natl Acad Sci U S A. 1999;96(6):2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177(2):1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 13.Zediak VP, Johnnidis JB, Wherry EJ, Berger SL. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol. 2011;186(5):2705–2709. doi: 10.4049/jimmunol.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 15.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14(5):509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol. 2003;170(3):1423–1429. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]
- 20.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 21.Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy. 2011;3(10):1223–1233. doi: 10.2217/imt.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33(1):96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179(1):36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohlmeier JE, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183(2):589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 27.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230(1):97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8(11):874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230(1):75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26(4):477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yago T, Fu J, McDaniel JM, Miner JJ, McEver RP, Xia L. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc Natl Acad Sci U S A. 2010;107(20):9204–9209. doi: 10.1073/pnas.1003110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4(5):325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 33.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9(6):881–890. doi: 10.1016/S1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 34.Carlow DA, Ardman B, Ziltener HJ. A novel CD8 T cell-restricted CD45RB epitope shared by CD43 is differentially affected by glycosylation. J Immunol. 1999;163(3):1441–1448. [PubMed] [Google Scholar]
- 35.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000;191(7):1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones AT, et al. Characterization of the activation-associated isoform of CD43 on murine T lymphocytes. J Immunol. 1994;153(8):3426–3439. [PubMed] [Google Scholar]
- 37.Moore KL, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128(4):661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tachibana K, et al. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology. 2006;16(1):46–53. doi: 10.1093/glycob/cwj038. [DOI] [PubMed] [Google Scholar]
- 39.Magnet AD, Fukuda M. Expression of the large I antigen forming β-1,6-N-acetylglucosaminyltransferase in various tissues of adult mice. Glycobiology. 1997;7(2):285–295. doi: 10.1093/glycob/7.2.285. [DOI] [PubMed] [Google Scholar]
- 40.Stone EL, et al. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol. 2009;29(13):3770–3782. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12(4):306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37(3):549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167(3):1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 44.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9(3):153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 45.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20(5):551–562. doi: 10.1016/S1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 46.Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol. 2008;181(9):5837–5841. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- 47.Wagers AJ, Waters CM, Stoolman LM, Kansas GS. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on α1, 3-fucosyltransferase VII gene expression. J Exp Med. 1998;188(12):2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim YC, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol. 1999;162(6):3193–3201. [PubMed] [Google Scholar]
- 49.White SJ, Underhill GH, Kaplan MH, Kansas GS. Cutting edge: differential requirements for Stat4 in expression of glycosyltransferases responsible for selectin ligand formation in Th1 cells. J Immunol. 2001;167(2):628–631. doi: 10.4049/jimmunol.167.2.628. [DOI] [PubMed] [Google Scholar]
- 50.Knibbs RN, et al. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161(11):6305–6315. [PubMed] [Google Scholar]
- 51.Carlow DA, Williams MJ, Ziltener HJ. Inducing P-selectin ligand formation in CD8 T cells: IL-2 and IL-12 are active in vitro but not required in vivo. J Immunol. 2005;174(7):3959–3966. doi: 10.4049/jimmunol.174.7.3959. [DOI] [PubMed] [Google Scholar]
- 52.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100(3):854–861. doi: 10.1182/blood.V100.3.854. [DOI] [PubMed] [Google Scholar]
- 53.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 54.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]