Abstract
Experimental autoimmune orchitis (EAO) is a model of immunologic male infertility and pathologically characterized by lymphocytic inflammation, which causes breakdown of the testicular immune privilege with spermatogenic disturbance. Generally, murine EAO is induced by immunization with testicular homogenate (TH) from the testes of donor mice + complete Freund's adjuvant (CFA) + Bordetella pertussigens (BP), and it has been considered that treatment with these two adjuvants is required to enhance the immune response against testicular antigens. However, there remains a possibility that CFA and BP may affect autoimmune responses against the testicular antigens without TH. In the present study, we examined this possibility using real-time RT-PCR, Western blotting and immunohistochemical staining. The results demonstrated that immunization with TH in combination with CFA and BP evoked more severe EAO than that with only TH. Real-time RT-PCR analyses revealed that Fas mRNA expression in TH+CFA+BP-induced EAO was significantly higher than that in TH-induced EAO. Interestingly, IL-6 mRNA expression dramatically increased in TH+CFA+BP-induced EAO; however, no apparent change in IL-6 mRNA expression occurred in TH-induced EAO. It was also noted that treatment with CFA and BP alone augmented autoimmune reactions against some testicular autoantigens. These results indicates that these adjuvants are helpful in evoking severe EAO, and treatment with the adjuvants alone can evoke autoimmune reactions against some testicular autoantigens despite the use of no TH.
Keywords: Adjuvant, Autoimmunity, Testis
In the testes, haploid germ cells (i.e., spermatids and spermatozoa) do not appear in the seminiferous epithelium until puberty, when immune tolerance has already been established. Therefore, they contain various autoimmunogenic materials that are recognized as foreign (non-self) by the immune system. A subcutaneous injection of testicular antigens can induce systemic immune responses against autoantigens of haploid germ cells [1]. However, the testes are immunologically privileged organs. In particular, the blood–testis barrier (BTB) formed by Sertoli cells separates autoimmunogenic spermatozoa from the self immune system [2, 3]. In addition, testicular macrophages and Leydig cells have been found to act as immune suppressors [4].
To overcome the testicular immune privilege, experimental autoimmune orchitis (EAO), a model of immunological male infertility, has been induced by immunization with testicular homogenate (TH) in complete Freund's adjuvant (CFA) and subsequent intravenous injections of Bordetella pertussigens (BP) in mice and rats [5,6,7]. EAO is accompanied by epididymio-vasitis and considered to be organ specific because mice injected with CFA+BP+liver homogenate do not develop inflammation [5, 6]. On the other hand, we established an EAO model induced by two immunizations with syngeneic testicular germ cells or TH alone in mice with a very high incidence [8, 9]. This model is unique in that CFA and BP are not necessary for EAO induction and epididymio-vasitis is hardly observed [8, 9]. In both TH+CFA+BP- and TH-induced EAO, inflammation is Th1 CD4+ cell dependent and involved in secretion of various cytokines and autoantibodies against testicular antigens, which causes damage to seminiferous tubules, namely, sloughing and apoptosis of germ cells [9, 10]. However, there has been no report focusing on the effects of CFA and BP on autoimmune responses against testicular antigens. In our previous study, we found that BP treatment alone induced systemic leukocytosis in mice with significant pathological changes in the ductuli efferentes, epididymis and prostate, but not in the testes [11]. The aim of the present study was to investigate the effects of CFA and BP on autoimmune responses against testicular antigens using real-time RT-PCR, Western blotting and immunostaining.
Materials and Methods
Animals
A/J mice (aged 8 weeks, n = 43) were purchased from Japan SLC (Shizuoka, Japan) and housed at the Laboratory Animal Center of Tokyo Medical University for 2 weeks before use. They were maintained at 22–24 C and 50–60% relative humidity with a 12 h light–dark cycle. Approval from the Tokyo Medical University Animal Committee (s-22020) was obtained for this study.
Experimental design
The 10-week-old mice were divided into four groups (one control group and three experimental groups) as follows: (a) Control group (n = 8), in which the mice were subcutaneously injected with 100 µl of phosphate-buffered saline (PBS) on days 0 and 14; (b) TH group (n = 8), in which the mice were subcutaneously injected with TH obtained from a testis of donor mice (n = 4) in 100 µl of PBS on days 0 and 14; (c) TH+CFA+BP group (n = 8), in which the mice were subcutaneously injected with TH obtained from a testis of donor mice (n = 4) in 100 µl of PBS emulsified with an equal volume of CFA (Sigma–Aldrich, St Louis, MO, USA) immediately followed by intravenous injection of 100 µl of BP solution (2 × 1010 dead microorganisms/animal, Wako, Osaka, Japan) on days 0 and 14; and (d) CFA+BP group (n = 8), in which the mice were injected subcutaneously with 100 µl of PBS emulsified with an equal volume of CFA followed immediately by intravenous injection of 100 µl of BP solution (2 × 1010 dead microorganisms/animal) on days 0 and 14. TH was prepared by homogenizing fresh decapsulated testes by ultrasonication for 5 min on ice. The amount of CFA and BP was based on the methods described by Kohno et al. [6]. On day 80, the mice were anesthetized with pentobarbital, and blood was collected from all the mice by cardiac puncture. Serum samples from individual mice were stored at −80 C until assayed. The testes were immediately removed from the sacrificed mice for histological and genetic examination.
Histological procedure
The right testes from each mouse of the four groups (n = 8) were examined. The testes were fixed with Bouin's solution and embedded in plastic (Technovit 7100; Kulzer & Co., Wehrheim, Germany) without cutting the organs to avoid artificial damage to the testicular tissue. Sections (3–4 µm) were obtained at 15–20-µm intervals and stained with Gill's hematoxylin III and 2% eosin Y for observation by light microscopy (200× magnification). Histopathological changes in spermatogenesis were evaluated using Johnsen's scoring system [12]. Briefly, scoring was as follows: 10) complete spermatogenesis with many spermatozoa, determined by head form, and an organized germinal epithelium of regular thickness, leaving an open lumen; 9) many spermatozoa present, but with a disorganized germinal epithelium and marked sloughing or obliteration of the lumen; 8) only a few spermatozoa present; 7) no spermatozoa, but many spermatids present; 6) no spermatozoa and only a few spermatids present; 5) no spermatozoa and no spermatids, but several or many spermatocytes present; 4) only a few spermatocytes (<5), but no spermatids or spermatozoa present; 3) spermatogonia were the only germ cells present; 2) no germ cells, but Sertoli cells were present; and 1) no cells in a tubular section. Twenty 1-mm2 areas were randomly examined, and more than 200 round- or oval-shaped seminiferous tubules were counted in each testis.
Tdt-mediated dUTP nick-end labeling (TUNEL) staining
The left testes from each mouse of the four groups (n = 4) were examined. The testes were fixed in 10% buffered formaldehyde for 3 days. After dehydration with ethanol, the testes were embedded in paraffin and 4-µm-thick sections were prepared. A commercially available kit (ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit; EMD Millipore, Billerica, MA, USA) was used to detect the 3′-OH ends of the DNA strands. Deparaffinized sections were treated with proteinase K (Dako, CA, USA) for 15 min at room temperature (RT) and then washed in distilled water for 4 min. Endogenous peroxidase activity was blocked by treating the sections with 3% H2O2 in PBS for 5 min at RT. The sections were incubated in a mixture of terminal deoxynucleotidyl transferase and digoxigenin-labeled dideoxynucleotides in a humidified chamber at 37 C for 1 h. After reacting with a stop buffer (ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit; EMD Millipore) for 10 min, the sections were incubated with an anti-digoxigenin peroxidase conjugate for 30 min. Peroxidase activity was detected by exposing the sections to a solution containing 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB). Negative controls were treated with distilled water in place of the TdT enzyme. For statistical analysis, more than 100 round- or oval-shaped seminiferous tubules were examined, and the number of TUNEL-positive germ cells per seminiferous tubule (mean ± SD) was determined in each mouse. Stained sections were counterstained with methyl green (Vector Laboratories, Burlingame, CA, USA).
Gene expression analysis
The left testes from each mouse of the four groups (n = 4) were examined. Total RNA was isolated from the testis using a TRIzol RNA extraction kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, and RNA pellets were dissolved in 10 µl of RNase-free distilled water. Total RNA was measured at 260/280 nm using a UV spectrophotometer and was stored at −80 C prior to use. cDNA was prepared from 10 µg of total RNA in a 100-µl reaction mixture using random primers according to a standard protocol (High-Capacity cDNA Archive Kit; PE Applied Biosystems, Foster City, CA, USA). The PCR reactions were performed in an iCycler thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA), and the mixtures were stored at −80 C before analysis. Real-time RT-PCR was performed on 3 ng of cDNA using a validated SYBR Green gene expression assay in combination with SYBR Premix Ex Taq II (TaKaRa, Bio, Ohtsu, Japan) for measuring murine Fas, Fas-L, IFN-γ, TNF-α, IL-6, IL-10 and GAPDH. All primers used in this study are listed in Table 1. Th1 cells (involved in delayed-type hypersensitivity) produce TNF-α and IFN-γ, whereas Th2-cells (involved in humoral immunity) produce IL-6 and IL-10 [13, 14]. Apoptosis is one of the main features characterizing germ cell death in the testes and is mediated by the Fas/Fas-L systems [15, 16]. Quantitative real-time PCR was performed in duplicate in a TP800 Thermal Cycler Dice Real Time System (TaKaRa), and the comparative Ct method (2∆∆Ct) was used to quantify gene expression levels. Data of the real-time PCR products were standardized to GAPDH, which was used as the internal control. To confirm the specific amplification of the target genes, each gene product was further separated on 1.5% agarose gel to detect any single bands and the theoretical product sizes.
Tabele 1. List of primers used in cloning.
| Primer name | Direction | Sequence 5-3´ |
| Fas | Forward | GCAGACATGCTGTGGATCTGG |
| Reverse | TCACAGCCAGGAGAATCGCAG | |
| Fas-L | Forward | TCCAGGGTGGGTCTACTTACTAC |
| Reverse | CCCTCTTACTTCTCCGTTAGGA | |
| IFN-γ | Forward | ATCTGGAGGAACTGGCAAAA |
| Reverse | TTCAAGACTTCAAAGAGTCTGAGGTA | |
| TNF-α | Forward | TCTTCTCATTCCTGCTTGTGG |
| Reverse | TCTGGGCCATAGAACTGATGA | |
| IL-6 | Forward | GCTACCAAACTGGATATAATCAGGA |
| Reverse | CCAGGTAGCTATGGTACTCCAGAA | |
| IL-10 | Forward | CAGAGCCACATGCTCCTAGA |
| Reverse | GTCCAGCTGGTCCTTTGTTT | |
| GAPDH | Forward | TGTGTCCGTCGTGGATCTGA |
| Reverse | TTGCTGTTGAAGTCGCAGGAG |
Protein isolation and Western blotting analysis
The right testes obtained from normal mice (n = 3) were homogenized in lysis buffer containing 10 mM of phosphate buffer (pH 7.2), 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 µg/ml of leupeptin and 1 µg/ml of chymostatin. Protein concentrations were determined by the Bradford method using BSA as a standard. Samples were boiled for 3 min in 0.125 M Tris–HCl, 10% 2-mercaptoethanol, 4% sodium dodecyl sulfate (SDS), 0.004% bromophenol blue and 10% sucrose and then electrophoretically separated on a 7.5% gradient gel (ATTO, Tokyo, Japan) with 50 µg protein per sample lane. Precision Plus Protein Standards (Bio-Rad Laboratories) were used as molecular mass markers. After electrophoresis, the proteins were electroblotted to polyvinylidene fluoride membranes (Immobilon-P Transfer Membranes; ATTO Corporation). After rinsing in PBS–Tween (PBS, 0.1% Tween-20), nonspecific binding was blocked by incubation of the blotted membranes in PBS–Tween containing 3% BSA (Sigma–Aldrich) for 1 h at RT. Thereafter, the membranes were incubated with each collected serum sample (diluted 1:100) in PBS–Tween at 4 C overnight. After washing in PBS–Tween, the blotted membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG (ECL) (diluted 1:10000; Amersham Biosciences, Buckinghamshire, UK) in PBS–Tween at RT for 1.h. The membranes were then washed five times with PBS–Tween and then examined using the ECL Plus Western Blotting Detection Reagents System (GE Healthcare).
Immunohistochemical examination
For the detection of serum autoantibodies, the left testes of the normal mice (n = 3) were placed in OCT compound (Miles Laboratories, IL, USA), frozen in liquid nitrogen and stored at –80 C until used. Sections (6 µm) were cut with a cryostat (CM1900; Leica, Wetzlar, Germany), dried in air, fixed in 95% ethanol for 10 min at −20 C, rinsed in PBS and then incubated with 50-fold serial dilutions of the collected serum samples for 60 min at RT. After rinsing in PBS, the cryostat sections were incubated for 60 min with HRP-conjugated goat anti-mouse IgG (1:500 dilution; ZyMax, CA, USA) at RT. After washing with PBS, the HRP-binding sites were detected with 0.05% DAB and 0.01% H2O2. The results were compared with those of background staining of the sections observed after incubation with HRP-conjugated antibodies in the absence of immune and nonimmune serum samples.
Data analysis
Data were expressed as means ± standard deviation (SD), and ANOVA followed by a Tukey-Kramer post hoc test was employed for statistical analysis. A P-value < 0.05 was considered statistically significant.
Results
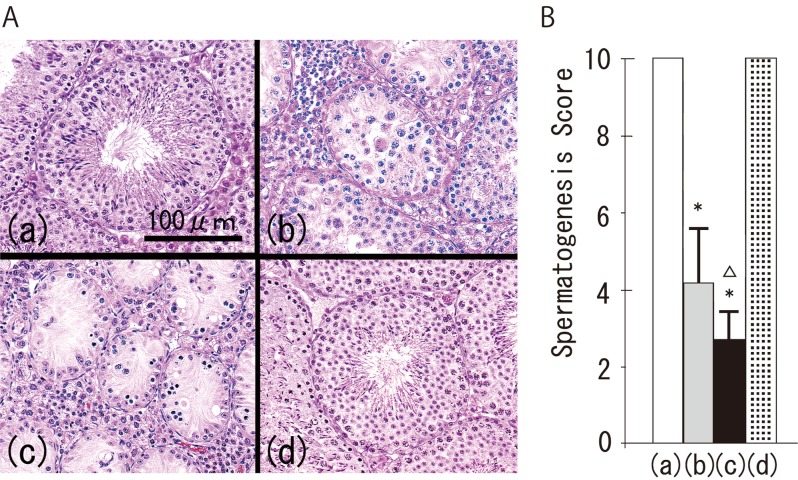
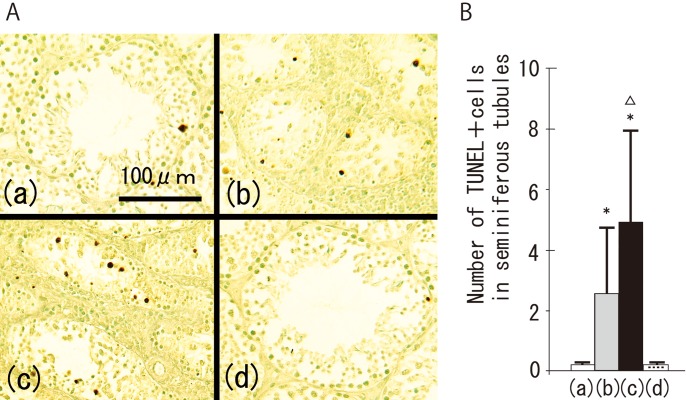
In the control group, no lymphocytes were observed in the testes (Fig. 1A (a)). However, lymphocytic infiltration with spermatogenic disturbance was found in all the mice in the TH and TH+CFA+BP groups (Figs. 1A (b) and (c)). Spermatogenic disturbance in the TH+CFA+BP group was significantly more severe than that in the TH group (Figs. 1B (b) and (c)). Histopathological changes were not observed in the CFA+BP group, as in the control group (Fig. 1A (d)). Immunohistochemically, TUNEL-positive germ cells were occasionally detected along the basement membrane of the seminiferous tubules in the control and CFA+BP groups. In contrast, many TUNEL-positive germ cells were consistently observed inside the seminiferous tubules in both the TH and TH+CFA+BP groups (Fig. 2A). There were significantly more positive cells in the TH+CFA+BP group compared with the TH group (Fig. 2B).
Fig. 1.
Histological views of testicular tissues (A) and spermatogenesis scores (B) of the control group (a), TH group (b), TH+CFA+BP group (c) and CFA+BP group (d). *P<0.05 vs. the control group; ∆P<0.05 vs. the TH group.
Fig. 2.
Tdt-mediated dUTP nick end labeling (TUNEL)-positive cells in the testicular tissues (A) and number of TUNEL-positive cells in seminiferous tubules (B) of the control group (a), TH group (b), TH+CFA+BP group (c) and CFA+BP group (d). *P<0.05 vs. the control group; ΔP<0.05 vs. the TH group.
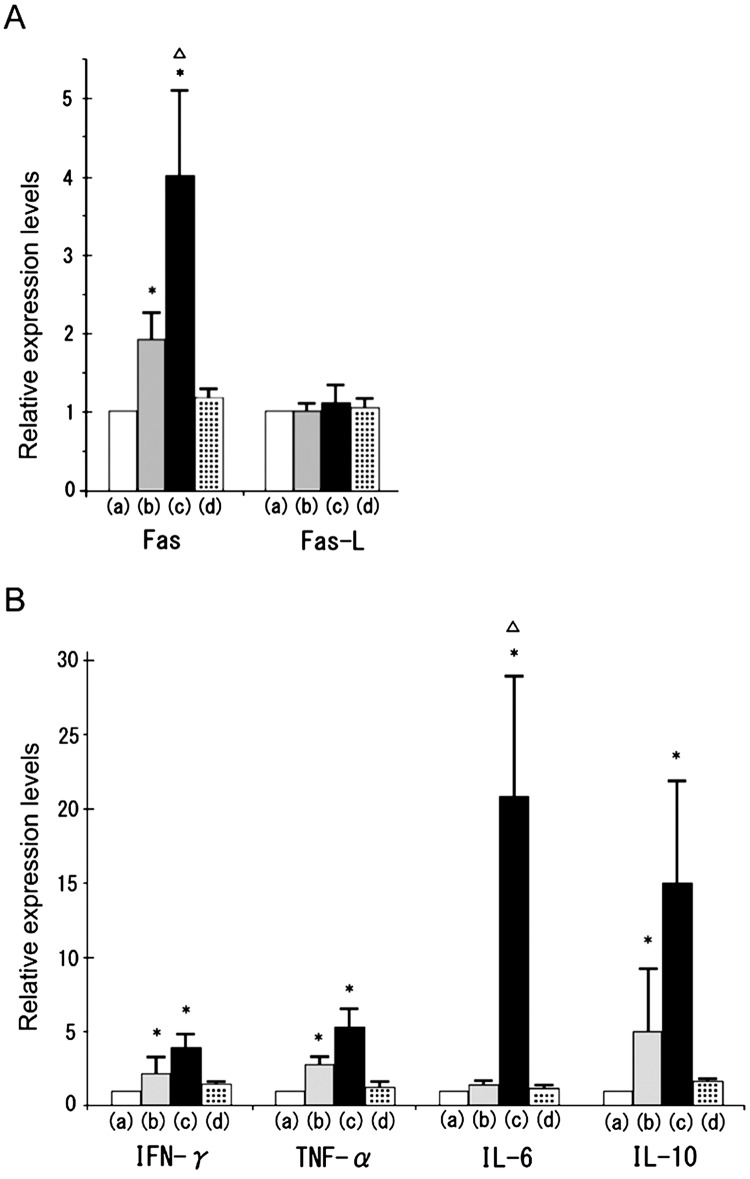
Real-time RT-PCR analyses revealed that Fas mRNA expression in both the TH and TH+CFA+BP groups significantly increased compared with the control group (Fig. 3). Furthermore, Fas expression in the TH+CFA+BP group was significantly higher than that in the TH group (Fig. 3A). In contrast, Fas-L expression did not show significant changes in the TH and TH+CFA+BP groups (Fig. 3A, P > 0.05 by ANOVA). Although no significant difference in IFN-γ, TNF-α and IL-10 expression was observed between the TH and TH+CFA+BP groups, there was a tendency for augmentation of these expressions in the TH+CFA+BP group compared with the TH group. Notably, IL-6 expression dramatically increased in the TH+CFA+BP group, but did not in the TH group (Fig. 3B). In the CFA+BP group, none of the examined mRNA expressions showed any significant changes (Fig. 3A and B).
Fig. 3.
Expression of apoptosis-related gene and cytokines. Fas and Fas-L (A), IFN-γ, TNF-α , IL-6, and IL-10 (B) mRNA expressions were analyzed by real-time RT-PCR in the testes of the control group (a), TH group (b), TH+CFA+BP group (c) and CFA+BP group (d). Relative intensity was calculated, and then the expression in the controls for the other groups was normalized to 1.0. Each bar represents a mean ± SD (n = 4). *P<0.05 vs. the respective control group; ∆P<0.05 vs. the respective TH group.
To identify the testicular antigens that specifically reacted with sera from each group, we performed SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting by reacting the sera with normal murine testicular homogenates (Fig. 4). In the control group, two immunoreactive bands corresponding to approximately 45 and 100 kDa were detected, showing the presence of natural autoantibodies against these two testicular antigens (Fig. 4a). In the three experimental groups (Figs. 4b, c and d), the two natural autoantibodies were more definitely detected, even in the CFA+BP group, despite the use of no TH (Fig. 4d). In the TH group, the serum samples also reacted with three additional autoantigens of approximately 25, 40 and 250 kDa (Fig. 4b). The serum samples obtained from the TH+CFA+BP group were reactive with more autoantigens than those from the TH group. Bands of approximately 15, 22, 60, 75 and 120 kDa were only observed in the TH+CFA+BP group. Compared with the control group, a band of approximately 40 kDa was additionally detected in the CFA+BP group (Fig. 4d). Four serum samples from each group were examined, and all produced similar results.
Fig. 4.
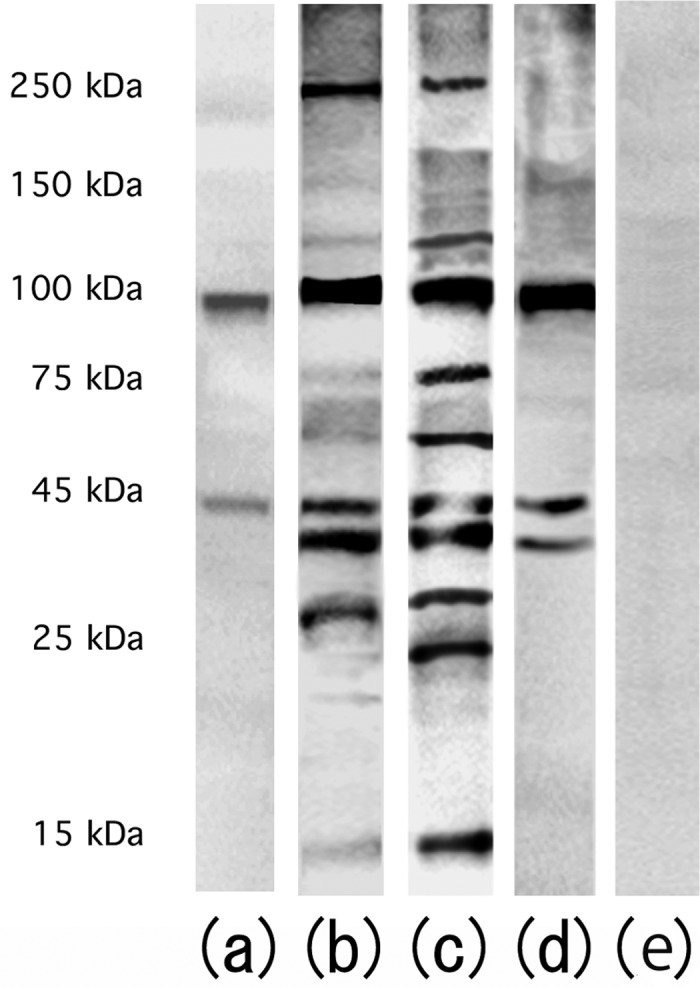
Western blotting of testicular antigens reacted with 100-fold diluted serum samples obtained from the control group (a), TH group (b), TH+CFA+BP group (c) and CFA+BP group (d) followed by reaction with anti-mouse IgG antibodies. (e) Anti-mouse IgG antibody instead of serum samples.
To identify the autoantibody-reacting sites, we performed immunohistochemical staining by reacting normal testicular sections with sera from each group (Fig. 5a). No significant staining was detected in the control group in reactions between the sera and frozen sections of testes from the control mice. Autoantibodies against only haploid cells were observed in the TH group (Fig. 5b), while autoantibodies against all components of the seminiferous epithelium and interstitial cells were found in the TH+CFA+BP group (Fig. 5c). In the CFA+BP group, immunostaining was weak; haploid cells and some cells around the basement membrane of seminiferous tubules were stained (Fig. 5d). Four serum samples from each group were examined, and all produced similar results.
Fig. 5.
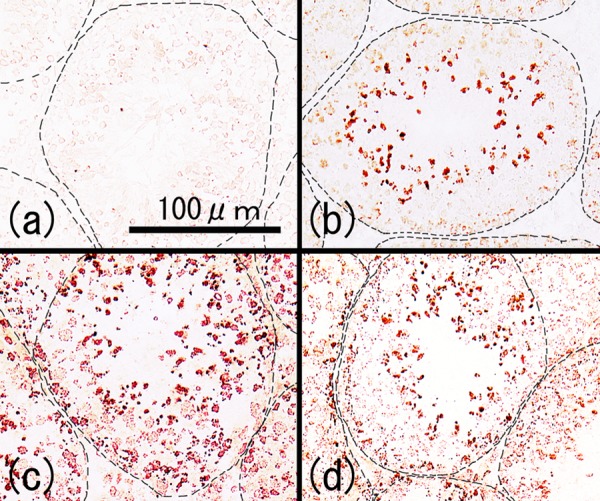
Cryostat sections of testes from normal mice (8 weeks of age). All the sections were reacted with 50-fold diluted serum samples obtained from the control group (a), TH group (b), TH+CFA+BP group (c) and CFA+BP group (d) followed by incubation with HRP-conjugated anti-mouse IgG antibody. The dashed line indicates the basal lamina of the seminiferous tubules.
Discussion
In the present study, we showed that immunization with TH in combination with CFA and BP evoked more severe autoimmune reactions compared with that with only TH in the testes of mice. Furthermore, we also showed that treatment with CFA and BP alone could evoke autoimmune reactions against some testicular autoantigens.
CFA and BP are ordinarily used as adjuvants to augment an immune response [17, 18]. The use of adjuvants is commonly essential in experiments on induction of organ-specific autoimmune diseases such as experimental autoimmune encephalomyelitis, neuritis, uveitis and thyroiditis in mice [19,20,21,22,23]. However, murine EAO can be induced by immunization with only testicular antigens, which contain various autoimmunogenic materials, without any aid of adjuvants. Damage to the BTB in one testis following an infection or trauma induces orchitis in the contralateral testis [24, 25]. Therefore, it is important to determine the effects of adjuvants on testicular autoimmunity. In the present study, immunization with TH in combination with CFA and BP induced more severe EAO compared with that with TH alone. Moreover, Fas mRNA expression in TH+CFA+BP-induced EAO was significantly higher than that in TH-induced EAO. It appears that the adjuvants led to severe apoptosis of germ cells in the TH+CFA+BP-induced EAO. Regarding cytokine-related mRNA expression, there was a tendency for the mRNA expressions of IFN-γ, TNF-α (Th-1-related cytokines) and IL-10 (a Th2 related cytokine) to be augmented in TH+CFA+BP-induced EAO compared with in TH-induced EAO. Interestingly, the IL-6 (Th2 related cytokine) dramatically increased in TH+CFA+BP-induced EAO, while it remained almost unchanged in TH-induced EAO. The in vitro experiments on seminiferous tubule cultures showed that IFN-γ and TNF-α induced apoptosis of germ cells through the Fas-FasL system [26, 27]. It was also demonstrated that IL-6 induced apoptosis of germ cells [28]. Therefore, our findings suggested a possibility that a combination of both TH and adjuvants augmented cytokine secretions and induced the severe apoptotic death of testicular germ cells.
In other studies, the employment of CFA and BP has proven to be valuable for indirect alteration of the BTB [17, 18]. Further, immunization with TH in combination with CFA and BP induced an autoimmune reaction against germ cells within the BTB (= autoimmunity against haploid cells) and also against testicular components outside the BTB (= autoimmunity against spermatogonia, Sertoli cells, Leydig cells and the basement membrane of the seminiferous tubule) [5, 29,30,31]. We also revealed that autoantibodies were detected only against haploid cell antigens in testicular germ cell-induced EAO sera [32]. The present data showed that an additional band of approximately 40 kDa appeared in the Western blot analysis of sera from the CFA+BP group compared with that of sera from the control group (Fig. 4d) and that the autoantibodies could be detected against both the haploid cells and some cells around the basement of seminiferous tubules by immunostaining (Fig. 5d). These results indicate that the adjuvants were helpful in evoking severe autoimmune reactions against testicular antigens and that the adjuvants alone can evoke autoimmune reactions against some testicular autoantigens despite the use of no TH. The results also suggest that treatment with CFA and BP may cause indirect damage to the BTB resulting in leakage of some autoantigens beyond the BTB. Although some researchers have tried to detect target EAO autoantigens using TH+CFA+BP-induced EAO sera, it remains unclear which proteins are the target antigens [33,34,35,36]. Autoantigen analyses using the TH group and/or CFA + BP group may contribute to the discovery of the target proteins of EAO.
Acknowledgment
This work was supported by the Japan Society for the Promotion of Science (KAKENHI 24791673 and 23500504). The authors wish to thank Ms M Kitaoka, Mr S Kawata, Ms Y Ogawa and Ms A Abo for excellent secretarial and technical support.
References
- 1.Tung KS, Teuscher C, Meng AL. Autoimmunity to spermatozoa and the testis. Immunol Rev 1981; 55: 217–255 [DOI] [PubMed] [Google Scholar]
- 2.Setchell BP, Voglmayr JK, Waites GM. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol 1969; 200: 73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3: 308–326 [DOI] [PubMed] [Google Scholar]
- 4.Itoh M, Terayama H, Naito M, Ogawa Y, Tainosho S. Tissue microcircumstances for leukocytic infiltration into the testis and epididymis in mice. J Reprod Immunol 2005; 67: 57–67 [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Hirokawa K, Hatakeyama S. Experimental allergic orchitis in mice. Histopathological and Immunological studies. Virchows Arch A Pathol Anat Histol 1981; 392: 147–158 [DOI] [PubMed] [Google Scholar]
- 6.Kohno S, Munoz JA, Williams TM, Teuscher C, Bernard CC, Tung KS. Immunopathology of murine experimental allergic orchitis. J Immunol 1983; 130: 2675–2682 [PubMed] [Google Scholar]
- 7.Tung KS, Yule TD, Mahi-Brown CA, Listrom MB. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol 1987; 138: 752–759 [PubMed] [Google Scholar]
- 8.Itoh M, Hiramine C, Hojo K. A new experimental model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. I. Immunological and histological studies. Clin Exp Immunol 1991; 83: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naito M, Itoh M. Patterns of infiltration of lymphocytes into the testis under normal and pathological conditions in mice. Am J Reprod Immunol 2008; 59: 55–61 [DOI] [PubMed] [Google Scholar]
- 10.Jacobo P, Guazzone VA, Theas MS, Lustig L. Testicular autoimmunity. Autoimmun Rev 2011; 10: 201–204 [DOI] [PubMed] [Google Scholar]
- 11.Itoh M, Chen XH, Takeuchi Y, Miki T. Morphological demonstration of the immune privilege in the testis using adjuvants: tissue responses of male reproductive organs in mice injected with Bordetella pertussigens. Arch Histol Cytol 1995; 58: 575–579 [DOI] [PubMed] [Google Scholar]
- 12.Johnsen SG. Testicular biopsy score count-a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1970; 1: 2–25 [DOI] [PubMed] [Google Scholar]
- 13.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136: 2348–2357 [PubMed] [Google Scholar]
- 14.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol 1987; 138: 3688–3694 [PubMed] [Google Scholar]
- 15.Theas MS, Rival C, Dietrich SJ, Guazzone VA, Lustig L. Death receptor and mitochondrial pathways are involved in germ cell apoptosis in an experimental model of autoimmune orchitis. Hum Reprod 2006; 21: 1734–1742 [DOI] [PubMed] [Google Scholar]
- 16.Kuerban M, Naito M, Hirai S, Terayama H, Qu N, Musha M, Ikeda A, Koji T, Itoh M. Involvement of Fas/Fas-L and Bax/Bcl-2 systems in germ cell death following immunization with syngeneic testicular germ cells in mice. J Androl 2012; 33: 824–831 [DOI] [PubMed] [Google Scholar]
- 17.Willson JT, Jones NA, Katsh S. Penetration of the testicular-tubular barrier by horseradish peroxidase induced by adjuvant. Anat Rec 1973; 176: 85–100 [DOI] [PubMed] [Google Scholar]
- 18.Pelletier RM, Nemirovsky MS, Calvert R, Hugon JS. Effects of immunization with Freund's complete adjuvant and isologous spermatozoa on the seminiferous epithelium and blood-testis barrier in guinea pigs. Anat Rec 1981; 199: 197–211 [DOI] [PubMed] [Google Scholar]
- 19.Munoz JJ, Mackay IR. Production of experimental allergic encephalomyelitis with the aid of pertussigen in mouse strains considered genetically resistant. J Neuroimmunol 1984; 7: 91–96 [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Nennesmo I, Deng GM, Levi M, Wahren B, Diab A, Mix E, Zhou JN, Ljunggren HG. Induction of experimental autoimmune neuritis in CD4-8-C57BL/6J mice. J Neuroimmunol 1999; 94: 196–203 [DOI] [PubMed] [Google Scholar]
- 21.Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol 2001; 70: 849–860 [PubMed] [Google Scholar]
- 22.Damotte D, Goulvestre C, Charreire J, Carnaud C. LPS and Freund's adjuvant initiate different inflammatory circuits in experimental autoimmune thyroiditis. Eur Cytokine Netw 2003; 14: 52–59 [PubMed] [Google Scholar]
- 23.Kitaichi N, Namba K, Taylor AW. Inducible immune regulation following autoimmune disease in the immune-privileged eye. J Leukoc Biol 2005; 77: 496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukasa A, Hiromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol 1995; 155: 2047–2056 [PubMed] [Google Scholar]
- 25.Naito M, Sakamoto Y, Terayama H, Hirai S, Qu N, Aota Y, Itoh M. Effect of unilateral testicular rupture on histopathology and germ cell delayed-type hypersensitivity in C3H/He and A/J mice. J Reprod Immunol 2009; 81: 55–61 [DOI] [PubMed] [Google Scholar]
- 26.Riccioli A, Starace D, D'Alessio A, Starace G, Padula F, De Cesaris P, Filippini A, Ziparo E. J TNF-alpha and IFN-gamma regulate expression and function of the Fas system in the seminiferous epithelium. J Immunol 2000; 165: 743–749 [DOI] [PubMed] [Google Scholar]
- 27.Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, Guazzone VA, Lustig L. Tumour necrosis factor-alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum Reprod 2008; 23: 1865–1872 [DOI] [PubMed] [Google Scholar]
- 28.Rival C, Theas MS, Guazzone VA, Lustig L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol 2006; 70: 43–58 [DOI] [PubMed] [Google Scholar]
- 29.Ichisohasama R, Hirokawa K, Hatakeyama S. Spermatogenic disturbance induced in mice by combined local injection of monoclonal antibodies to Sertoli cell and to basal lamina of seminiferous tubule. Am J Reprod Immunol 1986; 10: 158–165 [DOI] [PubMed] [Google Scholar]
- 30.Tung PS, Fritz IR. Histopathological changes in testes of adult inbred rats immunized against pachytene spermatocytes or Sertoli cells. Int J Androl 1987; 2: 459–481 [Google Scholar]
- 31.Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood-testis barrier. J Immunol 1988; 141: 1161–1167 [PubMed] [Google Scholar]
- 32.Itoh M, Miki T, Takeuchi Y, Miyake M, De Rooij DG. Immunohistological localization of autoantigens detected by serum autoantibodies from mice with experimental autoimmune orchitis without using adjuvants. Arch Androl 1994; 32: 45–52 [DOI] [PubMed] [Google Scholar]
- 33.Tung KS, Mahi-Brown CA, Yule TD. Immunologic mechanisms and target antigens in testicular autoimmunity. Ann N Y Acad Sci 1989; 564: 267–280 [DOI] [PubMed] [Google Scholar]
- 34.Teuscher C, Meeker ND, Livingstone KD, Sudweeks JD, Griffith JS, Wardell BB, Hickey WF. Experimental allergic orchitis in mice. VII. Preliminary characterization of the aspermatogenic autoanti- gens responsible for eliciting actively and passively induced disease. J Reprod Immunol 1994; 26: 233–249 [DOI] [PubMed] [Google Scholar]
- 35.Tung KS, Primakoff P, Woolman-Gamer L, Myles DG. Mechanism of infertility in male guinea pigs immunized with sperm PH-20. Biol Reprod 1997; 56: 1133–1141 [DOI] [PubMed] [Google Scholar]
- 36.Fijak M, Iosub R, Schneider E, Linder M, Respondek K, Klug J, Meinhardt A. Identification of immunodominant autoantigens in rat autoimmune orchitis. J Pathol 2005; 207: 127–138 [DOI] [PubMed] [Google Scholar]