Abstract
Developmental competence and quality of in vitro produced embryos has been demonstrated to be lower than in vivo derived embryos. This study aimed specifically to determine the effects of in vitro culture of feline embryos using various culture densities on developmental competence and expression of stress- and apoptotic-related genes in terms of heat shock protein 70 (HSP70) and apoptotic-related (BAX and BCL-2) gene expressions. In experiment 1, we characterized the inducible form of a feline-specific HSP70 mRNA sequence, as it has not been previously reported. The primers for feline HSP70 mRNA were synthesized and tested on heat-treated cat fibroblasts. In experiment 2, feline embryos were cultured at different culture densities (embryo:culture volume; 1:1.25, 1:5 and 1:20). The developmental competence was determined along with HSP70, BAX and BCL-2 transcript abundances using quantitative RT-PCR. In vivo derived embryos were used as a control group. A partial cat HSP70 mRNA sequence (190 bp) was characterized and exhibited high nucleotide identity (93 to 96%) with other species. Cleaved embryos cultured at high density (1:1.25) developed to blastocysts at a lower rate than those generated from lower densities. Irrespective of the culture densities used, in vitro cultured blastocysts showed increased levels of HSP70 and BAX transcripts compared with in vivo counterparts. Blastocysts derived from the highest culture density (1:1.25) showed higher levels of upregulation of HSP70 and BAX transcripts than those cultured at lower culture densities (1:5 and 1:20). In conclusion, increased levels of pro-apoptotic (BAX) and stress-response (HSP70) transcripts correlated with developmental incompetence of embryos cultured at high embryonic density, indicating that stress accumulated during in vitro embryo culture affected the fate for embryo development and quality.
Keywords: Apoptosis, Culture density, Embryo, Heat shock protein 70
Currently, the in vitro culture system is still suboptimal as a result of lower developmental competence and quality of in vitro produced embryos compared with in vivo counterparts [1]. Embryo density (embryo number: medium volume ratio) is one of many factors affecting the developmental competence of in vitro produced embryos in several species [2,3,4,5,6] including the domestic cat [6]. Although “group” embryonic culture supports development better than culture individually, an excessive culture density could negatively affect embryo development. This is suggested to be caused by increasing cell stress resulting in an aberration of gene expression of embryos [2]. Normally, most cellular stresses contribute to free radical formation, membrane destabilization, protein denaturation, DNA damage and apoptosis. To protect the cell from these stress factors, cytoprotective mechanisms including heat shock protein (HSP) response, DNA repair cycle checkpoint regulation and the antioxidant system are essentially activated [7]. Among the mechanisms associated with the stress responses, heat shock 70 kDa proteins (HSP70s) have been used as the most potential tool for assessing stress response in mammalian embryos during culture [8]. Inducible HSP70 (HSP70) plays essential roles in both normal cell development and protection against stress-induced damage [9,10,11]. This protein is actively involved in protein folding, assembly and translocation processes of several cytosolic proteins [12]. Under stress conditions, inducible HSP70 is markedly produced to stabilize damaged proteins, allowing them to be consequently repaired or degraded, hence minimizing cell damage [12, 13]. HSP70's upregulation in in vitro produced embryos has therefore been used to indicate the embryonic stress induced by an inappropriate culture condition [2, 14].
In addition to heat shock protein expression, apoptosis is also a common mechanism used to eliminate mutated, damaged and healthy unwanted cells [15]. Differences in apoptotic rate between in vitro cultured and in vivo produced embryos have been demonstrated in several species [15,16,17]. Basically, increasing incidence of apoptosis in cultured embryonic cells indicates a suboptimal culture condition [18]. To balance the homeostasis between life and death of cells, pro-apoptotic BAX (Bcl-2 associated X) and anti-apoptotic BCL-2 (B-cell lymphoma protein 2) proteins globally play an important role [19], and the ratio between BAX and BCL-2 (BAX/BCL-2 ratio) has commonly been used to predict cell fate [20, 21]. In fact, HSP70 also plays a role in anti-apoptotic function [22, 23]; it interacts with intrinsic and extrinsic pathways of apoptosis at a number of steps. These include the inhibition of translocation of BAX into mitochondria, release of cytochrome c from mitochondria, formation of the apoptosome and inhibition of activation of caspase activity. It also modulates JNK, NK-kB and Akt signaling pathways in the apoptotic cascade [23].
In feline species, expression analysis of genes associated with embryo development has become an important tool to understand the physiological response of embryos to their in vitro culture environment [24,25,26]. Though information for several genes has been provided, the change in gene expressions associated with stress-induced apoptosis is not entirely known in this species. To demonstrate the effects of culture density on stress responses, this study determined the expressions of heat shock protein 70 (HSP70) and apoptotic-related (BAX and BCL-2) genes in feline embryos cultured at various culture densities.
Materials and Methods
All chemicals used in the experiments were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Fibroblast cell culture and heat activation
Cat skin fibroblasts were cultured as monolayer in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (Gibco, Invitrogen, Carlsbad, CA, USA), 2 mM L-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin in a sealed cap tissue culture flask (25 cm2, BD FalconTM, Franklin Lakes, NJ, USA). The cells from passages 3 to 5 with 80% confluence were used for heat treatment by submerging the flask sealed with Parafilm under water in a water bath set at 45 C for 45 min (modified from Liu [27]). The cells from one culture flask were harvested immediately at the end of heat treatment (0 h). The other flasks were returned to the incubator and further cultured at 37 C in 5% CO2 for 3, 6 and 12 h. Non-heated cat fibroblasts served as a control group.
RNA extraction from feline fibroblasts
Following heat treatment, cat fibroblasts were dissociated using 0.25% (wt/vol) trypsin EDTA (Gibco) and centrifuged, and the cell pellets were finally stored at –80 C until use. Total RNA was extracted from cat fibroblasts using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Briefly, fibroblast pellets were lysed using RLT buffer and then vortexed for 1 min. An equal volume of 70% ethanol was added into the cell lysate. The mixture was then transferred to an RNA-binding column and centrifuged at ≥ 8,000 × g for 15 sec. The filtrate was discarded, and RW1 buffer was added into the reseated column. After centrifugation, the filtrate was discarded, and the reseated column was washed with RPE buffer twice and dried by centrifugation at ≥ 8,000 × g for 2 min. The RNA was eluted with 30 µl of RNase-free water. The purity and quantity of extracted RNA was assessed using a spectrophotometer (NanoDrop ND-2000, Wilmington, DE, USA), and only extracted RNA with an A260/A280 ratio ranging from 1.5 to 2.0 was immediately stored at –80 C for further analysis.
Reverse transcription polymerase chain reaction (RT-PCR) analysis of HSP70 transcript in feline fibroblasts
Reverse transcription (RT) was performed using a First-Strand cDNA Synthesis Kit (Omniscript Kit, Qiagen, CA, USA). The RT reaction (20 µl) was prepared following the kit manufacturer's protocol. Briefly, 8 µl of total RNA (1 µg) were mixed with 2 µl of random hexamers (100 µM), 2 µl of dNTP Mix (5 mM each dNTP), 2 µl of 10xRT buffer, 0.25 µl of RNaseOut (40 units/ µl), 1 µl of Omniscript RT and 4.65 µl of nuclease-free water. The reaction was incubated at 37 C for 60 min. Reverse transcription containing all RT-PCR reagents, except the reverse transcriptase, was run in parallel in RT-PCR as a minus-reverse transcriptase (“-RT”) control. The product was stored at –20 C for further use in PCR. The conserved region of the HSP70 mRNA sequence among the human, mouse, rat and canine was used to design the primers by using the rat HSP70 mRNA sequence as the template. PCR primers were designed from HSP70 mRNA, complete cDNA of the rat (accession number: L16764), using the LightCycler Probe Design Software Version 2.0 (Table 1). A primer of glyceraldehyde 3-phosphatedehydrogenase (GAPDH) transcripts designed from a previous study [28] was used in this study as the endogenous normalizer. The PCR was performed using the GoTaq Green Mastermix (Promega, Madison, WI, USA). Each PCR reaction (total volume of 25 µl) consisted of 2 µl of reverse transcription product and 23 µl of reaction mixture, which consisted of 12.5 µl of Gotaq Green Master Mix, 2 µl of both 10 µM forward and reverse primers and 6.5 µl of nuclease-free water. Thermal cycling conditions were as follows: 2 min at 95 C to activate Taq DNA polymerase; 30 cycles of 30 sec at 95 C for denaturing, 30 sec at 55 C for annealing and 30 sec at 72 C for extension; and 2 min at 72 C for the final extension. At the end of the program, PCR products were confirmed by electrophoresis. The amplified products were run in 2% (wt/vol) agarose gel (Bio-Rad, Hercules, CA, USA) prepared in 1 × TBE buffer (90 mM Tris, 90 mM boric acid and 2 mM EDTA, pH 8) containing 0.4 mg/ml ethidium bromide (Promega, Madison, WI, USA). The separated products in agarose gel were visualized under the UV light of a Gel Documentation system (Syngene, Cambridge, CB, UK). The band intensity of amplified RT-PCR products was converted into mean pixel per area using Scion Image Analysis software (Scion, Frederick, MD, USA). The PCR products of HSP70 were normalized with GAPDH at each condition.
Table 1. Description of forward (FP) and reverse (RP) primers used to assess expression of target genes in blastocysts acquired from different culture conditions.
| Genes | Sequence (5´-3´ orientation) | Fragment length (bp) | GenBank accession number or Reference |
| HSP701 | FP: ATCCAGGTGTACGAGGG RP: TGGTGATCTTGTTGGCCT |
190 | L16764 |
| BAX | FP: CCGATGGCAACTTCAACTGGG RP: GTCAGCACTCCCGCCACAAAG |
244 | [35] |
| BCL-2 | FP: GGAGGATTGTGGCCTTCT RP: GTTATCCTGGATCCAGGTGT |
143 | [36] |
| GAPDH | FP: GGAGAAAGCTGCCAAATATG RP: CAGGAAATGAGCTTGACAAAGTGG |
191 | [28] |
1HSP70 primers were used for feline fibroblast (experiment 1) and blastocysts (experiment 2).
Sequencing of partial feline-specific HSP70 mRNA
The PCR products acquired from gel electrophoresis were collected for sequencing, and the sequence was confirmed in GenBank. The amplicons were purified from agarose gel using NucleoSpin® Extract II kits (Macherey-Nagel, Düren, Germany). The purity of the eluted products was confirmed by running in a 1.5% agarose gel. After confirming the eluted products, both strands of each amplicon were sequenced using the same HSP70 primers. The sequences derived from both strands were assembled using BioEdit Version 7.0.8.0 (T.A. Hall Software, Raleigh, NC, USA). Assembled sequences were then blasted in GenBank to determine the nucleotide similarity to other species.
Oocyte recovery
The ovaries were obtained following ovariohysterectomy from domestic cats with unknown reproductive status. They were collected in a 0.9% (wt/vol) saline solution supplemented with 100 IU/ml penicillin and 100 µg/ml streptomycin. Within 2 h, they were washed and minced to obtain the cumulus-oocyte complexes (COCs) in holding medium (HM) consisting of Hepes-buffered M199, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin and 4 mg/ml bovine serum albumin (BSA, embryo tested) under a stereomicroscope (SMZ645 Nikon, Tokyo, Japan). Only COCs surrounded with compacted cumulus cells and containing with a homogeneous-dark ooplasm (Grades I and II) were used in this study [29].
Oocyte maturation and fertilization
In vitro oocyte maturation (IVM) and fertilization (IVF) were essentially performed as previously described [30]. Groups of 20–30 COCs were cultured for 24 h in 500 μl of IVM medium (NaHCO3-M199 supplemented with 1.0 mM sodium pyruvate, 2.0 mM L-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin, 4 mg/ml bovine serum albumin (BSA) and 25 ng/ml epidermal growth factor (EGF)) containing with 0.05 IU/ml recombinant human follicle stimulating hormone (rhFSH, Organon, The Netherlands). After oocyte culture, cumulus cells were partially removed by gentle pipetting, and a group of 8 to 10 oocytes were cultured in a 50 µl droplet of IVF medium (Tyrode's balanced salt solution containing 1% MEM nonessential amino acid, 6 mg/ml BSA, 100 IU/ml penicillin, 30 µg/ml heparin and IVF×100) [31]. IVF×100 stock consisted of 100 mM L-glutamine, 36.3 mM sodium pyruvate and 110.9 mM calcium lactate in a Tyrode's balanced salt solution.
The semen used in this study was collected from two fertility proven tom cats and then frozen according to Andersen [32] with minor modifications. In brief, the cats were anesthetized with 0.04 mg/kg atropine sulphate (A.N.B. Laboratories, Bangkok, Thailand), 3 mg/kg xylazine hydrochloride (Laboratorios Calier, Barcelona, Spain) and 10 mg/kg ketamine hydrochloride (Gedeon Richter, Budapest, Hungary). The semen was collected by electro-ejaculation [33]. It was cryopreserved by placing the straws horizontally 4 cm above liquid nitrogen vapors for 10 min and then plunging them into liquid nitrogen. After thawing at 70 C for 6 sec, the sperm were subjectively evaluated, and only sperm that had more than 50% progressive motility were used for in vitro fertilization. The frozen-thawed sperm were washed twice in IVF medium, and the sperm concentration was determined. To perform IVF, the IVM oocytes were co-incubated with sperm at a final concentration of 0.5 × 106 sperm/ml for 18 h at 38.5 C in a humidified condition of 5% CO2 in air.
In vitro embryo culture (IVC)
After co-incubation of the oocytes with sperm, cumulus cells were removed by gentle pipetting. Presumptive zygotes were then washed and cultured in a synthetic oviductal fluid (SOF) containing 4 mg/ml BSA, 100 µg/ml streptomycin and 100 IU/ml penicillin (IVC-1) [6]. After 24 h of culture, only cleaved embryos were washed and cultured in IVC-2 medium (SOF containing 10% (vol/vol) FCS (Gibco®, Invitrogen, Carlsbad, CA, USA)). Culture medium was changed every two days. In all cases, in vitro culture was performed at 38.5 C in a humidified condition of 5% CO2 in air.
In vivo produced embryos
Surgical collection of feline embryos was performed according to the Committee of Animal Ethics, Faculty of Veterinary Science, Chulalongkorn University (accession no. 11310032). Four queens were intramuscularly injected with 150 IU equine chorionic gonadotropin (eCG, Intervet/Schering-Plough, Boxmeer, The Netherlands). After eCG injection for 96 h, the queens received 150 IU human chorionic gonadotropin (hCG, Intervet/Schering-Plough) to ensure ovulation and simultaneously allowed to mate with a tom cat. Natural mating was performed three times a day at 3-h intervals on the first and second days (day 0) after hCG injection. Ovulation was expected to occur 24 to 28 h after mating/hCG injection [34]. On day 7 post hCG injection, the queens were anesthetized as previously described, and ovariohysterectomy was performed. Embryos were recovered from the uterine horns by gentle flushing with HM. The embryos classified as expanded blastocysts were washed twice in phosphate buffered saline (PBS) containing with 0.1% (wt/vol) BSA and immediately stored at –80 C in a minimum volume (less than 2 µl) for further analysis.
Assessment of embryo development and embryo quality
The percentages of cleaved embryos (2 to 16 cells), morula (≥16 cells without blastocele) and blastocysts (≥50 cells with blastocele formation) were evaluated on days 2, 5 and 7 of IVC, respectively. Morula and blastocyst rates were calculated relative to the cleaved embryos, while hatching rates were calculated relative to the number of blastocysts. To count the number of embryonic cells, blastocysts were first fixed in 4% (wt/vol) paraformaldehyde and kept at 4 C for 2 days before the staining procedure. They were then stained with 0.1 µg/ml 4',6-diamidino-2-phenylindole (DAPI) in PBS at 37 C for 10 min. Nuclear fragmentation of embryonic nuclei was calculated relative to the total embryonic cells under an epifluorescent microscope (BX51 Olympus, Shinjuku, Japan).
RNA extraction of feline embryos
An Absolutely RNA Nanoprep Kit (Stratagene, San Diego, CA, USA) was used to extract the total RNA from a pool of five expanded blastocysts following the manufacturer's instruction. Briefly, pooled blastocysts acquired from each experimental replicate were lysed using a lysis buffer containing with 0.7% (vol/vol) β-mercaptoethanol (β-ME). Cell lysate was added with an equal volume of 80% sulfolane. The mixture was then transferred to an RNA-binding nano-spin cup and was centrifuged at ≥ 12,000 × g for 60 sec. The filtrate was discarded, and a reseated spin cup was added with low-salt wash buffer. After centrifugation, the filtrate was discarded, and the cup was reseated and dried by centrifugation at ≥12,000 × g for 60 sec. DNase I (Stratagene, San Diego, CA, USA) was added onto the fiber matrix inside the cup, and the mixture was incubated at 37 C for 15 min. After incubation, the fiber matrix was washed once with a high-salt washing buffer and then twice with a low-salt washing buffer. RNA was eluted with 12 µl RNase-free water by centrifugation (≥12,000 × g for 5 min). The purity and quantity of extracted RNA was assessed using a spectrophotometer (NanoDrop ND-2000, Wilmington, DE, USA), and only extracted RNA with an A260/A280 ratio ranging from 1.5 to 2.0 was immediately stored at –80 C for further analysis.
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) analysis of HSP70, BAX and BCL-2 transcripts in embryos
RT reaction (20 µl) was performed using a First-Strand cDNA Synthesis Kit (SuperScript III Kit, Invitrogen, Carlsbad, CA, USA) according to the kit manufacturer's protocol. Initially, 8 µl of extracted RNA was incubated with 1 µl of random hexamers (50 ng/µl) and 1 µl of 10 mM dNTP mix at 65 C for 5 min, and then immediately placed on ice for at least 1 min. After chilling on ice, the reaction mixture was then added to master mix containing 2 µl of 10×RT buffer, 4 µl of 25 mM MgCl2, 2 µl of 0.1 M DTT, 1 µl of RNaseOut (40 U/µl) and 1 µl of SuperScript III RT (200 U/µl). The reaction mixture was further incubated at 50 C for 50 min followed by 85 C for 5 min. RNA template from the cDNA:RNA hybrid molecule was removed by digestion with 1 µl of RNase H (2 U/µl) after first-strand synthesis at 37 C for 20 min. Reverse transcription containing all RT-PCR reagents, except the reverse transcriptase, was run in parallel in RT-PCR as a minus-reverse transcriptase (“-RT”) control. The product was stored at –20 C for further use in PCR.
The mRNA transcript levels at steady state of individual target genes (HSP70, BAX and BCL-2) were normalized to the endogenous normalizer (GAPDH), and were run in separate wells. The PCR was performed using an ABI PRISM 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). Each PCR reaction (total volume of 20 µl) consisted of 2 µl of reverse transcription product (equivalent to 0.5 blastocysts) and 18 µl of reaction mixture, which was consisted of 10 µl of SYBR Green Master Mix, 1 µl of both 5 µM forward and reverse primers (Table 1) and 6 µl of nuclease-free water. Thermal cycling conditions were as follows: 10 min at 95 C to activate Taq DNA polymerase, 45 cycles of 15 sec at 95 C for denaturing, 30 sec at 55 C for annealing and 60 sec at 72 C for extension. PCR products were confirmed by melting curve analysis and gel electrophoresis. The amplified products were confirmed by running in 2% agarose gel (Bio-Rad, Hercules, CA, USA) prepared in 1 × TBE buffer (90 mM Tris, 90 mM boric acid and 2 mM EDTA, pH 8) containing 0.4 mg/ml ethidium bromide (Promega, Madison, WI, USA). The separated products in agarose gel were visualized under the UV light of a Gel Documentation System (Syngene, Cambridge, CB, UK). Three biological replicates of pooled embryos were analyzed for each gene. All PCRs were performed in a technical replicate of 20 µl for every transcript of interest.
The Sequence Detection System (SDS) Software Version 1.4 (Applied Biosystems, Carlsbad, CA, USA) was used to analyze the relative quantitation (RQ) by comparative Cq method using the in vivo derived (IVV) blastocysts as a control group. RQ was reported as relative abundance in relation to the control sample. The Δ Cq was calculated from the target gene Cq (HSP70, BAX and BCL-2) by subtracting the endogenous normalizer (GAPDH) Cq. ΔΔ Cq was formulated from Δ Cq(IVP embryo)– Δ Cq(IVV embryo), and the RQ was calculated from 2 –ΔΔ Cq.
Statistical analysis
Three to four biological replicates were performed in each experiment. The data were expressed as the mean ± standard error (SE). Values were analyzed using one-way ANOVA and Duncan's test for statistical differences among groups. Differences with P<0.05 were considered statistically significant.
Experimental design
Experiment 1–Identification of a partial feline HSP70 mRNA sequence in response to heat activation: This experiment was designated to identify feline HSP70 mRNA in heat-treated cat fibroblasts because the sequence is lacking in GenBank. In each replicate, 5 culture flasks of cat skin fibroblasts were used. One vessel was kept at 37 C in 5% CO2 incubator as a control group, and the others were heat treated (45 C) for 45 min. The heat-treated cells were harvested for RNA extraction at 0, 3, 6 and 12 h after heat treatment, while the control was harvested simultaneously with the cells heat-treated for 0 h. The cells were analyzed for expression of HSP70 using RT-PCR with designed primers. The acquired PCR products were sequenced and then blasted in GenBank in order to determine the nucleotide similarity to other species.
Experiment 2–Effect of embryo density on the transcripts of BAX, BCL-2 and HSP70 in relation to developmental competence: Cumulus-oocyte complexes (COCs) were matured and fertilized in vitro (day 0 = day of in vitro fertilization). Ten cleaved embryos were cultured in different culture volumes (12.5, 25, 50, 100 and 200 µl) to acquire the embryonic densities of 1:1.25, 1:2.5, 1:5, 1:10 and 1:20, respectively. In vitro developmental competence of embryos cultured at each embryonic density was determined by means of morula (day 5) and blastocyst (day 7) formation rates in relation to the number of cleaved embryos. On day 7, blastocysts were fixed and stained with a DNA labeling dye to count the total cell numbers and nuclear fragmentation. To examine the effect of embryonic culture density on HSP70, BAX and BCL-2 expressions, the culture densities of 1:1.25, 1:5 and 1:20 were selected as the representative “high,” “medium” and “low” densities. In vivo produced expanded blastocysts were included as a control group. Real-time PCR was used in this study due to the limited levels of gene expression in particular embryos. The mean values of BAX and BCL-2 transcripts were used to calculate the BAX/BCL-2 ratio.
Results
Experiment 1: Characterization of a partial feline HSP70 mRNA sequence in response to heat activation
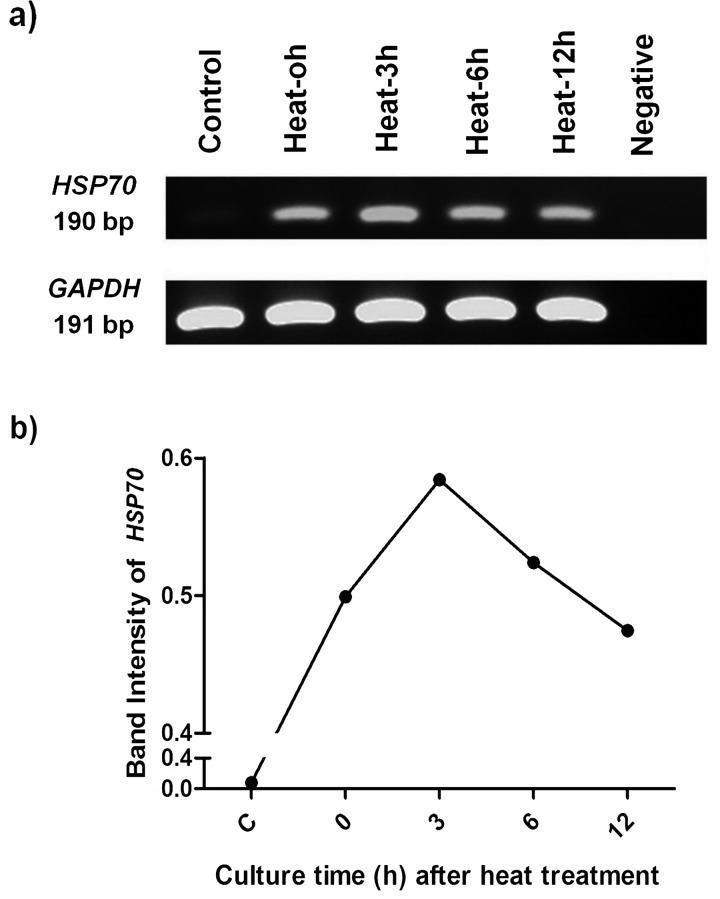
The heat treatment significantly upregulated the HSP70 transcript in cat skin fibroblasts. The acquired HSP70 transcript (190 bp) was transiently expressed in response to heat, gradually increasing from 0 to 3 h after heat activation and then gradually decreasing afterward (Fig. 1a and 1b). GAPDH transcripts were constantly expressed (Fig. 1a). The heat-response profile of HSP70 in this study was similar to that acquired from other species.
Fig. 1.
HSP70 transcript profiles of cat fibroblasts heat-treated at 45 C for 45 min followed by 0, 3, 6 and 12 h of culture (0, 3, 6 and 12 h, respectively) compared with non-heated control fibroblasts (C). PCR reaction without template served as a negative control (N). The amplified RT-PCR products (1a) and semi-quantitative analysis of HSP70 mRNA (1b) indicate the heat stress response of HSP70 of cat fibroblasts.
The acquired partial feline HSP70 mRNA sequence was subsequently determined and compared with the sequences previously reported in other species (Fig. 2 and Table 2). High similarity of HSP70 mRNA sequence between feline and other species was demonstrated (93 to 96% nucleotide identity) (Table 2). The designed HSP70 primers were then used for quantification of HSP70 transcripts of feline blastocysts in experiment 2.
Fig. 2.
The alignment between the acquired partial cat (Query) and rat (L16764.1) (Sbjct) heat shock protein 70 (HSP70) mRNA. The size of the feline mRNA product was 190 bp, and the position of the rat mRNA shown was from 1318 to 1507 bp. The vertical bar indicates the similarity of nucleotides between the sequences.
Table 2. Homology of HSPA1A mRNA between domestic cat and other species.
| Species | GenBank accession number | mRNA identity (%) |
| Rat (Rattus norvegicus) | L16764.1 | 94 |
| Mouse (Mus Musculus) | M35021.1 | 93 |
| Human (Homo sapiens) | DQ451402.1 | 93 |
| Canine (Canis lupus familiaris) | AB114672.1 | 95 |
| Bovine (Bos Taurus) | AY662497.1 | 96 |
| Swine (Sus scrofa) | M69100.1 | 95 |
| Buffalo (Bubalus bubalis) | EU099315.1 | 95 |
Experiment 2: HSP70, BCL-2 and BAX expressions related with developmental competence of embryos cultured at various embryonic densities
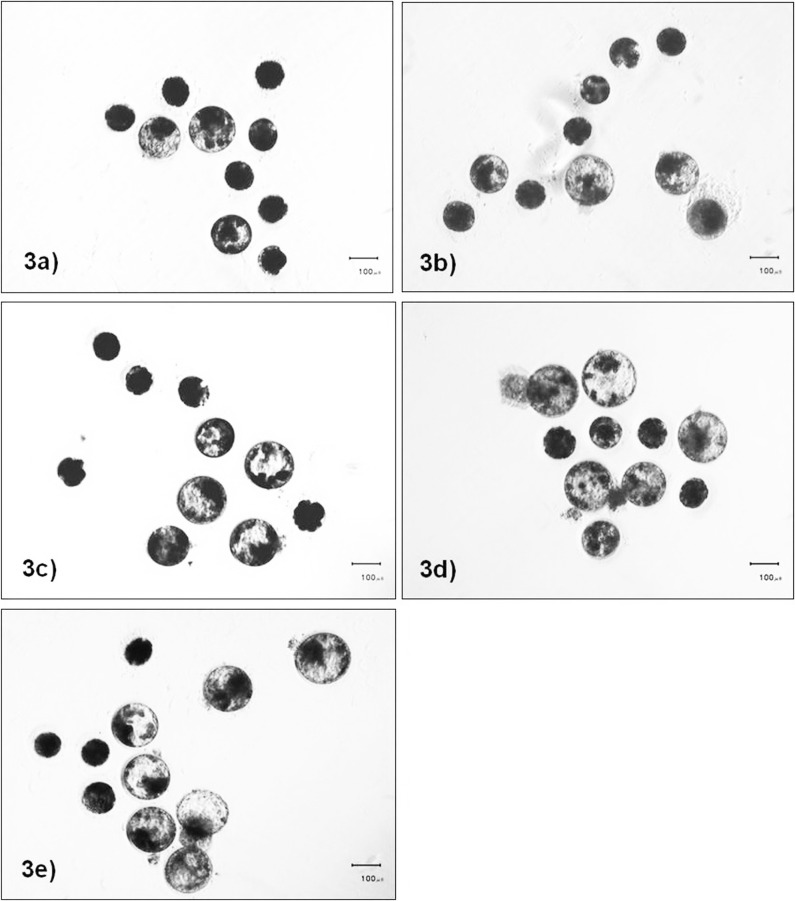
Following IVF, approximately 50 to 70% of inseminated oocytes cleaved (data not shown). Although the numbers of cleaved embryos that developed to the morula stage did not significantly differ among the embryonic culture densities (morula rates: ~80 to 95%, P> 0.05), high embryonic density during culture adversely affected the blastocyst formation rates. High embryonic culture density (1:1.25) significantly decreased the blastocyst formation rate when compared with those cultured at 1:5, 1:10 and 1:20 culture densities (Fig. 3 and Table 3). While blastocysts culture at a 1:1.125 culture density had lower cell numbers compared with other embryo culture densities, hatching and nuclear fragmentation rates of blastocysts were not significantly different among embryonic culture densities (Table 3).
Fig. 3.
Bright field images of day 7 cat embryos cultured at different densities (10 embryos per media volume). They were cultured at densities of 1:1.25 (3a), 1:2.5 (3b), 1:5 (3c), 1:10 (3d) and 1:20 (3e).
Table 3. Mean percent ± SE of developmental competence of cat embryos (3 replicates) cultured in groups of ten in different volume of medium to acquire different culture densities.
| Culture volume (µl) | Culture density | N | Cleaved embryos developed to | Hatching | Cell no. | Fragmentation | |
| Morula | Blastocyst | ||||||
| 12.5 | 1:1.25 | 50 | 81.7 ± 7.3a | 15.0 ± 10.4a | 0.0 ± 0.0a | 69.1 ± 42.7a | 19.5 ± 12.1a |
| 25 | 1:2.5 | 50 | 80.0 ± 7.6a | 20.0 ± 5.0ab | 13.3 ± 13.3a | 180.1 ± 41.8b | 22.5 ± 6.6a |
| 50 | 1:5 | 60 | 97.2 ± 1.5a | 39.4 ± 7.5bc | 21.0 ± 1.0a | 140.5 ± 15.4ab | 14.3 ± 1.4a |
| 100 | 1:10 | 50 | 86.7 ± 3.3a | 48.3 ± 1.7c | 14.1 ± 7.1a | 149.2 ± 14.9ab | 14.4 ± 1.5a |
| 200 | 1:20 | 60 | 90.6 ± 4.7a | 43.3 ± 3.3c | 19.4 ± 10.0a | 152.2 ± 28.0ab | 14.3 ± 1.2a |
a, b, c Within a column, values with different superscripts were significantly different (P<0.05). N = Total number of cleaved embryos used. Culture density = 10 embryos: medium volume (µl).
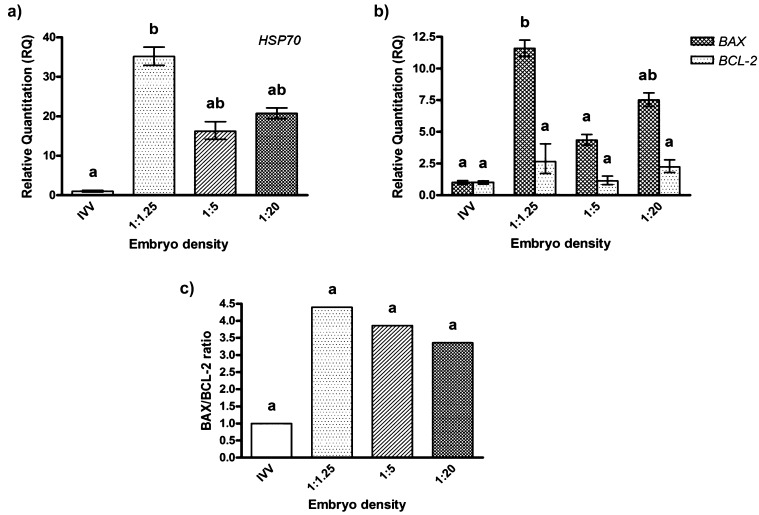
To examine the effect of embryonic culture density on HSP70, BAX and BCL-2 expressions, culture densities of 1:1.25, 1:5 and 1:20 were selected as the representative “high,” “medium” and “low” densities. In vivo produced blastocysts served as a control group. Overall, in vitro produced embryos, irrespective of the culture density employed, showed upregulated levels of HSP70 and BAX transcripts compared with in vivo derived feline embryos. However, only HSP70 and BAX transcripts from blastocysts cultured at a culture density of 1:1.25 were significantly higher than in in vivo embryos (P<0.05, Fig. 4a and Fig. 4b). Although expression of HSP70 was not significantly different among in vitro culture densities, BAX transcripts were significantly increased in embryos cultured at a culture density of 1:1.25 compared with a culture density of 1:5. Moreover, BAX/BCL-2 ratios appeared to decrease when the culture volume per embryo was increased (Fig. 4c).
Fig. 4.
Comparison of specific transcripts analyzed by real-time PCR in feline blastocysts produced in vivo (IVV) and in vitro at densities of 1:1.25, 1:5 and 1:20. Mean values were obtained from three independent replicates. Bars indicate the upper and lower bounds of values. a, b Different superscripts denote values that differ significantly (P<0.05).
Discussion
In this study, the partial sequence of feline HSP70 mRNA was firstly characterized and then further applied to evaluate the physiological response of embryos to the culture density. Embryos cultured at a high embryonic density showed upregulated levels of pro-apoptotic (BAX) and stress-response (HSP70) transcripts, and this adversely affected their developmental competence. This suggested possible stress accumulation that inhibited embryo development and probably induced apoptosis.
In response to stress, induced HSP70 protects essential cell components against heat damage and also allows a rapid resumption of normal cellular activities during the recovery period. HSP70 is rapidly and transiently induced in response to heat shock (around 40 to 50 C for mammalian cells) [37]. Because the structure and function of HSP70 proteins are highly conserved across species [38], conserved rat-specific HSP70 primers designed from human-mouse-rat-canine HSP70 mRNA alignments were used to amplify feline cDNA in the present study. In order to determine the inducible expression properties of HSP70, examination of heat response was firstly studied. Similar to previous studies [39,40], HSP70 response in cat fibroblasts in the current study was transiently increased during 0 to 3 h after heat treatment, and this was followed by a progressive decline (6 to12 h). However, the heat-response time of cat HSP70 in this study was different from that in a previous report in humans [39]. This appeared to be caused by the temperature- and cell-type specific response of HSP70per se [41,42]. Of note, this study only evaluated HSP70 transcription, and evaluation of the protein translation was not performed because of a limitation of the feline embryos produced.
According to our previous study [6], suboptimal in vitro culture density detrimentally affected the developmental competence of feline embryos. While paracrine and autocrine embryo-derived factors are critically important for their development [43,44,45], excessive embryo density could also, in turn, inhibit embryo development probably due to accumulation of stress from particular culture conditions (nutritional inadequacy, pH imbalance, etc.) [2]. Reduction of blastocyst formation rates was observed in embryos cultured at a high embryonic density (1:1.25). However, morphological study only is inadequate to clearly understand embryonic stress during in vitro culture. We therefore examined the HSP70, BAX and BCL-2 genes of embryos cultured at various densities in correlation with their morphology.
Similar to our previous report [6], high in vitro culture density (1:1.25) reduced developmental competence and quality of feline embryos in terms of blastocyst formation rates and quality. Interestingly, the low blastocyst development and quality of feline embryos derived from this high embryonic culture density coincided with an increase in HSP70 and pro-apoptotic (BAX) transcripts and BAX/BCL-2 ratio. The upregulation of these transcripts during embryo culture may indicate a stress response in the embryos when they were cultured at a high density. More importantly, it is clearly evident that the levels of the transcripts of in vitro produced embryos also increased to be higher than those obtained from in vivo embryos, even though the embryo density was optimal (density 1:20) in terms of the high blastocyst rates obtained (approximately 40 to 50%). The results thus suggested the detrimental effect of high embryonic culture density on developmental competence, probably via excessive cellular stresses.
Apart from the stress response of HSP70, apoptotic-related genes (BAX and BCL-2) also play an important role in adaptive response to stress condition by removal of damaged cells in embryos [16, 46]. Pro-apoptotic (BAX) expression and the BAX/BCL-2 ratio were increased in cat embryos cultured at high culture density (1:1.25) in the same manner as HSP70 expression. As previously described, upregulation of HSP70 appears to reduce pro-apoptotic activity of BAX, depending on the severity of cell damage [47]. In this study, high culture density may have severely induced embryonic stress, resulting in HSP70 not being able to overcome the BAX activation. This could contribute to the positive correlation between BAX and HSP70 expressions levels in densely cultured embryos (Fig. 4a and 4b). Although other apoptotic pathways may negatively contribute to embryo development, our results suggested the possible pathway of embryonic cell stress induced apoptosis from high embryonic culture density. Other pathways that trigger apoptosis in feline embryos still need to be further examined, since several types of stress from high embryo density involve the fate of embryo development. For example, oxidative stress in in vitro cultured embryos is commonly caused by excessive accumulation of embryotoxic elements secreted from crowded embryos such as radical oxygen species [48] and ammonium [2, 4, 49], an inadequacy of nutritional requirement and pH imbalance [50]. A large amount of reactive oxygen species (ROS) generated by this condition could possibly trigger HSP70 expression [51] and could also initiate apoptosis of cells [52]. In order to understand the importance of oxidative stress at a high culture density, further determination of its effects should be performed.
From our study, embryonic stress from high culture density results in developmental incompetency and affects gene expression patterns. The combination of morphological and molecular observations helps us to identify the probable mechanisms responsible for suboptimal culture conditions for feline embryos.
Acknowledgment
This work was financially supported by an RGJ-PhD grant, Thailand Research Fund, the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (HR1166IB-55); French Embassy; and “Integrated Innovation Academic Center: IIAC” Chulalongkorn University Centenary Academic Development Project (CU56-HR07). The authors would like to thank the Veterinary Public Health Division of the Bangkok Metropolitan Administration, Thailand, for sample collection and the Office of Academic Affairs, Chulalongkorn University, for linguistic editing of the manuscript.
References
- 1.Pope CE, Gomez MC, Dresser BL. In vitro production and transfer of cat embryos in the 21st century. Theriogenology 2006; 66: 59–71 [DOI] [PubMed] [Google Scholar]
- 2.de Oliveira AT, Lopes RF, Rodrigues JL. Gene expression and developmental competence of bovine embryos produced in vitro under varying embryo density conditions. Theriogenology 2005; 64: 1559–1572 [DOI] [PubMed] [Google Scholar]
- 3.Gardner DK, Lane M, Spitzer A, Batt PA. Enhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: amino acids, vitamins, and culturing embryos in groups stimulate development. Biol Reprod 1994; 50: 390–400 [DOI] [PubMed] [Google Scholar]
- 4.O'Doherty EM, Wade MG, Hill JL, Boland MP. Effects of culturing bovine oocytes either singly or in groups on development to blastocysts. Theriogenology 1997; 48: 161–169 [DOI] [PubMed] [Google Scholar]
- 5.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci USA 1990; 87: 4756–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sananmuang T, Tharasanit T, Nguyen C, Phutikanit N, Techakumphu M. Culture medium and embryo density influence on developmental competence and gene expression of cat embryos. Theriogenology 2011; 75: 1708–1719 [DOI] [PubMed] [Google Scholar]
- 7.Hansen PJ. To be or not to be--determinants of embryonic survival following heat shock. Theriogenology 2007; 68(Suppl 1): S40–S48 [DOI] [PubMed] [Google Scholar]
- 8.Mortensen CJ, Choi YH, Ing NH, Kraemer DC, Vogelsang MM, Hinrichs K. Heat shock protein 70 gene expression in equine blastocysts after exposure of oocytes to high temperatures in vitro or in vivo after exercise of donor mares. Theriogenology 2010; 74: 374–383 [DOI] [PubMed] [Google Scholar]
- 9.Luft JC, Dix DJ. Hsp70 expression and function during embryogenesis. Cell Stress Chaperones 1999; 4: 162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Günther E, Walter L. Genetic aspects of the hsp70 multigene family in vertebrates. Experientia 1994; 50: 987–1001 [DOI] [PubMed] [Google Scholar]
- 11.Yamashita M, Hirayoshi K, Nagata K. Characterization of multiple members of the HSP70 family in platyfish culture cells: molecular evolution of stress protein HSP70 in vertebrates. Gene 2004; 336: 207–218 [DOI] [PubMed] [Google Scholar]
- 12.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 2010; 11: 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol 2000; 59: 55–63 [DOI] [PubMed] [Google Scholar]
- 14.Edwards JL, Ealy AD, Hansen PJ. Regulation of heat shock protein 70 synthesis by heat shock in the preimplantation murine embryo. Theriogenology 1995; 44: 329–337 [DOI] [PubMed] [Google Scholar]
- 15.Hardy K. Cell death in the mammalian blastocyst. Mol Hum Reprod 1997; 3: 919–925 [DOI] [PubMed] [Google Scholar]
- 16.Hardy K. Apoptosis in the human embryo. Rev Reprod 1999; 4: 125–134 [DOI] [PubMed] [Google Scholar]
- 17.Pomar FJ, Teerds KJ, Kidson A, Colenbrander B, Tharasanit T, Aguilar B, Roelen BA. Differences in the incidence of apoptosis between in vivo and in vitro produced blastocysts of farm animal species: a comparative study. Theriogenology 2005; 63: 2254–2268 [DOI] [PubMed] [Google Scholar]
- 18.Fabian D, Koppel J, Maddox-Hyttel P. Apoptotic processes during mammalian preimplantation development. Theriogenology 2005; 64: 221–231 [DOI] [PubMed] [Google Scholar]
- 19.Giam M, Huang DC, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene 2008; 27 (Suppl 1): S128–S136 [DOI] [PubMed] [Google Scholar]
- 20.Perlman H, Zhang X, Chen MW, Walsh K, Buttyan R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ 1999; 6: 48–54 [DOI] [PubMed] [Google Scholar]
- 21.Yang MY, Rajamahendran R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim Reprod Sci 2002; 70: 159–169 [DOI] [PubMed] [Google Scholar]
- 22.Lanneau D, de Thonel A, Maurel S, Didelot C, Garrido C. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion 2007; 1: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci 2007; 32: 595–610 [DOI] [PubMed] [Google Scholar]
- 24.Filliers M, Goossens K, Van Soom A, Merlo B, Pope CE, de Rooster H, Smits K, Vandaele L, Peelman LJ. Gene expression profiling of pluripotency and differentiation-related markers in cat oocytes and preimplantation embryos. Reprod Fertil Dev 2012; 24: 691–703 [DOI] [PubMed] [Google Scholar]
- 25.Imsoonthornruksa S, Lorthongpanich C, Sangmalee A, Srirattana K, Laowtammathron C, Tunwattana W, Somsa W, Ketudat-Cairns M, Parnpai R. Abnormalities in the transcription of reprogramming genes related to global epigenetic events of cloned endangered felid embryos. Reprod Fertil Dev 2010; 22: 613–624 [DOI] [PubMed] [Google Scholar]
- 26.Waurich R, Ringleb J, Braun BC, Jewgenow K. Embryonic gene activation in in vitro produced embryos of the domestic cat (Felis catus). Reproduction 2010; 140: 531–540 [DOI] [PubMed] [Google Scholar]
- 27.Liu RY, Li X, Li L, Li GC. Expression of human hsp70 in rat fibroblasts enhances cell survival and facilitates recovery from translational and transcriptional inhibition following heat shock. Cancer Res 1992; 52: 3667–3673 [PubMed] [Google Scholar]
- 28.Sano J, Nagafuchi S, Yamazaki J, Oguma K, Kano R, Hasegawa A. Effect of antineoplastic drugs on the expression of Bcl-2 and Bcl-xL genes in the feline T-cell leukemia cell line. Res Vet Sci 2005; 79: 197–201 [DOI] [PubMed] [Google Scholar]
- 29.Wood TC, Wildt DE. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J Reprod Fertil 1997; 110: 355–360 [DOI] [PubMed] [Google Scholar]
- 30.Sananmuang T, Techakumphu M, Tharasanit T. The effects of roscovitine on cumulus cell apoptosis and the developmental competence of domestic cat oocytes. Theriogenology 2010; 73: 199–207 [DOI] [PubMed] [Google Scholar]
- 31.Pope CE. In vitro fertilization and embryo transfer in felids. Methods Mol Biol 2004; 254: 227–244 [DOI] [PubMed] [Google Scholar]
- 32.Andersen K. Insemination with frozen dog semen based on a new insemination technique. Zuchthygiene 1975; 10: 1–4 [DOI] [PubMed] [Google Scholar]
- 33.Howard JG, Bush M, Wildt DE. Semen collection, analysis and cryopreservation in nondomestic mammals. In: Morrow DA (ed.), Current Therapy in Theriogenology. Philadelphia: WB Saunders; 1986: 1047–1053.
- 34.Sojka NJ, Jennings LL, Hamner CE. Artificial insemination in the cat (Felis catus). Lab Anim Care 1970; 20: 198–204 [PubMed] [Google Scholar]
- 35.Zhao W, Zhao Q, Liu J, Xu XY, Sun WW, Zhou X, Liu S, Wang TH. Electro-acupuncture reduces neuronal apoptosis linked to Bax and Bcl-2 expression in the spinal cords of cats subjected to partial dorsal root ganglionectomy. Neurochem Res 2008; 33: 2214–2221 [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki J, Hasebe N, Nagafuchi S, Baba K, Tsujimoto H, Kano R, Hasegawa A. Expression of apoptosis-related gene mRNAs in feline T-cells infected with feline immunodeficiency virus (FIV). Vet Microbiol 2004; 101: 1–8 [DOI] [PubMed] [Google Scholar]
- 37.Burdon RH. Heat shock and the heat shock proteins. Biochem J 1986; 240: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett 2007; 581: 3702–3710 [DOI] [PubMed] [Google Scholar]
- 39.Lovell R, Madden L, Carroll S, McNaughton L. The time-profile of the PBMC HSP70 response to in vitro heat shock appears temperature-dependent. Amino Acids 2007; 33: 137–144 [DOI] [PubMed] [Google Scholar]
- 40.Pedersen CB, Gregersen N. Stress response profiles in human fibroblasts exposed to heat shock or oxidative stress. Methods Mol Biol 2010; 648: 161–173 [DOI] [PubMed] [Google Scholar]
- 41.Edwards JL, Ealy AD, Monterroso VH, Hansen PJ. Ontogeny of temperature-regulated heat shock protein 70 synthesis in preimplantation bovine embryos. Mol Reprod Dev 1997; 48: 25–33 [DOI] [PubMed] [Google Scholar]
- 42.Oehler R, Pusch E, Zellner M, Dungel P, Hergovics N, Homoncik M, Eliasen MM, Brabec M, Roth E. Cell type-specific variations in the induction of hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperones 2001; 6: 306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill JA. Maternal-embryonic cross-talk. Ann NY Acad Sci 2001; 943: 17–25 [DOI] [PubMed] [Google Scholar]
- 44.Stokes PJ, Abeydeera LR, Leese HJ. Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Dev Biol 2005; 284: 62–71 [DOI] [PubMed] [Google Scholar]
- 45.Viganò P, Mangioni S, Pompei F, Chiodo I. Maternal-conceptus cross talk– a review. Placenta 2003; 24 (Suppl B): S56–S61 [DOI] [PubMed] [Google Scholar]
- 46.Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod 1997; 56: 1088–1096 [DOI] [PubMed] [Google Scholar]
- 47.Beere HM. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 2004; 117: 2641–2651 [DOI] [PubMed] [Google Scholar]
- 48.Fujitani Y, Kasai K, Ohtani S, Nishimura K, Yamada M, Utsumi K. Effect of oxygen concentration and free radicals on in vitro development of in vitro-produced bovine embryos. J Anim Sci 1997; 75: 483–489 [DOI] [PubMed] [Google Scholar]
- 49.Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod 1993; 48: 377–385 [DOI] [PubMed] [Google Scholar]
- 50.Swain JE. Optimizing the culture environment in the IVF laboratory: impact of pH and buffer capacity on gamete and embryo quality. Reprod Biomed Online 2010; 21: 6–16 [DOI] [PubMed] [Google Scholar]
- 51.Li Q, Zhang M, Chen YJ, Wang YJ, Huang F, Liu J. Oxidative damage and HSP70 expression in masseter muscle induced by psychological stress in rats. Physiol Behav 2011; 104: 365–372 [DOI] [PubMed] [Google Scholar]
- 52.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3: 28 [DOI] [PMC free article] [PubMed] [Google Scholar]