Abstract
To identify embryos individually during in vitro development, we previously developed the well-of-the-well (WOW) dish, which contains 25 microwells. Here we investigated the effect of embryo density (the number of embryos per volume of medium) on in vitro development and gene expression of bovine in vitro-fertilized embryos cultured in WOW dishes. Using both conventional droplet and WOW culture formats, 5, 15, and 25 bovine embryos were cultured in 125 µl medium for 168 h. The blastocysts at Day 7 were analyzed for number of cells and expression of ten genes (CDX2, IFN-tau, PLAC8, NANOG, OCT4, SOX2, AKR1B1, ATP5A1, GLUT1 and IGF2R). In droplet culture, the rates of formation of >4-cell cleavage embryos and blastocysts were significantly lower in embryos cultured at 5 embryos per droplet than in those cultured at 15 or 25 embryos per droplet, but not in WOW culture. In both droplet and WOW culture, developmental kinetics and blastocyst cell numbers did not differ among any groups. IFN-tau expression in embryos cultured at 25 embryos per droplet was significantly higher than in those cultured at 15 embryos per droplet and in artificial insemination (AI)-derived blastocysts. Moreover, IGF2R expression was significantly lower in the 25-embryo group than in the 5-embryo group and in AI-derived blastocysts. In WOW culture, these expressions were not affected by embryo density and were similar to those in AI-derived blastocysts. These results suggest that, as compared with conventional droplet culture, in vitro development and expression of IFN-tau and IGF2R in the microwell system may be insensitive to embryo density.
Keywords: Bovine embryo, Developmental kinetics, Embryo density, Gene expression, Individual culture
Bovine in vitro-fertilized (IVF) embryos play a central role in modern dairy and beef production. The effectiveness of embryo production, genetic selection and crossbreeding schemes can be improved by using IVF embryos. In addition, emerging technologies offer the promise of improved embryo production and pregnancy rates in cattle with ovaries that are unresponsive to superovulation or show low fertility [1]. The number of bovine IVF embryos transferred to recipients has been increasing because of the worldwide diffusion of ovum pick-up (OPU) technology, which allows the collection of oocytes from specific donors that have high economic merit [2]. There are, however, limited numbers of oocytes that are actually collected in the commercial OPU setting [3]. Previous studies in Japanese Black and Holstein breeds showed that the practical numbers of oocytes per OPU section were 5 to 25 and 7 to 11, respectively [4, 5]. Furthermore, the embryos produced from an individual donor may vary in number and quality [6]. Hence, an optimized culture system that allows for practical culture of individual embryos and of various numbers of embryos is greatly needed for stable and efficient production of viable embryos.
We recently developed a polystyrene-based well-of-the-well (WOW) dish with 25 microwells that allows culture of up to 25 embryos under a single drop of medium and moreover allows tracking of individual embryos by time-lapse cinematography (TLC) [7]. Using this system, it is possible to track developmental progress and select healthy embryos using viability biomarkers [8]. No negative side effects were observed among WOW-derived embryos with respect to in vitro development, cell number or oxygen consumption [7]. Moreover, blastocysts derived from WOW culture had a lower incidence of apoptosis and produced a higher pregnancy rate than did those from conventional group culture [7]. The single-droplet design of the WOW microwell system still allows accumulation of suitable concentrations of autocrine factors such as insulin-like growth factor, tumor necrosis factor, platelet-activating factors, epidermal growth factors, fibroblast growth factors and platelet-derived growth factors, as well as diffusion of toxic substances (such as ammonium and oxygen-derived free radicals). Such diffusion of both positive and negative factors benefits the development of individual embryos [9, 10].
In conventional group culture, the effects of positive-acting autocrine/paracrine factors and negative-acting toxic by-products of embryo metabolism depend on droplet size [11], surface-to-volume ratio [11], distance between cultured embryos [12], quality of neighboring embryos [13] and embryo density (the number of embryos relative to medium volume) [14,15,16]. Embryo density has been considered a particularly critical factor, with some studies indicating that embryo density affects embryo development, the number of cells in the inner cell mass (ICM) and trophectoderm (TE) [17], interferon-tau secretion [18] and gene expression [15]. Such effects are generally attributed to autocrine/paracrine secretions from neighboring embryos [15, 17, 18]. In contrast, there is limited information about microwell cultures in general, or about the WOW culture system in particular, regarding the effect of embryo density on in vitro development or parameters of embryo quality such as cell number and gene expression. The objective of the present study was therefore to examine the effect of embryo density on in vitro development (including cleavage rates and blastocyst formation), developmental kinetics (analyzed by TLC), ICM and TE cell numbers and relative transcription of developmentally important genes involved in implantation/pregnancy (AKR1B1, aldo-keto reductase family 1 member B1; CDX2, caudal type homeobox 2; IFN-tau, interferon-tau; IGF2R, insulin-like growth factor 2 receptor; and PLAC8, placenta-specific 8), pluripotency (NANOG, nanog homeobox; OCT4, octamer-binding transcription factor 4; and SOX2, SRY [sex-determining region Y]-box containing gene 2) and metabolism (ATP5A1, ATP synthase, H+-transporting, mitochondrial F1 complex, alpha subunit 1, cardiac muscle, and GLUT1, glucose transporter 1) in bovine IVF embryos in WOW culture or conventional droplet culture using 125 µl medium.
Materials and Methods
Animal care and use
This study was approved by the Ethics Committee for the Care and Use of Experimental Animals at the National Livestock Breeding Center located in Nishigo, Japan. All animals received humane care according to law no. 105 and notification nos. 6 and 22 of the Japanese Guidelines for Animal Care and Use.
Design and fabrication of the WOW dishes
The WOW dishes were designed and fabricated as described previously [7, 19]. Each dish has 25 microwells surrounded by a circular wall in the center of a 35-mm culture dish. Each well is 287 µm in diameter and 168 µm deep, and the wells are arranged in a 5 × 5 grid. The wells are 150 µm apart between adjacent edges. The bottom of each well slopes downward at a 7.1° angle toward the center of the well. The circular wall is 7 mm in diameter and 1.5 mm high, and is used to form a single microdroplet of culture medium. The WOW dish was fabricated by the conventional injection molding method. A metal mold was fabricated by machining, and the WOW dishes were then produced by an injection molding machine using the mold. Polystyrene was chosen as the material for WOW dishes because of its non-toxicity in cell culture.
Preparation of WOW dishes
Preparation of WOW dishes was performed as described previously [7]. Briefly, each dish was rinsed with ethanol and then sterilized for 30 min using 2 ml ethanol (Wako, Osaka, Japan, 057-00456). The ethanol was then removed, and the dish was air-dried on a warm plate and sterilized under ultraviolet light for 10 min. After sterilization, the interior of the circular wall was filled with 125 µl Charles Rosenkrans 1 medium with amino acids (CR1aa) [20] supplemented with 5% newborn calf serum (CS; Gibco, Grand Island, NY, USA, 16010-159). This was covered with paraffin oil, and air bubbles inside the microwells were flushed out by tapping the exterior of the dish with an awl. The WOW dish was incubated at 38.5 C in 5% O2, 5% CO2 and 90% N2 with saturated humidity for at least 3 h before use.
Oocyte collection and in vitro maturation
Collection of bovine cumulus-oocyte complexes (COCs) was performed as described previously [21]. Ovaries from Japanese Black or Holstein breeds were collected at a local slaughterhouse, transported to the laboratory, washed and stored in physiological saline supplemented with 50 µg/ml gentamicin (Sigma Chemical, St. Louis, MO, USA, G1397) at 20 C for ~20 h. COCs were aspirated from small follicles (2–6 mm in diameter) using a 5-ml syringe equipped with a 19-gauge needle. The in vitro maturation (IVM) medium was 25 mM HEPES-buffered TCM199 (M199; Gibco, 12340-030) supplemented with 5% CS. COCs were washed twice with IVM medium and incubated in 600 µl IVM medium (60–80 COCs/droplet) covered with paraffin oil (Paraffin Liquid; Nacalai Tesque, Kyoto, Japan, 26114-75) in 35-mm Petri dishes (Nunclon Multidishes; Nalge Nunc International, Roskilde, Denmark) for 22 h at 38.5 C in a humidified atmosphere of 5% CO2 in air.
In vitro fertilization
In vitro fertilization (IVF) was carried out as described previously [21]. Briefly, at the end of IVM, ejaculated sperm samples from a Japanese Black bull that were frozen in 0.5-ml straws were thawed in a 37 C water bath for 30 sec and then centrifuged in 3 ml 90% Percoll solution (GE Healthcare, Uppsala, Sweden, 17-0891-01) at 750 × g for 10 min. The pellets were resuspended in 6 ml sperm-washing medium (Brackett and Oliphant [BO] solution [22] supplemented with 10 mM hypotaurine [Sigma, H1384]) and 4 U/ml heparin [Novo-Heparin Injection 1000; Aventis Pharma, Tokyo, Japan, B068]) and centrifuged at 550 × g for 5 min. The resulting pellets were resuspended in sperm-washing medium and BO solution supplemented with 20 mg/ml BSA (crystallized and lyophilized; Sigma, A7030) to achieve a final concentration of 3 × 106 spermatozoa/ml. Fertilization droplets of this suspension (100 μl each) were placed in 35-mm dishes and covered with paraffin oil. COCs were removed from the maturation medium, washed twice in BO supplemented with 10 mg/ml BSA and added to the fertilization droplets at 20 COCs/droplet. Fertilization droplets were then cultured for 6 h at 38.5 C in 5% CO2/air with saturated humidity.
In vitro culture
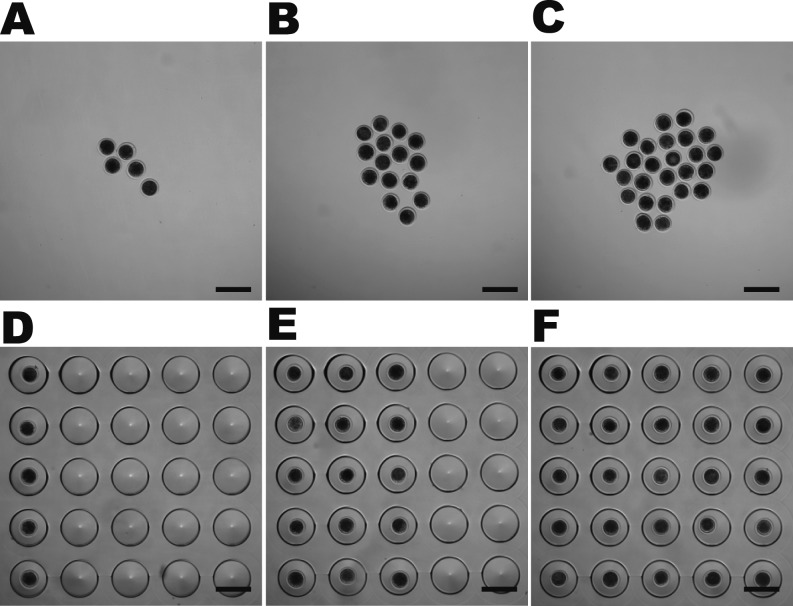
After insemination, putative zygotes were completely denuded from cumulus cells and spermatozoa by gentle pipetting with a fine glass pipet in in vitro culture (IVC) medium (CR1aa medium supplemented with 5% CS). Zygotes with two obvious polar bodies were placed in 125-ml droplets of IVC medium in microwells of WOW dishes (one zygote per microwell; 5, 15 or 25 per dish) or in conventional 125-ml droplets in 35-mm dishes (one droplet per dish; 5, 15 or 25 zygotes per droplet) as shown in Fig. 1. Droplets were covered with paraffin oil, and IVC was performed for 168 h at 38.5 C in 5% O2, 5% CO2 and 90% N2 with saturated humidity.
Fig. 1.
Conventional droplet and WOW culture systems and embryo densities for IVC of bovine embryos. Groups of 5 (A, D), 15 (B, E) and 25 (C, F) IVF embryos were cultured for 168 h in a droplet (A−C) or WOW dish (D−F), each of which contained 125 μl medium. Bar = 300 μm.
Time-lapse cinematography
Analysis of developmental kinetics using time-lapse cinematography (TLC) was performed as described previously [8]. In vitro development was monitored with a Real-Time Cultured Cell Monitoring System with Multiple-Point Imaging Capture (CCM-MULTI; Astec, Fukuoka, Japan). During the 168-h culture period, 673 photographs of embryos were taken at 15-min intervals using a plan objective with 4× magnification. Image stacks were analyzed using the CCM version 2.1.0.4 software (Astec). The time of the first appearance of the following cleavage or embryo stages was recorded for zygotes in focus with identifiable blastomeres: 2-, 3- or 4-, 5- to 8- and 9- to 16-cell stages; morulae; and blastocysts. If proper evaluation of specific cleavage events was not possible, these events were treated as missing data points. The cleavage stages of embryos in which one or more blastomeres stopped further cleavage were categorized according to the number of cell cycles observed in the healthy blastomeres. From the fourth cell cycle onward, individual blastomeres could not be observed directly. Thus, passage from the fourth and fifth cell cycles was defined by combining direct chronological observation of cleaving blastomeres or the initiation of intensive movements within the cell mass created by blastomere cleavage, the cessation of all cleavages and movement followed by a resting period, and the start of a new cleavage round, which creates new movement. The presence of a morula was characterized by the first signs of compaction, but with blastomeres still clearly distinguishable on the surface. The appearance of a blastocyst was characterized by the first appearance of a stable, confluent blastocoel. Finally, blastocysts were characterized as expanded when the diameter of the zona pellucida was increased as a result of expansion of the blastocoel.
Collection of embryos derived by artificial insemination
As physiological control of gene expression analysis, artificial insemination (AI)-derived embryos were collected from superovulated Japanese Black cows as described by Hashiyada et al. [23]. Superovulation was performed by injecting 20 AU of follicle-stimulating hormone (Antrin-R•10; Kyorisu Seiyaku, Kawasaki, Japan, 401101) and 2 ml prostaglandin F2α (RESIPRON-C; ASKA Pharmaceutical, Tokyo, Japan, 3021210A) followed by AI. On day 8 after AI, at which point blastocysts and expanded blastocysts can be observed as the dominant population, embryos were recovered by uterine flushing. Blastocysts and expanded blastocysts were selected and stored in Dulbecco's phosphate-buffered saline (Gibco, 14287) containing 20% CS until subsequent analysis.
Differential staining of ICM and TE cells
The cellular composition of blastocysts was assessed by differential staining of ICM and TE cells as described previously [24]. Briefly, TE cells of blastocysts were stained for 40 s with 100 μg/ml propidium iodide (Sigma, P4170) in a permeabilizing solution of 0.2% (v/v) Triton X-100 (Sigma, T9284). Blastocysts were then counterstained and simultaneously fixed for 5 min in 25 μg/ml Hoechst 33342 (Calbiochem, La Jolla, CA, USA, 382065) in 99.5% ethanol. Whole fixed and stained blastocysts were mounted and assessed for cell number using an epifluorescence microscope (IX-71; Olympus, Tokyo, Japan). ICM and TE nuclei were blue and pinkish red, respectively.
Quantitative real-time reverse-transcription PCR
The following genes were analyzed with real-time reverse-transcription RT-PCR (RT-PCR) as described previously [25]: CDX2, IFN-tau, PLAC8, NANOG, OCT4, SOX2, AKR1B1, ATP5A1, GLUT1 and IGF2R. Histone H2A family, member Z (H2AFZ) was used as the endogenous control gene. Individual blastocysts were lysed in 50 µl extraction buffer (Arcturus, Carlsbad, CA, USA, KIT0204), incubated at 42 C for 30 min and stored at −80 C. Total RNA was extracted from each sample using a PicoPure RNA Isolation Kit (Arcturus, KIT0204). Residual genomic DNA was removed with recombinant RNase-free DNase I (Roche, Mannheim, Germany, 4716728). RNA was eluted from the purification column using 11 µl elution buffer (Arcturus, KIT2024). RNA was reverse-transcribed into cDNA using a ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan, FSQ-101). Each 20-µl reaction product was diluted with nuclease-free water to a final volume of 40 µl. Quantitative real-time RT-PCR was performed using the StepOnePlus System (Applied Biosystems, Foster City, CA, USA) in a 20-µl reaction volume containing 2 µl cDNA, 0.5 µl each of forward and reverse primers (Table 1) [25], 7 µl nuclease-free water and 10 µl Fast SYBR Green PCR Master Mix (Applied Biosystems, 4385612). In each set of PCR reactions, duplicate cDNA samples were run to control for the reproducibility of the real-time RT-PCR results. Universal thermal cycling parameters (initial step 20 sec at 95 C, followed by 45 cycles of 3 sec at 95 C, 10 sec at 60 C and 20 sec at 72 C) were used to quantify the expression of all genes. At the end of real-time PCR, melting curve analysis was carried out on the real-time cycler to check the specificity of the reaction. A standard curve was generated for both the target gene and the endogenous control gene (H2AFZ) in every PCR run using serial 10-fold dilutions of amplified cDNA derived from blastocysts. Final quantitative analysis was performed using the relative standard method, and results were reported as relative expression after normalization to H2AFZ, which was found to be stably expressed regardless of the experimental group.
Table 1. Sequences of primers for real-time RT-PCR.
| Gene name | GenBank accession number | Forward (F) and reverse (R) sequences | Amplicon size (bp) |
| H2AFZ | NM_174809 | F: 5´-ACAGCTGTCCAGTGTTGGTG-3´ | 125 |
| R: 5´-GCAGAAATTTGGTTGGTTGG-3´ | |||
| CDX2 | XM_871005 | F: 5´-GCCACCATGTACGTGAGCTAC-3´ | 140 |
| R: 5´-ACATGGTATCCGCCGTAGTC-3´ | |||
| IFN-tau | X65539 | F: 5´-TGTCTGAGGACCACATGCTAGGT-3´ | 121 |
| R: 5´-ACCATCTCCTGAGGAAGACCAA-3´ | |||
| PLAC8 | NM_016619 | F: 5´-CGGTGTTCCAGAGGTTTTTCC-3´ | 163 |
| R: 5´-AAGATGCCAGTCTGCCAGTCA-3´ | |||
| NANOG | DQ069776 | F: 5´-GTCCCGGTCAAGAAACAAAA-3´ | 107 |
| R: 5´-TGCATTTGCTGGAGACTGAG-3´ | |||
| OCT4 | NM_174580 | F: 5´-CCACCCTGCAGCAAATTAGC-3´ | 68 |
| R: 5´-CCACACTCGGACCACGTCTT-3´ | |||
| SOX2 | NM_001105463 | F: 5´-GGTTGACATCGTTGGTAATTTATAATAGC-3´ | 88 |
| R: 5´-CACAGTAATTTCATGTTGGTTTTTCA-3´ | |||
| AKR1B1 | NM_001012519.1 | F: 5´-CGTGATCCCCAAGTCAGTGA-3´ | 152 |
| R: 5´-AATCCCTGTGGGAGGCACA-3´ | |||
| ATP5A1 | NM_174684 | F: 5´-CTCTTGAGTCGTGGTGTGCG-3´ | 184 |
| R: 5´-CCTGATGTTGGCTGATAACGTG-3´ | |||
| GLUT1 | NM_174602 | F: 5´-CAGGAGATGAAGGAGGAGAGC-3´ | 258 |
| R: 5´-CACAAATAGCGACACGACAGT-3´ | |||
| IGF2R | NM_174352.2 | F: 5´-GCTGCGGTGTGCCAAGTGAAAAAG-3´ | 201 |
| R: 5´-AGCCCCTCTGCCATTGTTACCT-3´ |
Experimental studies
First, the rate of cleavage and development to blastocysts were calculated at 48 and 168 h (hour of IVF start was defined as 0 h) of IVF, respectively. Some of the embryos that developed to the blastocyst stage were collected for analyzing cell numbers or gene expression. TLC was not used in this experiment.
Next, developmental kinetics in embryos developed to the blastocyst stage were analyzed by TLC. Some of the embryos developed into blastocysts were collected for analyzing cell numbers or gene expression.
Finally, to analyze whether the rate of cleavage and blastocyst formation of embryos in WOW dishes is affected by neighbors, 5 embryos were cultured with different distances between embryos (300 µm and 1260 µm), as shown in Supplementary Fig. 1 (online only).
Statistical analysis
All values were compared among embryo density groups in the same culture system. Gene expression data were analyzed nonparametrically by the Kruskal-Wallis test followed by Scheffé's post hoc test. All other data were analyzed using ANOVA followed by Fisher's protected least significant difference test. All percentage data were arcsine transformed. For all data, P<0.05 was considered significant. All analyses were conducted using StatView (SAS Institute, Cary, NC, USA).
Results
Cleavage and blastocyst formation
The rates of cleavage at Day 2 and blastocyst formation at Day 7 were analyzed in embryos in both droplet and WOW cultures (Table 2). In droplet cultures, the rates of formation for >4-cell embryos and blastocysts in the 5-embryo group were lower than those in the 15- and 25-embryo groups (P<0.05). In contrast, no significant differences in the rates of cleavage and blastocyst formation were observed between embryo densities in WOW culture. In the 5-embryo group in WOW culture, there was no significant difference between embryo distances for the rates as shown in Supplementary Table 1 (online only). For expanded blastocyst formation, there was no significant difference among embryo densities for either culture format.
Table 2. Effect of embryo density on in vitro development of bovine embryos in droplet or WOW culture*.
| Culture system | Embryos/dish | Total embryos (10 replicate dishes) |
Number of embryos (%)a | ||||
| Cleavage stage | Blastocyst stage | ||||||
| Total | >4-cell | Total | Expanded | ||||
| Droplet | 5 | 50 | 43 (86.0 ± 4.3) | 28 (56.0 ± 5.8)b | 14 (28.0 ± 7.4)b | 11 (22.0 ± 4.7) | |
| 15 | 150 | 135 (90.0 ± 3.0) | 102 (68.0 ± 3.3)c | 58 (38.7 ± 4.2)c | 33 (22.0 ± 2.8) | ||
| 25 | 250 | 232 (92.8 ± 1.2) | 193 (77.2 ± 2.4)c | 102 (40.8 ± 2.8)c | 60 (24.0 ± 2.3) | ||
| WOW | 5 | 50 | 46 (92.0 ± 4.4) | 35 (70.0 ± 6.8) | 24 (48.0 ± 5.3) | 14 (28.0 ± 3.3) | |
| 15 | 150 | 137 (91.3 ± 3.0) | 95 (63.3 ± 6.4) | 61 (40.1 ± 5.1) | 37 (24.7 ± 4.0) | ||
| 25 | 250 | 233 (93.2 ± 1.7) | 178 (71.2 ± 4.6) | 104 (41.6 ± 2.5) | 62 (24.8 ± 3.4) | ||
* Values were created with embryos from 10 replicate trials. a Cleavage and blastocyst stage embryos were counted at Days 2 and 7 of IVC, respectively. Percent values reflect the mean ± SEM. b,c Values are significantly different among embryo density groups in same culture system (P<0.05).
Developmental kinetics
The appearance times of the 2-cell, 3- to 4-cell, 5- to 8-cell, 9- to 16-cell, morula, blastocyst and expanded blastocyst stages were determined by TLC (Table 3). In both droplet and WOW culture, there was no significant difference between embryo densities for the time at which any of the examined cell stages occurred.
Table 3. Effect of embryo density on developmental kinetics of bovine embryos that formed blastocysts in droplet or WOW culture*.
| Culture system | Embryos/dish | Total embryos examined |
Time of appearance for each cell stagea | ||||||
| 2-cell | 3- to 4-cell | 5- to 8-cell | 9- to 16-cell | Morula | Blastocyst | Expanded blastocyst | |||
| Droplet | 5 | 14 | 25.8 ± 1.5 | 36.3 ± 2.5 | 44.2 ± 2.8 | 74.2 ± 15.1 | 103.9 ± 7.6 | 146.5 ± 12.5 | 158.8 ± 10.9 |
| 15 | 22 | 26.7 ± 2.4 | 36.4 ± 4.2 | 44.3 ± 3.9 | 77.8 ± 19.2 | 112.8 ± 7.1 | 148.4 ± 10.7 | 164.9 ± 4.6 | |
| 25 | 36 | 27.0 ± 4.2 | 36.5 ± 4.0 | 43.0 ± 3.5 | 67.3 ± 20.6 | 110.7 ± 10.7 | 148.8 ± 10.5 | 162.7 ± 10.2 | |
| WOW | 5 | 14 | 25.5 ± 2.4 | 35.2 ± 2.9 | 42.6 ± 2.6 | 70.0 ± 18.5 | 111.8 ± 9.6 | 143.1 ± 10.1 | 161.9 ± 10.3 |
| 15 | 25 | 25.0 ± 2.3 | 34.9 ± 3.3 | 41.8 ± 2.7 | 73.6 ± 19.8 | 111.0 ± 10.8 | 145.8 ± 13.4 | 156.3 ± 10.0 | |
| 25 | 45 | 25.4 ± 4.1 | 34.5 ± 2.9 | 42.6 ± 3.7 | 68.9 ± 20.9 | 108.3 ± 10.6 | 143.4 ± 13.5 | 155.2 ± 17.2 | |
* Values were created with embryos from 5 replicate trials. a Values are in hours and indicate the mean ± SD.
Cell numbers
Numbers of ICM, TE, and total cells and the percentage of ICM cells relative to total cells were examined by differential staining (Table 4). In both droplet and WOW cultures, there was no significant difference between embryo densities in terms of cell numbers or the percentage of ICM cells.
Table 4. Effect of embryo density on the number of cells in blastocysts in droplet or WOW cultures*.
| Culture system | Embryos/dish | Total embryos examined |
Number of cells/blastocyst a | ICM% (ICM/total)a | ||
| Total | ICM | TE | ||||
| Droplet | 5 | 19 | 97.7 ± 53.6 | 32.5 ± 18.9 | 65.3 ± 37.2 | 34.0 ± 9.5 |
| 15 | 25 | 91.3 ± 40.0 | 36.4 ± 15.3 | 54.8 ± 26.9 | 40.8 ± 8.2 | |
| 25 | 33 | 107.1 ± 37.0 | 39.4 ± 17.9 | 67.7 ± 29.2 | 37.5 ± 13.1 | |
| WOW | 5 | 19 | 109.2 ± 33.2 | 36.4 ± 13.3 | 72.8 ± 27.4 | 33.8 ± 9.9 |
| 15 | 30 | 122.1 ± 37.8 | 46.1 ± 18.3 | 75.9 ± 25.3 | 37.5 ± 7.7 | |
| 25 | 43 | 107.1 ± 38.2 | 37.2 ± 20.6 | 70.8 ± 25.7 | 33.1 ± 12.2 | |
* Values were created with embryos from 5 replicate trials. a Values indicate the mean ± SD. ICM, inner cell mass; TE, trophectoderm.
Relative gene expression
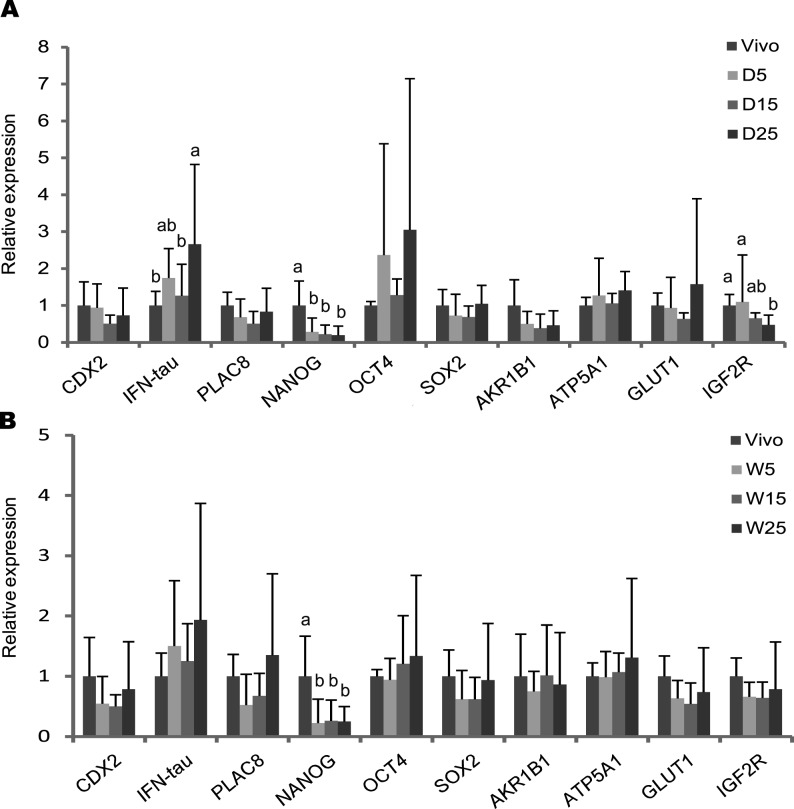
The expression levels of ten genes (CDX2, IFN-tau, PLAC8, NANOG, OCT4, SOX2, AKR1B1, ATP5A1, GLUT1, and IGF2R) in day 7 blastocysts derived from droplet (Fig. 2A) and WOW (Fig. 2B) cultures were examined by real-time RT-PCR. In droplet culture, the relative expression of IFN-tau was significantly higher in the 25-embryo group than in the 15-embryo group and AI-derived blastocysts (Fig. 2A). Moreover, in droplet culture, IGF2R expression was significantly lower in the 25-embryo group than in the 5-embryo group and AI-derived blastocysts (Fig. 2A). On the other hand, in WOW cultures, no significant difference in IFN-tau or IGF2R expression was found between embryo densities (Fig. 2B) and on comparison with AI-derived blastocysts (Fig. 2B). No differences in expression for the other eight genes were found among embryo density in either culture system (Fig. 2A and 2B).
Fig. 2.
Effect of embryo density on gene expression in bovine blastocysts from droplet (D) or WOW cultures (W). Expression of ten genes was measured by real-time RT-PCR in blastocysts derived at densities of 5, 15 or 25 embryos in droplet (A) and WOW (B) cultures and in artificial insemination (AI)-derived embryos. Relative expression was presented as the fold change from the mean value of seven AI-derived blastocysts, which was set at 1. Each value was created with 12 individual blastocysts produced from 5 replicate trials. Eleven genes including H2AFZ were analyzed in a single blastocyst. Values indicate the mean ± SD. a,b Different superscripts are significantly different among embryo density groups at P<0.05.
Discussion
In the present study, we examined the effect of embryo density (the number of embryos per volume of medium) on the rate of in vitro development and gene expression in bovine embryos cultured in conventional droplets or WOW dishes. WOW cultures did not show the same effects from embryo density on the in vitro development rate or gene expression as observed in conventional droplet cultures. These findings indicate that blastocyst formation and transcription may not be affected by embryo density in this microwell culture system in the way they are affected by embryo density in conventional droplet culture.
In conventional group culture within a droplet, paracrine factors released by early embryos promote growth of neighboring embryos [12, 13]. On the other hand, WOW culture could allow autocrine factors to accumulate in the microwell, resulting in improvement of in vitro development by the factors [10]. In the present study, embryos cultured at 5 embryos per group (i.e., 1 per 25 µl) had lower rates of >4-cell stage and blastocyst formation as compared with embryos cultured in groups of 15 (1 per 8.3 µl) and 25 embryos (1 per 5 µl) in droplet culture. This is supported by a previous observation that bovine blastocyst formation is higher at an embryo density of 1 per 10 µl as compared with 1 per 35 µl [15]. Gopichandran and Leese showed that blastocyst formation in group culture declines significantly when the distance between the embryos is increased, reaching zero blastocysts when the distance is >540 µm; this result demonstrates that embryo development is influenced by a decrease in the availability of autocrine/paracrine factors [12]. In WOW cultures, increasing the distance between embryos from 300 µm to 1260 µm (Suppl Fig. 1: on-line only) had no impact on the rate of blastocyst formation (Suppl Table 1: online only). Thus, WOW dishes could promote in vitro development of embryos via autocrine factors from the embryos themselves without the participation of paracine factors from neighbors. This also suggests that WOW culture provides a suitable concentration of autocrine factors surrounding each embryo to support blastocyst formation, while it may be difficult to provide the autocrine factors in conventional droplet culture. To our knowledge, this is the first report to examine the effect of embryo density on developmental kinetics. Previous studies have evaluated how developmental kinetics are affected by other culture conditions. For instance, decreasing the oxygen tension from 20% to 5% improved developmental kinetics [26, 27], and platelet-derived growth factors (PDGFs), which regulate mitogenesis and act in an autocrine/paracrine fashion to stimulate embryonic development in vitro, may stimulate cell proliferation during the fourth cell cycle in bovine [28]. From these observations, we speculated that a difference in embryo density might alter oxygen tension and autocrine/paracrine factors surrounding each embryo and thus might affect developmental kinetics. However, we observed no significant differences in developmental kinetics among embryo densities in either droplet or WOW culture. One possible explanation for this is that potential differences in developmental kinetics were masked because embryos were cultured in both the droplet and WOW format at a hypoxic oxygen tension (5%). In droplet cultures, the rate of >4-cell formation at day 2 was significantly lower at 5 embryos/droplet than at 15 and 25 embryos/droplet, but there was no such difference in WOW cultures, suggesting that inferior >4-cell formation was affected by deficient autocrine/paracrine function only at the lowest density and only in droplet culture. In both droplet and WOW culture, embryo density had no significant effect on cell numbers or the percentage of ICM cells.
Gene expression of bovine blastocysts is sensitive to the culture environment [29,30,31], which includes the embryo density [14, 32]. In present study, the examined metabolism-related genes, ATP5A1 and GLUT1, were unaffected by embryo density in both droplet and WOW culture, suggesting that embryo density had no impact on embryo metabolism such as oxidative phosphorylation and glucose consumption. Also, we observed no difference of pluripotency related genes, NANOG, OCT4 and SOX2. This is complimentary to the present data that showed that there was no difference in the number and percentage of ICM cells between embryo densities. However, expression of NANOG was downregulated in in vitro-cultured blastocysts compared with in vivo-derived blastocysts in both droplet and WOW culture. This suggests a problem of in vitro culture that cannot overcome by WOW system and thar NANOG is a potent marker for optimizing in vitro culture systems.
On the other hand, in droplet culture, gene expression levels of both IFN-tau and IGF2R depended on embryo density. Upregulated IFN-tau expression and downregulated IGF2R expression were observed in embryos cultured at 25 embryos per group. In contrast, no difference was observed in WOW culture. One speculation for the effect of embryo density on IFN-tau and IGF2R may be the paracrine actions of neighboring embryos rather that the autocrine actions of the embryos themselves. Alternatively, aberrant expression of IFN-tau and IGF2R may have resulted from increased by-products of toxic metabolites. Increased embryo density appears to enhance the accumulation of some toxic by-products of embryo metabolism, such as ammonium [33]. Ammonium induces aberrant expression of the imprinting gene H19 in mice blastocysts, but not affects the rate of blastocyst formation [33]. In contrast, the fact that the WOW dish did not allow participation of neighboring embryos may explain why aberrant gene expression did not occur even at the highest embryo density in droplet culture. Fujita et al. reported that an embryo density of one per 5 µl is most beneficial [14]. However, this density may be harmful to embryos in conventional droplet culture, as such a high density may lead to aberrant gene expression. This is supported by another study in which an embryo density of one per 5 µl had a negative effect on gene expression in bovine embryos [4]. Upregulated IFN-tau, which is key for pregnancy reorganization, could be an indicator of poor embryo quality; the expression of IFN-tau is significantly higher in in vitro-derived blastocysts than in in vivo-derived blastocysts [34]. Epigenetic changes such as downregulation of IGF2R are associated with fetal overgrowth in sheep embryos [35]. Therefore, when using higher embryo densities, use of the WOW dish could prevent epigenetic aberrations that may affect subsequent development after embryo transfer or even neonatal growth. In fact, blastocysts derived from a WOW dish had higher viability after transfer compared with droplet culture when embryos were cultured at 25 embryos per dish [7].
In conclusion, the WOW dish may be useful for improving embryo homogeneity by allowing comparable rates of blastocyst formation and gene expression regardless of embryo density. This microwell system could prove to be a suitable culture environment for 5 to 25 embryos without the negative side effects on in vitro development and expression of IFN-tau and IGF2R that are observed in conventional droplet culture at low and high embryo densities.
Supplementary Material
Acknowledgment
We thank M Oguro and S Oishi for technical assistance and N Hitomi for transportation of ovaries. This work was supported by the Research and Developmental Program for New Bio-industry Initiatives.
References
- 1.Hansen PJ. Realizing the promise of IVF in cattle––an overview. Theriogenology 2006; 65: 119–125 [DOI] [PubMed] [Google Scholar]
- 2.van Wagtendonk-de Leeuw AM. Ovum pick up and in vitro production in the bovine after use in several generations: a 2005 status. Theriogenology 2006; 65: 914–925 [DOI] [PubMed] [Google Scholar]
- 3.Merton JS, Ask B, Onkundi DC, Mullaart E, Colenbrander B, Nielen M. Genetic parameters for oocyte number and embryo production within a bovine ovum pick-up-in vitro production embryo-production program. Theriogenology 2009; 72: 885–893 [DOI] [PubMed] [Google Scholar]
- 4.Blondin P, Bousquet D, Twagiramungu H, Barnes F, Sirard MA. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol Reprod 2002; 66: 38–43 [DOI] [PubMed] [Google Scholar]
- 5.Hirata T, Sato M, Sasaki S, Sasaki O, Osawa T. Effect of suckling on embryo production by repeated ovum pick-up before and after timed artificial insemination in early postpartum Japanese black cows. J Reprod Dev 2008; 54: 346–351 [DOI] [PubMed] [Google Scholar]
- 6.Machado SA, Reichenbach HD, Weppert M, Wolf E, Gonçalves PB. The variability of ovum pick-up response and in vitro embryo production from monozygotic twin cows. Theriogenology 2006; 65: 573–583 [DOI] [PubMed] [Google Scholar]
- 7.Sugimura S, Akai T, Somfai T, Hirayama M, Aikawa Y, Ohtake M, Hattori H, Kobayashi S, Hashiyada Y, Konishi K, Imai K. Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biol Reprod 2010; 83: 970–978 [DOI] [PubMed] [Google Scholar]
- 8.Sugimura S, Akai T, Hashiyada Y, Somfai T, Inaba Y, Hirayama M, Yamanouchi T, Matsuda H, Kobayashi S, Aikawa Y, Ohtake M, Kobayashi E, Konishi K, Imai K. Promising system selecting healthy in vitro-fertilized embryos in cattle. PLoS ONE 2012; 7: e36627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoelker M, Rings F, Lund Q, Phatsara C, Schellander K, Tesfaye D. Effect of embryo density on in vitro developmental characteristics of bovine preimplantative embryos with respect to micro and macroenvironments. Reprod Domest Anim 2010; 45: e138–145 [DOI] [PubMed] [Google Scholar]
- 10.Vajta G, Peura TT, Holm P, Paldi A, Greve T, Trounson AO, Callesen H. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev 2000; 55: 256–264 [DOI] [PubMed] [Google Scholar]
- 11.Ferry L, Mermillod P, Massip A, Dessy F. Bovine embryos cultured in serum-poor oviduct-conditioned medium need cooperation to reach the blastocyst stage. Theriogenology 1994; 42: 445–453 [DOI] [PubMed] [Google Scholar]
- 12.Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006; 131: 269–277 [DOI] [PubMed] [Google Scholar]
- 13.Stokes PJ, Abeydeera LR, Leese HJ. Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Dev Biol 2005; 284: 62–71 [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Umeki H, Shimura H, Kugumiya K, Shiga K.Effect of group culture and embryo-culture conditioned medium on development of bovine embryos. J Reprod Dev 2006; 52: 137–142 [DOI] [PubMed] [Google Scholar]
- 15.Hoelker M, Rings F, Lund Q, Ghanem N, Phatsara C, Griese J, Schellander K, Tesfaye D. Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction 2009; 137: 415–425 [DOI] [PubMed] [Google Scholar]
- 16.Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 2000; 54: 741–756 [DOI] [PubMed] [Google Scholar]
- 17.Vutyavanich T, Saeng-Anan U, Sirisukkasem S, Piromlertamorn W. Effect of embryodensity and microdrop volume on the blastocyst development of mouse two-cell embryos. Fertil Steril 2011; 95: 1435–1439 [DOI] [PubMed] [Google Scholar]
- 18.Larson MA, Kubisch HM. The effects of group size on development and interferon-tau secretion by in-vitro fertilized and cultured bovine blastocysts. Hum Reprod 1999; 14: 2075–2079 [DOI] [PubMed] [Google Scholar]
- 19.Rosat DV, Rosat DV, Rosat MG. Injection Molding Handbook, 2nd ed. Berlin: Springer-Verlag; 2000 [Google Scholar]
- 20.Rosenkrans CF, Jr, Zeng GQ, GT MC, Schoff PK, First NL. Development of bovine embryos in vitro as affected by energy substrates. Biol Reprod 1993; 49: 459–462 [DOI] [PubMed] [Google Scholar]
- 21.Imai K, Tagawa M, Yoshioka H, Matoba S, Narita M, Inaba Y, Aikawa Y, Ohtake M, Koyayashi S. The efficiency of embryo production by ovum pick-up and in vitro fertilization in cattle. J Reprod Dev 2006; 52(Suppl): 19–29 [Google Scholar]
- 22.Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod 1975; 12: 260–274 [DOI] [PubMed] [Google Scholar]
- 23.Hashiyada Y, Okada M, Imai K. Transition of the pregnancy rate of bisected bovine embryos after co-transfer with trophoblastic vesicles prepared from in vivo-cultured in vitro-fertilized embryos. J Reprod Dev 2005; 51: 749–756 [DOI] [PubMed] [Google Scholar]
- 24.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online 2001; 3: 25–29 [DOI] [PubMed] [Google Scholar]
- 25.Sugimura S, Kobayashi S, Hashiyada Y, Ohtake M, Kaneda M, Yamanouchi T, Matsuda H, Aikawa Y, Watanabe S, Nagai T, Kobayashi E, Konishi K, Imai K. Follicular growth-stimulated cows provide favorable oocytes for producing cloned embryos. Cell Reprogram 2012; 14: 29–37 [DOI] [PubMed] [Google Scholar]
- 26.Lequarre AS, Marchandise J, Moreau B, Massip A, Donnay I. Cell cycle duration at the time of maternal zygotic transition for in vitro produced bovine embryos: effect of oxygen tension and transcription inhibition. Biol Reprod 2003; 69: 1707–1713 [DOI] [PubMed] [Google Scholar]
- 27.Wale PL, Gardner DK. Time-lapse analysis of mouse embryo development in oxygen gradients. Reprod Biomed Online 2010; 21: 402–410 [DOI] [PubMed] [Google Scholar]
- 28.Larson RC, Ignotz GG, Currie WB. Platelet derived growth factor (PDGF) stimulates development of bovine embryo during fourth cell cyce. Development 1992; 115: 821–826 [DOI] [PubMed] [Google Scholar]
- 29.Corcoran D, Fair T, Park S, Rizos D, Patel OV, Smith GW, Coussens PM, Ireland JJ, Boland MP, Evans AC, Lonergan P. Suppressed expression of genes involved in transcription and translation in in vitro compared with in vivo cultured bovine embryos. Reproduction 2006; 131: 651–660 [DOI] [PubMed] [Google Scholar]
- 30.Rizos D, Lonergan P, Boland MP, Arroyo-Garcia R, Pintado B, de la Fuente J, Gutierrez-Adan A. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol Reprod 2002; 66: 589–595 [DOI] [PubMed] [Google Scholar]
- 31.Wrenzycki C, Herrmann D, Keskintepe L, Martins A, Jr, Sirisathien S, Brackett B, Niemann H. Effects of culture system and protein supplementation on mRNA expression in pre-implantation bovine embryos. Hum Reprod 2001; 16: 893–901 [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira AT, Lopes RF, Rodrigues JL. Gene expression and developmental competence of bovine embryos produced in vitro under varying embryo density conditions. Theriogenology 2005; 64: 1559–1572 [DOI] [PubMed] [Google Scholar]
- 33.Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod 2003; 69: 1109–1117 [DOI] [PubMed] [Google Scholar]
- 34.Lonergan P, Rizos D, Gutierrez-Adán A, Moreira PM, Pintado B, de la Fuente J, Boland MP. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 2003; 69: 1424–1431 [DOI] [PubMed] [Google Scholar]
- 35.Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet 2001; 27: 153–154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.