Abstract
Pollutants from burning of diesel fuel are hazardous to human health. Nanoparticles in diesel exhaust potentially have profound impact on fetal development and maternal endocrine function during pregnancy due to their ability to penetrate deeply into the body. To investigate the effects of nanoparticle-rich diesel exhaust (NR-DE) on pregnancy, pregnant rats were exposed to NR-DE, filtered diesel exhaust (F-DE) or clean air for 19 days of gestation. Relative weights of maternal liver and spleen to body weight were significantly lower in the NR-DE and F-DE groups than those in the control group. The serum concentration of maternal progesterone was significantly lower, while those of luteinizing hormone (LH) and corticosterone were significantly higher in the NR-DE and F-DE groups than those in the control group. The serum concentration of estradiol-17β was significantly higher in the F-DE group than that in the control group. The levels of cytochrome P450 side-chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase and LH receptor mRNA in the corpus luteum were significantly lower in the NR-DE and F-DE groups than those in the control. In fetuses, body weight and crown-rump length were significantly greater and shorter, respectively, in both males and females in the NR-DE and F-DE groups than those in the control group. These results demonstrate that exposure of pregnant rats to NR-DE and F-DE suppresses the function of corpora lutea and stimulates the function of the adrenal cortex, suggesting a risk of spontaneous abortion associated with maternal hormonal changes.
Keywords: Diesel exhaust, Hormonal regulation, Luteal function, Nanoparticle, Pregnancy, Rat
Diesel exhaust particles (DEP) emitted from diesel engines are the major component of ambient particulate matter (PM), which contains small particles with diameters ranging from nanometers [nanoparticles (NPs)] to micrometers (course particles) [1, 2]. People living near roadsides and underground mines are commonly exposed to diesel-derived NPs, and may be impacted by their toxicity [3]. Hazardous effects of NPs have been observed in laboratory animals [4, 5]. NPs are thought to be able to penetrate deeply into the respiratory tract because of their relative large surface area [6]. They can cross the pulmonary epithelium and reach the interstitium, causing widespread damage in the cardiopulmonary system [7]. Furthermore, NPs can enter the blood stream and be transported to liver, kidney, spleen, brain and heart [5, 8, 9]. Recently, the potential reproductive toxicity of NPs has been recognized. Our previous studies have shown that nanoparticle-rich diesel exhaust (NR-DE) increases testosterone levels in the serum and testis of adult male rats [10, 11] and mice [12] and disrupts adrenocortical function in adult male mice [13]. We have also shown that prenatal exposure to NR-DE leads to endocrine disruption in offspring and suppresses testicular function in immature male rats [14].
During pregnancy, the corpus luteum is the major source of progesterone in the rat. Other sources of progesterone include the ovary, adrenal gland and placenta [15]. In the corpus luteum, both the small and large luteal cells possess steroidogenic capability, with the large luteal cells being the major steroid producer [16]. During luteal steroidogenesis, cholesterol precursor is taken up by cells from circulating cholesterol-rich lipoproteins and transported into mitochondria by steroidogenic acute regulatory protein (StAR), which controls the translocation from the outer to inner mitochondrial membrane. Cholesterol is converted into pregnenolone by cytochrome P450 side-chain cleavage enzyme (P450scc) and then further transformed into progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD) [17].
Emerging evidence has suggested potential adverse effects of NPs on fetal and child development [18]. However, the underlying mechanism remains to be delineated. The aim of the present study was to address these questions using established NR-DE and filtered diesel exhaust (F-DE) exposure systems in a rat model.
Materials and Methods
Animals
Pregnant Fischer rats (F344/DuCrlCrli; day 0 of pregnancy = day of sperm positivity) were obtained from Charles River Laboratories Japan (Tokyo, Japan) and housed individually in wire-mesh cages in whole-body exposure chambers (2.25 m3; Sibata Science Technology, Tokyo, Japan) in a facility with a 12:12 h light:dark cycle and air flow of 1 m3/min at 23 C and 50% humidity. The rats were provided with a commercial diet (CE-2, Japan Clea, Tokyo, Japan) and water ad libitum. The use of animals in this study was approved by the Animal Care and Use Committee of the Japanese National Institute for Environmental Studies.
Experimental design
Pregnant rats were divided into three groups (n = 6 per group) and exposed to clean air (Group 1; control), NR-DE (Group 2; 148.86 μg/m3, 1.83 × 106 particles/cm3) and F-DE (Group 3; 3.10 μg/m3, 2.66 particles/cm3) for 5 h daily from day 1 to 19 of gestation. Body weight was measured on days 8, 15 and 20. On day 20, the rats were euthanized under pentobarbital anesthesia. Blood samples were collected and centrifuged at 1700 × g for 15 min at 4 C. Serum was collected for assessing the levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), testosterone, progesterone, estradiol-17β, corticosterone and immunoreactive (ir)-inhibin. Maternal organs (liver, spleen, kidneys, adrenals, uterus and ovaries) were dissected and immediately weighed. Corpora lutea were dissected from the ovaries under a stereoscopic microscope using forceps and scissors. Total RNA was isolated from the corpora lutea of ovaries of 3 rats. Other ovaries were fixed in 10% neutral phosphate-buffered formalin (pH 7.4; Wako Pure Chemical Industries, Osaka, Japan) for histology.
Nanoparticle-rich diesel exhaust generation
NR-DE was generated using an 8-Litter diesel engine (J08C, Hino Motors, Hino, Japan) as described previously [10]. Briefly, the engine was operated under a steady-state condition for 5 h/d using low-sulfur diesel fuel (JIS No. 2 light oil) at a speed of 2000 rpm. The engine torque was set to be 0 Newton meter (Nm), which readily generates high concentrations of nanoparticles [19]. The exhaust was diluted immediately with clean air to prevent particles from coagulating prior to delivery to the exposure chambers. A control chamber (Group 1) received “clean air,” which was filtered through HEPA and charcoal filters. Groups 2 and 3 were exposed to NR-DE and F-DE (3.10 μg/m3, 2.66 particles/cm3), respectively. Gaseous concentrations were monitored using a gas analyzer (Horiba, Kyoto, Japan).
Particle size and concentration were measured using a scanning mobility particle sizer (SMPS 3034, TSI, Shoreview, MN, USA) and a condensation particle counter (CPC 3025A, TSI), respectively [14]. Particles were collected using a Teflon filter (FP-500, Sumitomo Electric, Osaka, Japan) and quartz fiber filter (2500 QAT-UP, Pall, Pine Bush, NY, USA). Particle mass concentration was measured using a Teflon filter. Particle weight was measured using an electrical microbalance (M5P-F, Sartorius, Tokyo, Japan) in an air-conditioned chamber (CHAM-1000, Horiba) with controlled temperature and humidity (25 C, 50%). Analysis of particle composition showed a higher percentage of organic carbon (79–63%) than elemental carbon (21–37%). The average diameter of NPs ranges from 22 to 27 nm [14]; NPs of these diameters are often observed at traffic intersections in urban regions in Japan [20] and in gaseous components during the exposure experiments.
Radioimmunoassay (RIA)
Serum concentrations of LH, FSH and PRL were measured using NIDDK rat RIA kits (Torrance, CA, USA) with anti-rat LH-S-11, anti-rat FSH-S-11 and anti-rat PRL-S-9 sera. The intra- and interassay coefficients of variation were 5.4 and 6.9% for LH, 4.3 and 10.3% for FSH and 3.4 and 5.2% for PRL, respectively. Serum concentration of ir-inhibin was measured as described previously [21]. Iodinated 32-kDa bovine inhibin was prepared using a rabbit antibody against bovine inhibin (TNDH-1). The intra- and interassay coefficients of variation were 8.8 and 14.4%, respectively. Serum concentrations of progesterone, estradiol-17β, testosterone and corticosterone were determined using a double-antibody RIA system with 125I-labeled radioligands, as described previously [22, 23]. The antisera against progesterone (GDN 337) [24], estradiol-17β (GDN 244) [25] and testosterone (GDN 250) [26] were kindly provided by Dr GD Niswender, Colorado State University (Fort Collins, CO, USA). The intra- and interassay coefficients of variation were 6.9 and 11.2% for progesterone, 4.8 and 5.8% for estradiol-17β, 6.3 and 7.2% for testosterone and 9.5 and 16.4% for corticosterone, respectively.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from the corpora lutea at day 20 of gestation using an RNeasy Mini kit (Qiagen, Tokyo, Japan). cDNA was prepared using a PrimeScript First-Strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan). Briefly, 1 μg total RNA was mixed with 50 μM oligo-dT primer and 10 μM dNTPs in DNase- and RNase-free water to a final volume of 10 μl. RNA and primer were denatured at 65 C and then cooled immediately on ice. Reverse transcription was performed by using 10 μl of a master mixture comprised of 5× PrimeScript buffer (Takara Bio), RNase inhibitor (40 U/μl), PrimeScript reverse transcriptase (200 U/μl; Takara Bio) and DNase- and RNase-free water. The reaction was carried out at 42 C for 60 min and terminated by incubation at 75 C for 15 min.
Polymerase chain reaction (PCR) primers (Table 1) were designed using the Primer Express 1.0 software (Applied Biosystems, Singapore). Quantitative real-time PCR was performed using a Thermal Cycler Dice Real Time System (TP800, Takara Bio) according to the manufacturer's instructions. The PCR reaction was programmed with an initial 10 sec at 95 C followed by 40 cycles of 95 C for 5 sec and 60 C for 30 sec. Melting curves were optimized to gain desired amplicons and eliminate any primer dimers or products from DNA contamination. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping control for data analysis. Levels of mRNA in the samples transcribed from all genes of interest were normalized to that of GAPDH.
Table 1. Primers used in real-time PCR.
| Gene | Forward primer (5´ to 3´) | Reverse primer (5´ to 3´) |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| StAR | CTGCAGCAAGCACTGTGTGG | GGGATAACAGCTCAGACGGTAGAGA |
| P450scc | GGAGGAGATCGTGGACCCTGA | TGGAGGCATGTTGAGCATGG |
| 3β-HSD | AGCAAAAAGATGGCCGAGAA | GGCACAAGTATGCAATGTGCC |
| LHR | CTGCGCTGTCCTGGCC | CGACCTCATTAAGTCCCCTGAA |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; StAR, steroidogenic acute regulatory protein; P450scc, cytochrome P450 side-chain cleavage enzyme; 3β-HSD, 3β-hydroxysteroid dehydrogenase; LHR, luteinizing hormone receptor.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM) and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. Statistical analysis was performed using the GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Differences were considered statistically significant when the P value was less than 0.05.
Results
Maternal organ and body weights
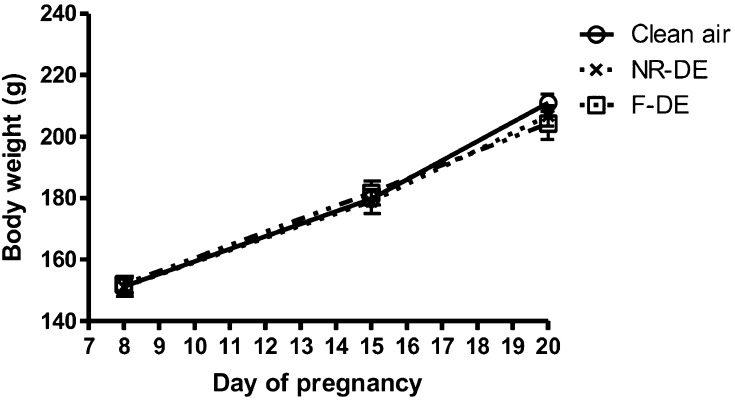
No deaths, miscarriages or other signs of general toxicity were observed in rats exposed to NR-DE or F-DE. Body weights showed no significant difference between the NR-DE or F-DE group and the control group (Fig. 1).
Fig. 1.
Body weight changes of pregnant rats exposed to nanoparticle-rich diesel exhaust (NR-DE), filtered exhaust (F-DE) or clean air from day 1 to day 19 of pregnancy. Each bar represents the mean ± SEM (n=6).
The relative weights of the liver and spleen, measured at day 20, were significantly lower (P<0.001 and P<0.05) in the NR-DE and F-DE groups than those in the control group (Table 2). There were no differences in relative weight for other organs, including the kidney, adrenal gland, uterus and ovary.
Table 2. Body weights and relative organ weights on gestation day 20 in pregnant rats exposed to clean air, NR-DE or F-DE from day 1 to day 19 of pregnancy.
| Exposure groups | |||
| Clean air (Control) |
Nanoparticle-rich diesel exhaust (NR-DE) |
Filtered diesel exhaust (F-DE) |
|
| Number of animals | 6 | 6 | 6 |
| Body weight (g) | 221.0 ± 2.8 | 206.8 ± 3.3 | 204.3 ± 5.1 |
| Weight gain (g) | 59.7 ± 2.6 | 55.7± 2.2 | 52.4 ± 3.2 |
| Liver weight (g) | 8.5 ± 0.1 | 7.3 ± 0.1*** | 7.1 ± 0.2*** |
| Liver/body weight (mg/g) | 40.2 ± 0.5 | 31.5 ± 0.4*** | 34.6 ± 0.2*** |
| Spleen weight (g) | 0.55 ± 0.01 | 0.50 ± 0.02 | 0.48 ± 0.01** |
| Spleen/body weight (mg/g) | 2.60 ± 0.04 | 2.43 ± 0.05* | 2.35 ± 0.02** |
| Kidney weight (g) | 1.21 ± 0.02 | 1.18 ± 0.02 | 1.17 ± 0.03 |
| Kidney/body weight (mg/g) | 5.75 ± 0.12 | 5.72 ± 0.06 | 5.76 ± 0.11 |
| Adrenal weight (mg) | 53.52 ± 0.89 | 50.32 ± 1.12 | 52.27 ± 1.51 |
| Adrenal/body weight (mg/g) | 0.254 ± 0.004 | 0.243 ± 0.004 | 0.256 ± 0.006 |
| Uterus weight (g) | 2.53 ± 0.14 | 2.51 ± 0.10 | 2.48 ± 0.15 |
| Uterus/body weight (mg/g) | 12.1 ± 0.6 | 12.2 ± 0.5 | 12.1 ± 0.6 |
| Ovary weight (g) | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 |
| Ovary/body weight (mg/g) | 0.48 ± 0.06 | 0.54 ± 0.02 | 0.51 ± 0.03 |
*P<0.05, **P<0.01 and ***P<0.001 compared with the value for the control group (Tukey's multiple comparison test). Values are expressed as the mean ± SEM (n = 6).
Serum concentrations of hormones
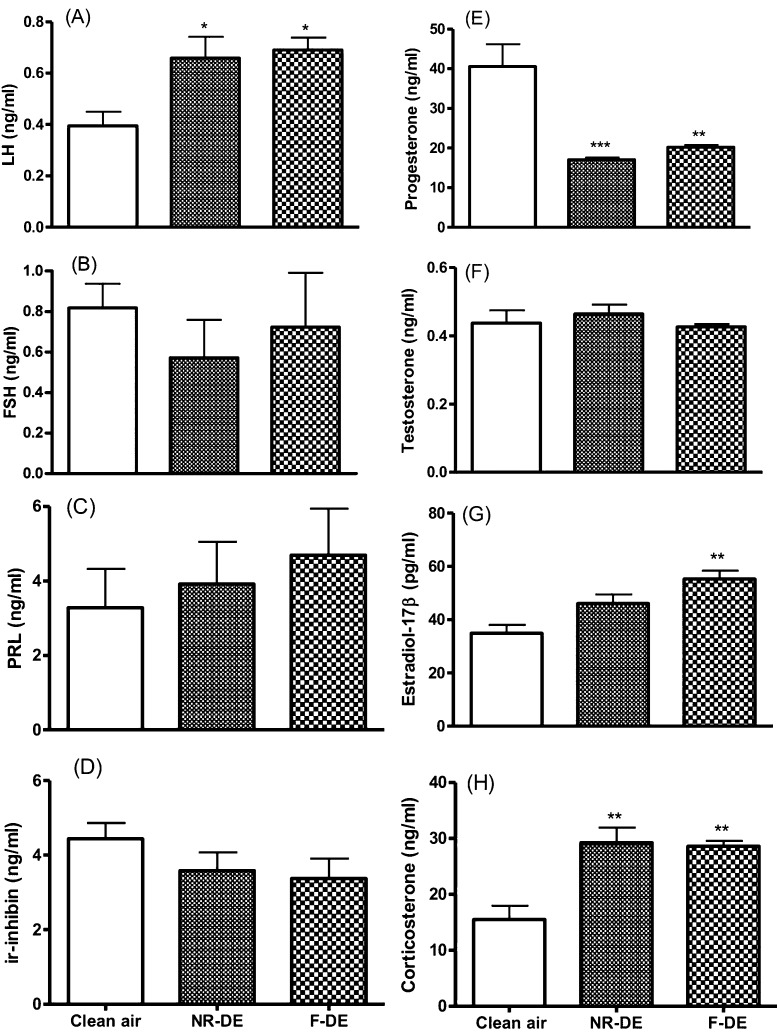
While serum concentrations of FSH (Fig. 2B), PRL (Fig. 2C), ir-inhibin (Fig. 2D), and testosterone (Fig. 2F) in the NR-DE and F-DE groups showed no difference from those in the controls, those of LH (Fig. 2A) and corticosterone (Fig. 2H) were significantly higher (P<0.05; P<0.01), and those of progesterone (Fig. 2E) were significantly lower (P<0.01). The concentration of estradiol-17β was significantly higher (P<0.01) in the F-DE group than in the control group (Fig. 2G).
Fig. 2.
Serum concentrations of LH (A), FSH (B), PRL (C), immunoreactive (ir) inhibin (D), progesterone (E), testosterone (F), estradiol-17β (G) and corticosterone (H) at gestation day 20 in pregnant rats exposed to nanoparticle-rich diesel exhaust (NR-DE), filtered exhaust (F-DE) or clean air from day 1 to day 19 of pregnancy. Each bar represents the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001 compared with the control group (n=6).
mRNA expression of StAR, P450scc, 3β-HSD and LHR in the corpus luteum
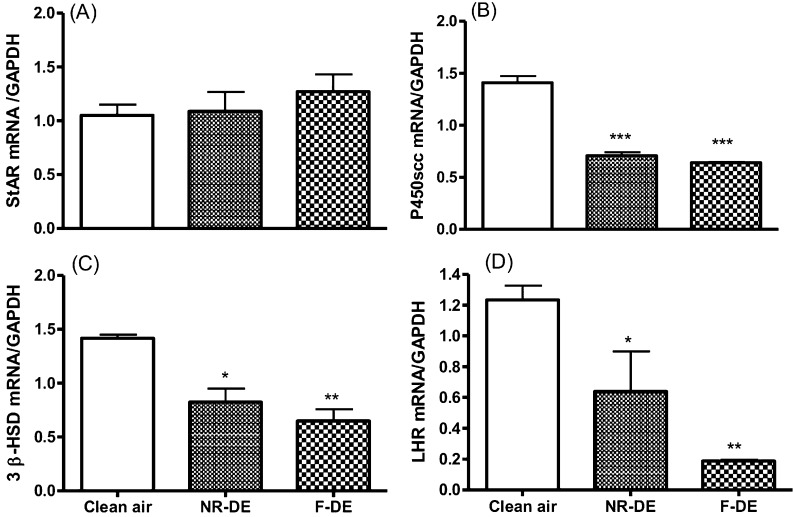
Expression levels of P450scc, 3β-HSD and LHR mRNA were significantly lower in the NR-DE and F-DE groups than those in the control group (Fig. 3B, C and D; P<0.05). The mRNA levels of StAR showed no difference between the control and the exposed groups (Fig. 3A).
Fig. 3.
Expression of StAR (A), P450scc (B), 3β-HSD (C) and LH receptor (LHR; D) mRNA in the corpus luteum at day 20 of gestation in pregnant rats exposed to nanoparticle-rich diesel exhaust (NR-DE), filtered exhaust (F-DE) or clean air from day 1 to day 19 of pregnancy. Each bar represents the mean ± SEM. *P<0.05, **P<0.01 and ***P<0.001 compared with the control group (n=3).
Litter parameters
Fetal body weights were significantly greater in both males and females in the NR-DE and F-DE groups than in the control group (Table 3). Fetal crown-rump lengths were significantly lower in both males and females in the NR-DE and F-DE groups than in the control group (Table 3). There were no significant differences in fetal morphology and sex ratio between control and treated litters (Table 3).
Table 3. Parameters of gestation day 20 fetuses from mother rats exposed to clean air, NR-DE or F-DE from day 1 to day 19 of pregnancy.
| Exposure groups | ||||
| Clean air | Nanoparticle-rich diesel exhaust |
Filtered diesel exhaust |
||
| Control | NR-DE | F-DE | ||
| Number of fetuses | Males | 23 | 30 | 19 |
| Females | 22 | 23 | 28 | |
| Body weight of fetuses (g) | Males | 3.39 ± 0.06 | 3.58 ± 0.05* | 3.72 ± 0.05*** |
| Females | 3.14 ± 0.06 | 3.30 ± 0.03* | 3.52 ± 0.03***# | |
| Crown-rump length (mm) | Males | 37.6 ± 0.3 | 35.9 ± 0.5** | 36.0 ± 0.4* |
| Females | 37.3 ± 0.3 | 34.8 ± 0.3*** | 35.2 ± 0.5** | |
| Anogenital distance (mm) | Males | 5.7 ± 0.2 | 6.0 ± 0.1 | 6.0 ± 0.2 |
| Females | 4.1 ± 0.2 | 4.0 ± 0.1 | 4.5 ± 0.1 | |
| Anogenital index (mm/g1/3) | Males | 3.77 ± 0.21 | 3.81 ± 0.15 | 3.89 ± 0.11 |
| Females | 2.79 ± 0.11 | 2.72 ± 0.08 | 2.98 ± 0.08 | |
*P<0.05, **P<0.01 and ***P<0.001 compared with the value for the control group; #P<0.01 compared with the value for the NR-DE group (Tukey's Multiple Comparison Test). Values are expressed as the mean ± SEM (n = 19 to 30 per group). Anogenital index (mm/g1/3) represents the ratio of the anogenital distance to the cube root of the body weight.
Discussion
The present study clearly demonstrates that exposure to NR-DE or F-DE disrupts hormonal regulation in pregnant rats, as indicated by decreased progesterone and increased LH, estradiol-17β and corticosterone. In pregnant rats, the corpus luteum, but not the placenta, is the major site of progesterone production [15], indicating that it is essential for pregnancy. Similarly, the corpus luteum, but not the placenta, also secretes estradiol-17β, primarily in the middle of gestation [15]. At the end of gestation, the granulosa cells of growing antral follicles produce estradiol-17β for postpartum ovulation [15]. Pituitary prolactin maintains progesterone secretion in the corpus luteum during the first half of pregnancy, whereas placental lactogens maintain it during the second half of gestation [27]. LH is also an important luteotropic hormone in the rat. In addition, at the end of pregnancy, high levels of circulating prolactin cause structural luteolysis in the corpus luteum [28].
In rodents, luteolysis is induced by 20α-hydroxysteroid dehydrogenase (20α-HSD) [15]. This enzyme converts progesterone to 20α-dihydroprogesterone, an inactive metabolite of progesterone. It is well known that prolactin maintains secretion of progesterone in the corpora lutea by inhibiting 20α-HSD in female rats [29]. At the end of pregnancy, activity of 20α-HSD and circulating levels of 20α-dihydroprogesterone increase with a concomitant decrease in progesterone [15]. The decline in circulating progesterone in conjunction with an increase in estradiol-17β induces delivery. The key role of 20α-HSD as a critical enzyme of luteolysis at the end of pregnancy has been clearly demonstrated using a 20α-HSD knockout mouse model, in which the circulating progesterone level remains elevated and parturition is delayed [30].
In the present study, the decrease in mRNA levels of 3β-HSD, P450scc and LH receptor and increase in estradiol-17β mRNA in the corpus luteum suggest an impairment in luteal function. These results strongly suggest that the mode of action of NR-DE or F-DE in the disruption of secretion of progesterone is due to suppression of the activity of steroid enzymes and production of LH receptors in corpora lutea. In addition to these hormone-producing enzymes, an elevation of estrogen was observed in late gestation. Estrogen upregulates expression of oxytocin receptors [31] and relaxin [32] in the uterus, which are required for delivery. The present results suggest that exposure of pregnant animals to NR-DE or F-DE increases the risk of spontaneous abortion.
The higher levels of corticosterone observed in animals exposed to NR-DE and F-DE in this study suggest that androgenization of the sexually dimorphic nucleus of the preoptic area (SDN-POA) may be disrupted in the male fetus. This structure has been shown to be susceptible to stress in pregnancy, resulting in suppression of testosterone secretion from fetal testes [33].
The present study also suggests that maternal liver function is affected by NR-DE and F-DE, as the liver weight was significantly decreased. Future work is warranted to unravel the underlying mechanisms, for example, whether hepatic inflammation and dyslipidemia are involved [34]. The present study also showed a significant decrease in weight of the spleen in the NR-DE- and F-DE-treated rats. However, the circulating level of corticosterone was increased, suggesting that corticosterone influences cytolysis of spleen cells.
It is an important to point out that no difference was observed in the levels of progesterone and corticosterone between the NR-DE and F-DE treatments. These results suggest that NPs exert no significant effect on the function of the corpora luteum and adrenal cortex. Diesel exhaust contains thousands of chemicals, including nitrophenols, nitrogen oxide, dioxin-like compounds and polycyclic aromatic hydrocarbons [35]. In our previous studies, nitrophenols from DEP have been shown to disrupt gonadal and adrenal function in rats and Japanese quails [36,37,38,39,40,41,42,43,44,45,46, 47]. Taken together, it is suggested that toxic chemicals in F-DE may be responsible for dysfunction of the corpora lutea and the adrenal cortex.
In conclusion, the present study shows, for the first time, that inhalation of NR-DE or F-DE disrupts steroid hormone production in the corpus luteum and adrenal cortex in pregnant rats. Such adverse effects may induce abortion and suppress androgenization of the SDN-POA in the brain of male fetuses. This study, therefore, suggests that exposure to NR-DE in the environment may have detrimental effects on pregnancy and fetal growth in wildlife and humans.
Acknowledgment
We are grateful to the National Hormone and Pituitary Program, NIDDK, NIH, Torrance, CA, USA, and Dr AF Parlow for the rat LH, FSH and PRL RIA kits and to Dr GD Niswender, Animal Reproduction and Biotechnology Laboratory, Colorado State University (Fort Collins, CO, USA), for providing antiserum to progesterone (GDN 337), testosterone (GDN 250) and estradiol-17β (GDN 244).
This study was supported in part by Project of the National “Twelfth-Five Year” Research Program of China (2012BAD39B02), Grants-in-Aid for Scientific Research (P07582) from the Japan Society for the Promotion of Science (JSPS), the Ministry of the Environment, Japan (C-0901), and the Fundamental Research Funds for the Central Universities (KYJ200910).
References
- 1.Kittelson DB. Engines and nanoparticles: a rewiew. J Aerosol S 1998; 29: 575–588 [Google Scholar]
- 2.Kittelson DB. On-road particles-characteristics and measurement. In: Proceedings of the International Workshop on Vehicle-related Nonoparticles and Environmental Health Seoul, Korea; 2007: 47–71.
- 3.Biswas P, Wu CY. Nanoparticles and the environment. J Air Waste Manag Assoc 2005; 55: 708–746 [DOI] [PubMed] [Google Scholar]
- 4.Kreyling WG, Semmler M, Möller W. Dosimeter and toxicology of ultrafine particles. J Aerosol Med 2004; 17: 140–152 [DOI] [PubMed] [Google Scholar]
- 5.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles followering whole-body inhalation exposure of rats. J Toxicol Environ Health A 2002; 65: 1531–1543 [DOI] [PubMed] [Google Scholar]
- 6.Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, Yoshikawa T. Effects of nano particles on antigen-related airway inflammation in mice. Respir Res 2005; 6: 106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utell MJ, Frampton MW. Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med 2000; 13: 355–359 [DOI] [PubMed] [Google Scholar]
- 8.Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, Heyder J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect 2001; 109 (Suppl 4): 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Win-Shwe TT, Fujimaki H. Nanoparticles and neurotoxicity. Int J Mol Sci 2011; 12: 6267–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Taneda S, Taya K, Watanabe G, Li X, Fujitani Y, Ito Y, Nakajima T, Suzuki AK. Effects of inhaled nanoparticle-rich diesel exhaust on regulation of testicular function in adult male rats. Inhal Toxicol 2009; 21: 803–811 [DOI] [PubMed] [Google Scholar]
- 11.Ramdhan DH, Ito Y, Yanagiba Y, Yamagishi N, Hayashi Y, Li C, Taneda S, Suzuki AK, Watanabe G, Taya K, Kamijima M, Nakajima T. Nanoparticle-rich diesel exhaust may disrupt testosterone biosynthesis and metabolism via growth hormone. Toxicol Lett 2009; 191: 103–108 [DOI] [PubMed] [Google Scholar]
- 12.Li C, Li X, Jigami J, Hasegawa C, Suzuki AK, Zhang Y, Fujitani Y, Nagaoka K, Watanabe G, Taya K. Effect of nanoparticle-rich diesel exhaust on testosterone biosynthesis in adult male mice. Inhal Toxicol 2012; 24: 599–608 [DOI] [PubMed] [Google Scholar]
- 13.Li C, Li X, Suzuki AK, Fujitani Y, Jigami J, Nagaoka K, Watanabe G, Taya K. Effects of exposure to nanoparticle-rich diesel exhaust on adrenocortical function in adult male mice. Toxicol Lett 2012; 209: 277–281 [DOI] [PubMed] [Google Scholar]
- 14.Li C, Taneda S, Taya K, Watanabe G, Li X, Fujitani Y, Nakajima T, Suzuki AK. Effects of in utero exposure to nanoparticle-rich diesel exhaust on testicular function in immature male rats. Toxicol Lett 2009; 185: 1–8 [DOI] [PubMed] [Google Scholar]
- 15.Taya K, Greenwald GS. In vivo and in vitro ovarian steroidogenesis in the pregnant rat. Biol Reprod 1981; 25: 683–691 [DOI] [PubMed] [Google Scholar]
- 16.Nelson SE, McLean MP, Jayatilak PG, Gibori G. Isolation, characterization and culture of cell subpopulations forming the pregnant rat corpus luteum. Endocrinology 1992; 130: 954–966 [DOI] [PubMed] [Google Scholar]
- 17.Christenson LK, Devoto L. Cholesterol transport and steroidogenesis by the corpus luteum. Reprod Biol Endocrinol 2003; 1: 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacasaña M, Esplugues A, Ballester F. Exposure to ambient air pollution and prenatal and early childhood health effects. Eur J Epidemiol 2005; 20: 183–199 [DOI] [PubMed] [Google Scholar]
- 19.Fujitani Y, Hirano S, Kobayashi S, Tanabe K, Suzuki A, Furuyama A, Kobayashi T. Characterization of dilution conditions for diesel nanoparticle inhalation studies. Inhal Toxicol 2009; 21: 200–209 [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa S, Hirabayashi M, Kobayashi S, Moriguchi Y, Kondo Y, Tanabe K, Wakamatsu S.Size distribution and characterization of ultrafine particles in roadside atmosphere. J Environ Sci Health A Tox Hazard Subst Environ Eng 2004; 39: 2671–2690 [DOI] [PubMed] [Google Scholar]
- 21.Hamada T, Watanabe G, Kokuho T, Taya K, Sasamoto S, Hasegawa Y, Miyamoto K, Igarashi M. Radioimmunoassay of inhibin in various mammals. J Endocrinol 1989; 122: 697–704 [DOI] [PubMed] [Google Scholar]
- 22.Kanesaka T, Taya K, Sasamoto S. Radioimmunoassay of corticosterone using 125I-labeled radioligand. J Reprod Dev 1992; 38: 85–89 [Google Scholar]
- 23.Taya K, Watanabe G, Sasamoto S. Radioimmunoassay for progesterone, testosterone, and estradiol-17β using 125I-iodohistamine radioligands. Jpn J Anim Reprod 1985; 31: 186–197 [Google Scholar]
- 24.Gibori G, Antczak E, Rothchild I. The role of estrogen in the regulation of luteal progesterone secretion in the rat after day 12 of pregnancy. Endocrinology. 1977; 100: 1483–1495 [DOI] [PubMed] [Google Scholar]
- 25.Korenman SG, Stevens RH, Carpenter LA, Robb M, Niswender GD, Sherman BM. Estradiol radioimmunoassay without chromatography: procedure, validation and normal values. J Clin Endocrinol Metab 1974; 38: 718–720 [DOI] [PubMed] [Google Scholar]
- 26.Gay VL, Kerlan JT. Serum LH and FSH following passive immunization against circulating testosterone in the intact male rat and in orchidectomized rats bearing subcutaneous silastic implants of testosterone. Arch Androl 1978; 1: 257–266 [DOI] [PubMed] [Google Scholar]
- 27.Voogt JL, Pakrasi PL, Johnson DC, Dey SK. Evidence for pituitary regulation of placental lactogen in the rat. J Endocrinol 1985; 107: 121–126 [DOI] [PubMed] [Google Scholar]
- 28.Taya K, Greenwald GS. Effect of hypophysectomy on day 12 of pregnancy on ovarian steroidogenesis in the rat. Biol Reprod 1981; 25: 692–698 [DOI] [PubMed] [Google Scholar]
- 29.Albarracin CT, Parmer TG, Duan WR, Nelson SE, Gibori G. Identification of a major prolactin-regulated protein as 20 alpha-hydroxysteroid dehydrogenase: coordinate regulation of its activity, protein content, and messenger ribonucleic acid expression. Endocrinology 1994; 134: 2453–2460 [DOI] [PubMed] [Google Scholar]
- 30.Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20alpha-hydroxysteroid dehydrogenase. Mol Endocrinol 2005; 19: 431–440 [DOI] [PubMed] [Google Scholar]
- 31.Murata T, Narita K, Honda K, Higuchi T. Changes of receptor mRNAs for oxytocin and estrogen during the estrous cycle in rat uterus. J Vet Med Sci 2003; 65: 707–712 [DOI] [PubMed] [Google Scholar]
- 32.Bryant-Greenwood GD. Relaxin as a new hormone. Endocr Rev 1982; 3: 62–90 [DOI] [PubMed] [Google Scholar]
- 33.Anderson DK, Rhees RW, Fleming DE. Effects of prenatal stress on differentiation of the sexually dimorphic nucleus of the preoptic area (SDN-POA) of the rat brain. Brain Res 1985; 332: 113–118 [DOI] [PubMed] [Google Scholar]
- 34.Ito Y, Ramdhan DH, Yanagiba Y, Yamagishi N, Kamijima M, Nakajima T. Exposure to nanoparticle-rich diesel exhaust may cause liver damage. Nihon Eiseigaku Zasshi 2011; 66: 638–642 [DOI] [PubMed] [Google Scholar]
- 35.Clunies-Ross C, Stanmore BR, Millar GJ. Dioxins in diesel exhaust. Nature 1996; 381: 379 [DOI] [PubMed] [Google Scholar]
- 36.Furuta C, Suzuki AK, Taneda S, Kamata K, Hayashi H. Mori Yoki, Li C, Watanabe G, Taya K. Estrogenic activities of nitrophenols in diesel exhaust particles. Biol Reprod 2004; 70: 1527–1533 [DOI] [PubMed] [Google Scholar]
- 37.Furuta C, Noda S, Li C, Suzuki AK, Taneda S, Watanabe G, Taya K. Nitrophenols isolated from diesel exhaust particles regulate steroidogenic gene expression and steroid synthesis in the human H295R adrenocortical cell line. Toxicol Appl Pharmacol 2008; 229: 109–120 [DOI] [PubMed] [Google Scholar]
- 38.Furuta C, Suzuki AK, Watanabe G, Li C, Taneda S, Taya K. Nitrophenols isolated from diesel exhaust particles promote the growth of MCF-7 breast adenocarcinoma cells. Toxicol Appl Pharmacol 2008; 230: 320–326 [DOI] [PubMed] [Google Scholar]
- 39.Li C, Takahashi S, Taneda S, Furuta C, Watanabe G, Suzuki AK, Taya K. Impairment of testicular function in adult male Japanese quail (Coturnix japonica) after a single administration of 3-methyl-4-nitrophenol in diesel exhaust particles. J Endocrinol 2006; 189: 555–564 [DOI] [PubMed] [Google Scholar]
- 40.Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Anti-androgenic activity of 3-methyl-4-nitrophenol in diesel exhaust particles. Eur J Pharmacol 2006; 543: 194–199 [DOI] [PubMed] [Google Scholar]
- 41.Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Estrogenic and anti-androgenic activities of 4-nitrophenol in diesel exhaust particles. Toxicol Appl Pharmacol 2006; 217: 1–6 [DOI] [PubMed] [Google Scholar]
- 42.Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Effects of 3-methyl-4-nitrophenol in diesel exhaust particles on the regulation of testicular function in immature male rats. J Androl 2007; 28: 252–258 [DOI] [PubMed] [Google Scholar]
- 43.Li C, Takahashi S, Taneda S, Furuta C, Watanabe G, Suzuki AK, Taya K. Effects of 3- methyl-4-nitrophenol in diesel exhaust particles on the regulation of reproductive function in immature female Japanese quail (Coturnix japonica). J Reprod Dev 2007; 53: 673–678 [DOI] [PubMed] [Google Scholar]
- 44.Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Effects of 3-methyl-4-nitrophenol on the suppression of adrenocortical function in immature male rats. Biol Pharm Bull 2007; 30: 2376–2380 [DOI] [PubMed] [Google Scholar]
- 45.Li C, Suzuki AK, Takahashi S, Taneda S, Watanabe G, Taya K. Effects of 3-methyl-4-nitrophenol on the reproductive toxicity in female Japanese quail (Coturnix japonica). Biol Pharm Bull 2008; 31: 2158–2161 [DOI] [PubMed] [Google Scholar]
- 46.Li X, Li C, Suzuki AK, Taneda S, Watanabe G, Taya K. 4-nitrophenol isolated from diesel exhaust particles disrupts regulation of reproductive hormones in immature male rats. Endocrine 2009; 36: 98–102 [DOI] [PubMed] [Google Scholar]
- 47.Li X, Li C, Suzuki AK, Watanabe G, Taneda S, Taya K. Endocrine disruptive effect of 3-methyl-4-nitrophenol isolated from diesel exhaust particles in Hershberger assay using castrated immature rats. Biosci Biotechnol Biochem 2009; 73: 2018–2021 [DOI] [PubMed] [Google Scholar]