Abstract
Human embryos for hESC derivation are often donated at the cleavage stage and of reduced quality. Poor quality embryos have lower efficiency for hESC derivation. However, cleavage stage mouse embryos develop into higher quality expanded blastocysts if they are cultured with insulin, suggesting that this approach could be used to improve hESC derivation from poor quality cleavage stage embryos. The present study used a mouse model to examine this approach. In particular we examined the effect of insulin on the number of epiblast cells in blastocysts on days 4, 5 and 6 using Oct4 and Nanog co-expression. Second we examined the effect of insulin on the frequency with which outgrowths can be derived from these. Finally, we tested whether prior culture in the presence of insulin results in blastocysts with increased capacity to generate ESC colonies. Culture of cleavage stage embryos with insulin increased the number of Oct4 and Nanog positive cells in blastocysts at all time points examined. Prior culture with insulin had no effect on outgrowths generated from blastocysts plated on days 4 or 5. However, insulin treatment of blastocysts plated on day 6 resulted in increased numbers of outgrowths with larger epiblasts compared with controls. 13% of insulin treated day 6 blastocysts produced primary ESC colonies compared with 6% of controls. In conclusion, treatment with insulin can improve epiblast cell number in mice leading to an increase with which primary ESC colonies can be generated and may improve hESC isolation from reduced quality embryos donated at the cleavage stage.
Keywords: Blastocyst, Embryonic stem cell, Epiblast, Insulin, Pluripotency, Preimplantation development
Embryonic stem cells (ESCs) are pluripotent cells whose progenitors are the epiblast cells of the inner cell mass (ICM) of blastocysts [1,2,3]. In a fully developed mouse blastocyst ICM cells are marked by Oct4 expression, while epiblast cells are the only cells that also co-express the pluripotency marker Nanog [4]. Those ICM cells which do not express Nanog are deemed to be primitive endoderm [5].
ESCs can be differentiated to any cell type found in the adult body. As such human ESCs (hESCs) have potential applications in regenerative medicine. However, the efficiency with which hESCs can be derived is generally lower [6, 7] than that reported for mice [8, 9]. One key difference between mESCs and hESCs, is that mouse embryos for ESC derivation are usually cultured in vivo, while many human embryos used for ESC research are frozen assisted reproduction technology (ART) embryos donated often up to 5–10 years after they were produced [10] due to regulatory requirements. Prior to cryopreservation these human embryos were frequently cultured in vitro in conditions now known to perturb embryo viability [11,12,13]. Culture of embryos in vitro has been shown to reduce epiblast cell numbers in blastocysts [14]. The number of epiblast cells in the blastocyst has been shown to be a major determinant of the efficiency with which ESCs can be isolated from an embryo [7].
The majority of embryos donated for hESC derivation have been cryopreserved at the cleavage stage [15,16,17]. Hence one approach to improving hESC isolation efficiencies from these embryos is to culture them to the blastocyst stage in media which increase epiblast cell number. We have developed a mouse model of human embryo culture to examine how culture from the 8-cell stage can be used to improve blastocyst quality. Using this model we have shown that culture from the 8-cell stage in G2 medium supplemented with 1.7 ρM insulin increases the percentage of epiblast cells in the ICM of in vitro cultured blastocysts [18].
The aim of the present study was to examine this effect further using the same mouse model. In particular we examined the effect of insulin on the progression of Oct4 and Nanog restriction in the developing blastocyst, whether treatment with insulin could increase outgrowth formation, ESC progenitor cell number and the efficiency with which primary mESC colonies could be derived.
Materials and Methods
All procedures were approved by The University of Adelaide Animal Ethics Committee, in compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Unless otherwise stated chemicals used were purchased from Sigma-Aldrich, St Louis, MO, USA. All chemicals and consumables used for standard embryo culture were tested in a 1-cell mouse embryo assay for compatibility with embryo development [19]. Mice were fed ad libitum, and kept in a 14:10 light:dark cycle.
Embryo culture
Embryos were collected from female mice (C57BL/6) aged 3–4 weeks given a 5 IU equine chorionic gonadotropin (eCG; Folligon, Intervet Australia, Bendigo, Victoria) intraperitoneal injection, then 5 IU human chorionic gonadotropin (hCG; Pregnyl, Organon, Sydney, Australia) 48 h later to induce ovulation. Female mice were then placed with a male mouse of the same strain, and mating was assessed the following morning by the presence of a vaginal plug. Zygotes were flushed from oviducts into MOPS-G1 [20] 22 h post-hCG. Surrounding cumulus cells were denuded from the zygotes using 50 IU/ml hyaluronidase for up to 2 min.
Zygotes were washed in MOPS-G1 and cultured in 20 µl drops of G1.2 medium in groups of ten at 37 C in 6% CO2, 5% O2, 89% N2 from 0–48 h. Medium was overlaid with paraffin oil (Merek KGaA, Darmstadt, Germany). Embryos that reached the 8-cell stage by 48 h were transferred to 20 µl drops of G2.2 supplemented with 0 or 1.7 ρM insulin, and cultured individually at 37 C in 6% CO2, 5% O2, 89% N2 to 70–75 h (day 4), 96 h (day 5) or 115 h (day 6).
Experiment 1: Effect of insulin on blastocyst development
The aim of Experiment one was to determine the timing of Oct4 and Nanog restriction to the epiblast in embryos cultured in vitro as well as the effect of insulin on Oct4 and Nanog co-expression in the blastocyst. Control and insulin treated embryos were placed in G2.2 at Day 3 (48 h) and then fixed on days 4, 5 and 6, and immunocytochemistry was performed. Embryos in all groups which began cavitation prior to 70 h or had not cavitated by 75 h were discarded to avoid confounding effects. As such all blastocysts in the day 5 and day 6 groups were known to have cavitated between 70 and 75 h and to have on time development. Individual culture was used to minimize potentially confounding interference from paracrine growth factor secretion [21].
Experiment 2: Effect of insulin on outgrowth formation
The aim of Experiment two was to determine whether prior culture with insulin could increase outgrowth formation rate and epiblast cell number. As such, three experiments were carried out where control and insulin treated blastocysts were plated on day 4, day 5 and then day 6. Then, a fourth experiment was undertaken to confirm an apparent increase in epiblast cell number observed for plating blastocysts on day 6 compared to day 4 or day 5, where control cultured blastocysts from the same pool were plated on days 4, 5 and 6 .
Blastocysts had their zona pellucidae removed by incubation in acid Tyrode's solution, followed by plating onto organ well dishes coated with Sv129 derived mouse embryonic fibroblasts (MEFs). Media used was α-MEM with glutamax, ribo- and deoxyribonucleosides, supplemented with 10% knockout serum replacement (Invitrogen, Carlsbad, CA, USA), 1× non-essential amino acids (Invitrogen), 55 µM β-mercaptoethanol (Invitrogen), 1× insulin-transferrin-selenium (Invitrogen, Carlsbad, CA), 20 ng/ml bFGF (Chemicon, Temecula CA, USA), 20 ng/ml Activin-A (R and D systems), 20 ng/ml human recombinant EGF (Invitrogen), 100 ng/ml Vitronectin and 10 ng/ml mouse LIF at 37 C in 6% CO2, 5% O2, 89% N2. Attachment was assisted by gently pressing blastocysts into the surface of the MEFs, using a 30 G needle. Following 48 h of culture, attachment was assessed, outgrowths were fixed, and immunocytochemistry was performed.
Experiment 3: Effect of insulin on the derivation of primary ESC colonies from day 6 blastocysts
The aim of Experiment three was to determine if insulin could increase the efficiency with which primary ESC colonies could be derived. Outgrowths from control and insulin treated blastocysts plated on day 6 were generated as discussed above, with the exception that all embryos were used, not just those which cavitated between 70 and 75 h. Additionally, due to individual culture of embryos and the pressing method of outgrowth, it was possible to track a developing embryo's morphology and examine which embryos gave rise to a primary ESC colony. Embryo development was scored on day 4, day 5 and day 6. Embryos were scored as arrested (those embryos that had failed to develop from the 8-cell stage), early blastocyst (embryos with a blastocoel cavity less than two thirds their volume), blastocyst (embryos with a blastocoel cavity greater than two thirds their volume), hatching blastocyst (embryos which were herniating from the zona pellucidia) and hatched blastocyst (embryos that were completely free of the zona pellucidia). We also scored which 8-cell embryos had begun to compact when the pool was first split between control and insulin treatments.
Outgrowths were trypsinised with 0.05% trypsin (Invitrogen) and replated on MEFs. Cells were allowed to grow for two days, then colonies with a domed shape, multiple layers and a smooth undifferentiated ESC morphology were picked and fixed in 4% paraformaldahyde in PBS overnight at 4 C prior to immunocytochemistry.
Immunocytochemistry
The expression of Oct4 and Nanog in blastocysts was measured using immunocytochemistry. Blastocysts were fixed in 4% paraformaldahyde overnight at 4 C, and then incubated in 0.1 M glycine in PBS for 5 min at room temperature (RT). Permeabilisation was performed in PBS with 0.25% TritonX-100 (PBS-TX) for 30 min at RT. Blastocysts were then blocked in 10% Normal Donkey Serum (Sapphire Bioscience, Redfern, New South Wales, Australia) for 1 h at RT, followed by incubation with Nanog rabbit anti mouse polyclonal antibody (Sapphire, Cat#120-21603 or Cosmo Bio, Tokyo, Japan, Cat#REC-RCAB0002P-F) at 1:200 and Oct-3/4 goat anti mouse polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA, inc sc-8628) at 1:100 in PBS-TX overnight at 4 C. After washing the next day in PBS-TX, blastocysts were incubated with Donkey anti rabbit secondary antibody (1:100) conjugated to FITC (Australian Laboratory Services, Homebush New South Wales, Australia) and donkey anti goat secondary antibody (1:100) conjugated to Rhodamine (Jackson ImmunoResearch, West Grove, PA, USA, 705-025-003) for 2 h at 37 C. Finally, embryos were washed and then incubated with 3nM of nuclear stain 4'-6-Diamidino-2-phenylindole (DAPI) at RT for 2–3 min, which stains all cell nuclei, before imaging using confocal microscopy (Calcium Lecia SP5, Lecia SP5 software, Wetzlar, Germany). A negative control was performed where primary antibody was not applied.
The procedure for staining outgrowths was performed as described for blastocysts above, but with the following alterations; permeabilization was reduced to 5 min in 0.05% TX-100, primary antibodies were applied with blocking solution, the wash step between primary and secondary antibody application was extended to 3 h, the wash step between the secondary antibodies and DAPI was increased to 3 rinses at 5 min each and DAPI staining was increased to 5 min. Primary ESC colonies were stained using the same methodology as blastocysts.
Statistics
For quantitative analyses treatment group was fitted as a fixed factor and replicate was fitted as a cofactor. Data was expressed as mean ± SEM and analysed using Univariate General Linear Model or an independent samples t-test using PASW Statistics 17. Between treatment differences were assessed using the Least Significant Difference (LSD) method. Categorical data was analysed by chi square or Fischer's exact tests. Values of P<0.05 were considered significant.
Results
Experiment 1: Effect of insulin on blastocyst development
Representative images of day 4, 5 and 6 blastocysts stained for Oct4 and Nanog are shown in (Fig. 1). In day 4 blastocysts Oct4 and Nanog co-expression was not restricted to the ICM (Fig. 1B). However, there were more Oct4 and Nanog positive cells in the insulin treated embryos compared with controls (Table 1). There was no difference in the number of Oct4 positive cells between the insulin treated and control groups, however blastocysts from the insulin treated group had a higher total cell number (TCN) compared with the control group (Table 1).
Fig. 1.
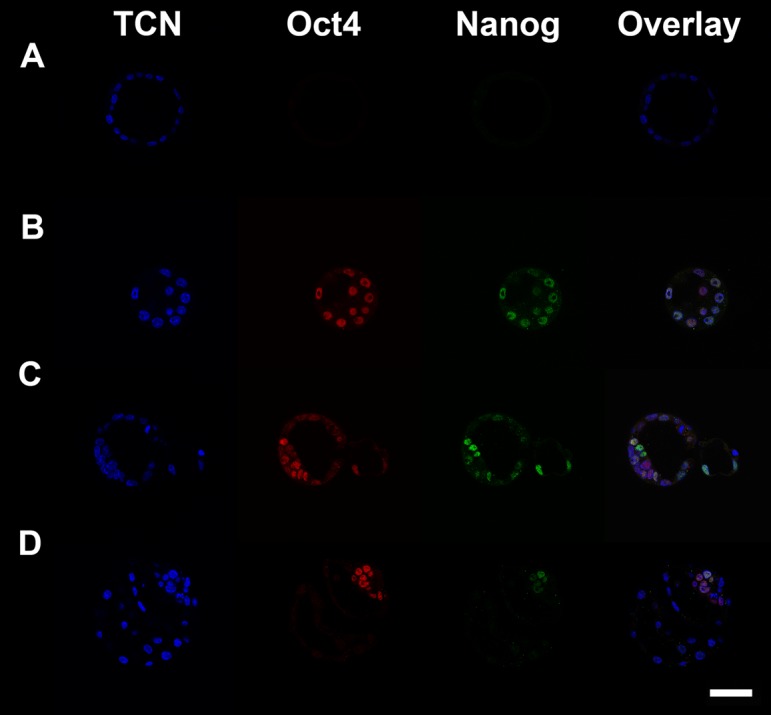
Blastocysts stained for TCN (DAPI; blue), Oct4 (Rhodamine; red) and Nanog (FITC; green). Scale bar is 50 µm. A. Negative control where staining was performed without primary antibodies. B. A day 4 blastocyst fixed as it began cavitation. C. A day 5 blastocyst. D. A day 6 blastocyst.
Table 1. The effect insulin at 1.7 ρM on day 4, 5 and 6 blastocyst Oct4 and Nanog expression.
| TCNA | Oct4B | Oct4+NanogC | |
| Day 4 control | 39.4 ± 1.6* | 36.4 ± 1.6 | 25.4 ± 1.4* |
| Day 4 insulin | 43.6 ± 1.1* | 39.5 ± 1.0 | 31.9 ± 1.2* |
| Day 5 control | 89.1 ± 3.1‡ | 56.2 ± 2.3‡ | 33.7 ± 2.2‡ |
| Day 5 insulin | 110.5 ± 3.3‡ | 63.9 ± 2.3‡ | 48.2 ± 2.1‡ |
| Day 6 control | 153.4 ± 6.3 | 27.8 ± 2.9 | 4.8 ± 0.5† |
| Day 6 insulin | 155.6 ± 5.2 | 29.1 ± 2.9 | 6.8 ± 0.6† |
A Total cell number of blastocysts on the day indicated. B Oct4 positive cell number of blastocysts on the day indicated. C Oct4 and Nanog positive cell number of blastocysts on the day indicated. Data is mean ± SEM. Like pairs a *, ‡ and †, are significantly different within columns. N≥28 (3 replicate experiments).
In day 5 blastocysts, Oct4 and Nanog continued to be expressed in cells outside the ICM and as such could not be used to determine epiblast cell number (Fig. 1C). Insulin treated blastocysts contained more Oct4 and Nanog positive cells than controls (Table 1). Furthermore, the number of Oct4 positive cells and TCN were also increased in blastocysts from the insulin treated group compared with controls (Table 1).
In day 6 blastocysts Oct4 and Nanog co-expression was restricted to the epiblast (Fig. 1D) and hence could be used to determine the number of epiblast cells. Insulin had no effect on Oct4 positive cell number or TCN but significantly increased epiblast cell number (Table 1). This resulted in a trend for an increased proportion of ICM that was epiblast (23.8 ± 1.8 vs. 18.9 ± 1.8. P=0.059, N≥28, 3 replicates).
Experiment 2: Effect of insulin on outgrowth formation
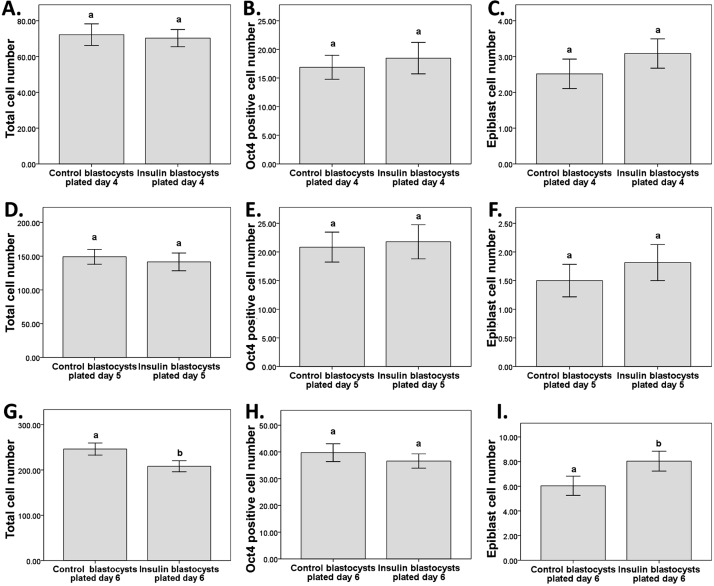
There was no difference in the epiblast cell number of outgrowths plated on day 4 between control and insulin and treatments, as determined by Oct 4 and Nanog co-staining (Fig. 2C). Additionally, there was no effect on TCN (Fig. 2A) or Oct4 positive cell number (Fig. 2B). This was the same for outgrowths plated on day 5 (Fig. 2D–F). For outgrowths plated on day 6, there were significantly more epiblast cells in the insulin treated group than the control group (Fig. 2I). However Oct4 cell number was not affected (Fig. 2H), while TCN was decreased (Fig. 2G). Representative images of outgrowths from blastocysts plated on days 4, 5 and 6 and stained for Oct4 and Nanog 48 h after plating are shown in (Fig. 3).
Fig. 2.
The effect insulin at 1.7 ρM in embryo culture media from the compacting stage of development on the outgrowths of early blastocysts plated on day 4, blastocysts plated on day 5 and blastocysts plated on day 6 on outgrowth cell number and differentiation. A: Total cell number of outgrowths plated day 4. B: Oct4 positive cell number of outgrowths plated day 4. C: Epiblast cell number of outgrowths plated day 4. D: Total cell number of outgrowths plated day 5. E: Oct4 positive cell number of outgrowths plated day 5. F: Epiblast cell number of outgrowths plated day 5. G: Total cell number of outgrowths plated day 6. H: Oct4 positive cell number of outgrowths plated day 6. I: Epiblast cell number of outgrowths plated day 6. Data is mean ± SEM. N≥27 (3 replicate experiments). Superscripts a and b differ at P<0.05
Fig. 3.
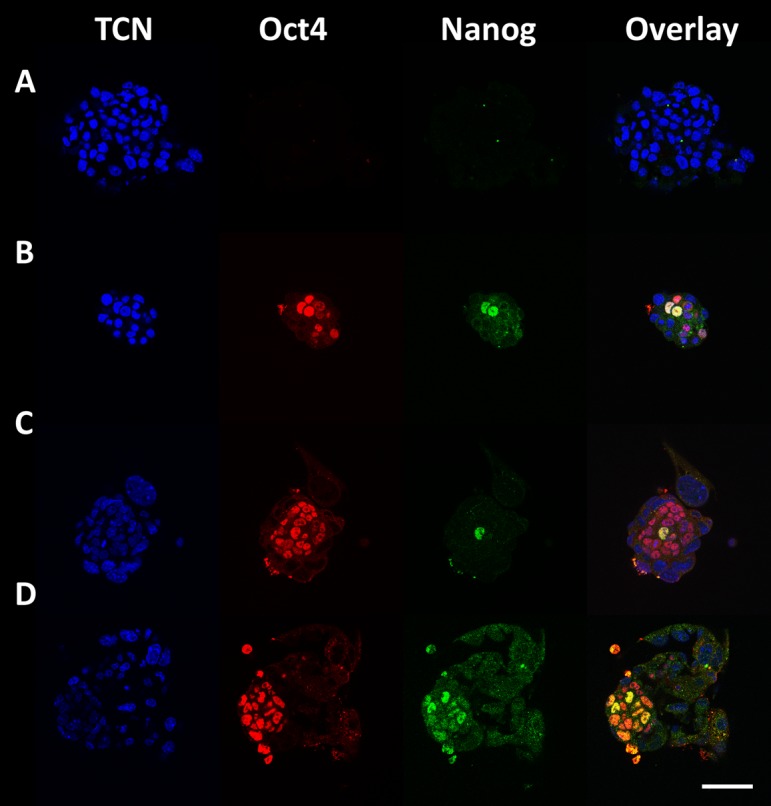
Outgrowths stained for TCN (DAPI; blue), Oct4 (Rhodamine; red) and Nanog (FITC; green). Scale bar is 50 µm. A: Negative control where staining was performed without primary antibodies. B: An outgrowth from a blastocyst plated on day 4. C: An outgrowth from a blastocyst plated on day 5. D: An outgrowth from a blastocyst plated on day 6.
As it is possible to obtain an ESC line from an outgrowth containing as little as one ESC progenitor cell, we examined the effect of prior culture in the presence of insulin on the percentage of outgrowths which contained at least one epiblast cell. No significant effect was seen on the percentage of outgrowths containing an epiblast when blastocysts were plated on days 4 or 5 between control and insulin treated groups (64.5% vs. 83.3%, and 62.5% vs. 70.3%, respectively, N≥27, 3 replicates) however, insulin increased the proportion of outgrowths containing an epiblast if they were plated on day 6 (100% vs. 86.5%, P<0.01 N≥27, 3 replicates).
Furthermore, because not all blastocysts attach and/or produce outgrowths when plated, we examined the effect of insulin on the efficiency of outgrowth formation. Day 4 blastocysts from the insulin group were more likely to give rise to outgrowths than the control group (61.4% vs. 43.4%, P<0.05, N≥64, 3 replicates), however no difference was found for day 5 blastocysts (67.2% vs. 64.1%, N≥64, 3 replicates). Insulin treated blastocysts plated on day 6 also gave rise to outgrowths at higher efficiencies compared with controls (97.1% vs. 87.9%, P<0.05, N≥64, 3 replicates).
To confirm the apparent effect of day of plating on epiblast cell number seen in the first three experiments we undertook a fourth experiment where control embryos from the same pool were plated on days 4, 5 and 6. The results of this experiment confirmed that primary outgrowths from blastocysts plated on days 4 or 5 had fewer epiblast cells present in their outgrowths and that these were also less likely to contain an epiblast compared with day 6 plated outgrowths. The efficiency of attachment was also reduced in day 4 and 5 blastocysts (Table 2).
Table 2. Presence of epiblast cells in outgrowths plated at different times.
| Blastocysts plated | Outgrowths (%)A | Epiblast cell numberB | Outgrowths with an epiblast (%)C |
| Day 4 | 60.6a | 1.9 ± 0.4a | 68.4a |
| Day 5 | 60.0a | 2.0 ± 0.4a | 70.6a |
| Day 6 | 87.5b | 4.9 ± 0.4b | 92.5b |
A Total number of outgrowths 48 h after plating blastocysts on the day indicated as a proportion of number of blastocysts plated, data shown as a percentage. B Mean number of epiblast cells in outgrowths as indicated by the co-expression of Oct4 and Nanog 48 h after plating blastocysts on the day indicated, data is mean ± SEM. C Total number of outgrowths with at least one epiblast cell as indicated by the co-expression of Oct4 and Nanog 48 h after plating blastocysts on the day indicated expressed as a percentage of the number of outgrowths stained. Superscripts a and b differ at P<0.05, N≥34 (3 replicate experiments).
Experiment 3: Effect of insulin on the derivation of primary ESC colonies from day 6 blastocysts
Control and insulin treated embryos were cultured from the 8-cell stage to day 6, then plated and allowed to outgrow. Outgrowths were then picked, trypsinised and replated. Resultant colonies were stained for Oct4 and Nanog to confirm their pluripotency (Fig. 4) if they possessed an ESC like morphology (Fig. 5).
Fig. 4.
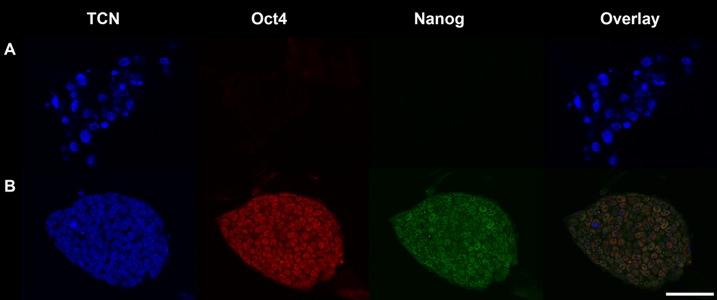
Primary ESC colonies stained for TCN (DAPI; blue), Oct4 (Rhodamine; red) and Nanog (FITC; green). A: Negative control where staining was performed without primary antibodies. B: A primary ESC colony which is positive for both Oct4 and Nanog. Scale bar is 50 µm.
Fig. 5.
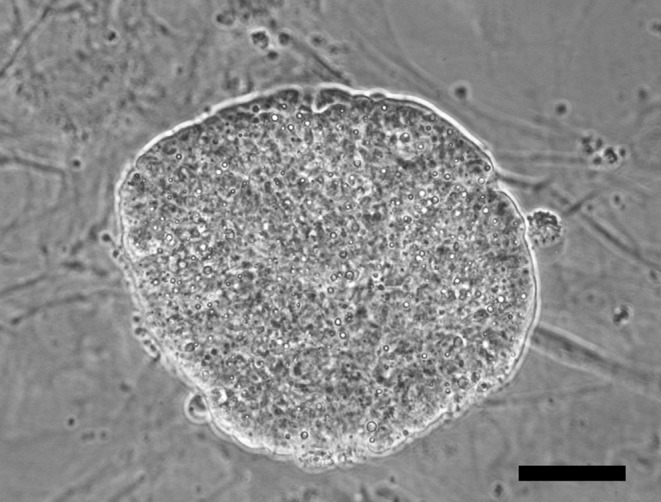
A bright field image of a primary colony generated by the trypsinization of a day 6 plated outgrowth showing an undifferentiated morphology. Scale bar is 50 µm.
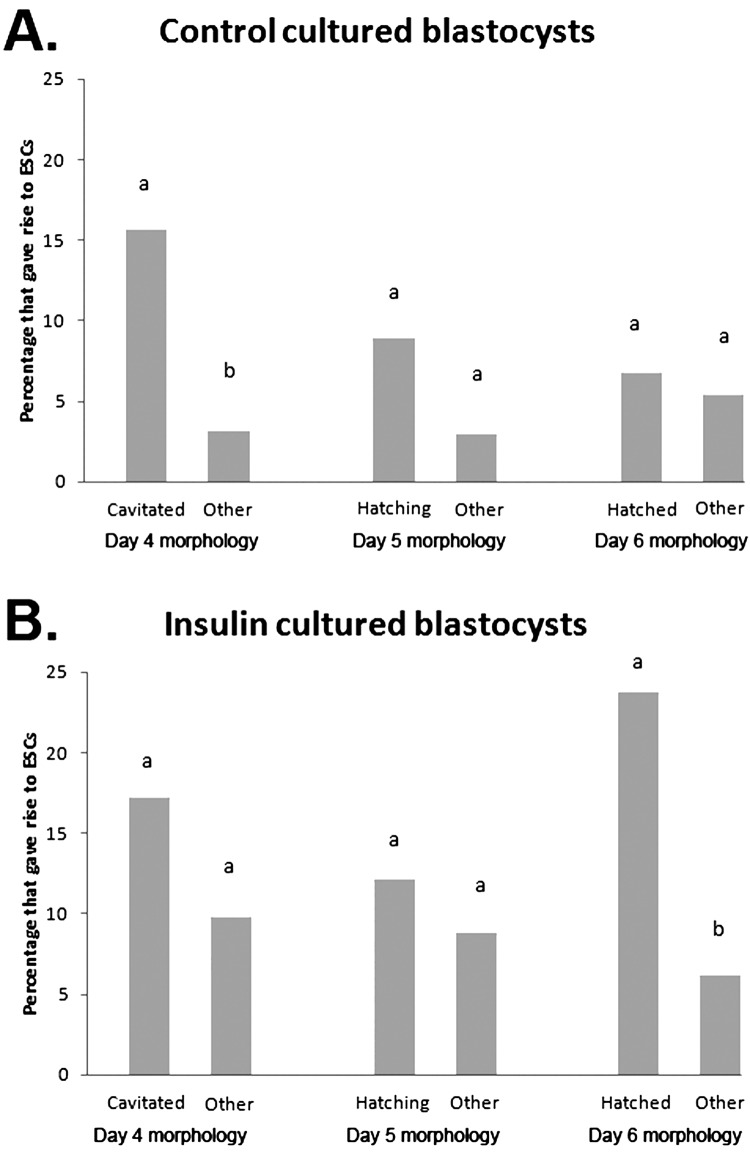
Embryos which were cultured in the presence of insulin were more likely to give rise to a primary ESC colony compared with control treated embryos (13% vs. 6%, P<0.05, N=156, 9 replicates). As embryos were cultured individually from the 8-cell stage to day 6 we were able to monitor each embryo's initial morphology, the developmental stages it reached on days 4, 5, and 6, whether or not it outgrew, and if it was able to generate a primary ESC colony.
Insulin increased the rate of development of embryos, as more embryos from the insulin group had cavitated on day 4 (41% vs. 21%, P<0.001, N=156, 9 replicates), and completed hatching by day 6 (38% vs. 28%, P<0.05, N=156, 9 replicates) compared with control embryos. No difference was observed for commencement of hatching on day 5 between control and insulin treated groups. Embryos which had cavitated on day 4 were more likely to give rise to ESC colonies (17% vs. 6%, P<0.01, N≥96, 9 replicates), as were embryos which had hatched on day 6 (17% vs. 6%, P<0.01, N≥103, 9 replicates), compared with those which had not cavitated or hatched respectively. Control embryos which cavitated on day 4 were significantly more likely to give rise to ESC colonies than those which had not cavitated, but the difference for insulin treated embryos was non-significant (Fig. 6). Following plating on day 6, insulin treated blastocysts which had hatched were significantly more likely to give rise to ESC colonies than those which had not hatched but the difference for the control treatment was non-significant (Fig. 6). Additionally, hatched blastocysts from the insulin group were more likely to give rise to an ESC colony compared with hatched blastocysts from the control group (24% vs. 7%, P<0.05, N≥44, 9 replicates).
Fig. 6.
The effect of morphological state on days 4, 5 and 6 on the likelihood of blastocysts giving rise to ESC colonies. A: Percentage of blastocysts cultured in control conditions at the indicated morphological states which ultimately gave rise to a primary ESC colony, B: Percentage of blastocysts cultured with insulin at the indicated morphological states which ultimately gave rise to a primary ESC colony. Data are the number of blastocysts which generated ESCs expressed as a percentage of all blastocysts at that morphological stage. N≥44 (9 replicate experiments). Superscripts a and b within paired columns differ at P<0.05.
When we restricted our analysis to embryos that were of poorer quality (had not begun compaction when they were placed in treatment groups), the beneficial effect of insulin on ESC colony generation was still observed (13% vs. 6%, P<0.05, N≥136, 9 replicates).
Discussion
Human embryos for hESC derivation are typically donated at the cleavage stage [15,16,17], frequently 5–10 years after cryopreservation [10, 22] or after the identification of poor morphology [23, 24]. These embryos are often of reduced quality [23,24,25,26], and give rise to ESC lines with low efficiency as a result [6, 7]. The aim of the present study was to examine the effect of insulin treatment on blastocyst development, outgrowth formation, ESC progenitor cell number and the derivation of primary ESC colonies from cleavage stage embryos using a mouse model, as a first step towards improving hESC isolation efficiencies from embryos which have a reduced quality.
The results of experiment one demonstrated that Oct4 and Nanog are expressed in cells outside the epiblast in day 4 and 5 in vitro cultured blastocysts but are restricted to the epiblast in day 6 blastocysts (Fig. 1). Treatment with insulin increased the number of cells positive for both OCT4 and Nanog in day 4 and day 5 blastocysts, as well as epiblast cell number in day 6 blastocysts compared with controls. Interestingly, although insulin increased the TCN of blastocysts on days 4 and 5, there was no effect on day 6. Analysis of the relative proportions of cells which co-expressed OCT4 and Nanog on days 4 and 5 showed an increase in the insulin treated group (data not shown), demonstrating that the increase in the number of OCT4 and Nanog positive cells was not a concomitant result of the general increase in total cell number, and suggesting an effect by insulin to increase OCT4 and Nanog protein levels.
Furthermore, the TCN finding suggests that there is a plateau effect occurring where insulin stimulates mitosis during the earlier stages of development, but that after a certain cell number or number of divisions has been reached the effect subsides and control blastocysts are essentially able to catch up, supporting our conclusion from previous work [18] that insulin's role is more than a mitogenic stimulator. This mitogenic plateau may explain an apparent contradiction between our work and that of Harvey and Kaye [27] who found a significant increase in ICM cell number in the mouse blastocyst for culture with insulin which was not observed in our system. Harvey and Kaye assessed ICM cell number at approximately 80 h (although embryos were flushed at the 2-cell rather than zygote stage, which adds a confounding factor with regards to timing, but not insulin exposure as mouse embryos do not express the insulin receptor until the 8-cell stage [28]), whereas in this study ICM cell number was only assessed when Oct4 was restricted to the ICM on day 6 (115 h). As such, in assessing ICM cell number on day 6, after the proposed plateau was reached, we may have missed observing the ICM increase reported by Harvey and Kaye despite it potentially having been present earlier in our system, as evidenced by the TCN increases seen on days 4 and 5.
In the mouse blastocyst, embryonic cells commit to the trophectoderm or ICM cell fates when cavitation begins [29]. While the trophectoderm marker Cdx2 is restricted to the outer cells, Oct4 and Nanog are not restricted to the developing ICM [29,30,31]. The ICM then forms the epiblast and primitive endoderm [4, 30, 31] with Oct4 and Nanog co-expression eventually being restricted to the epiblast, which has previously been reported to happen at or before day 5 [4, 32]. However in the present study Oct4 and Nanog co-expression was not restricted to the epiblast until day 6. The reason for this difference is unclear, but it may relate to the fact that we cultured our embryos in vitro from the zygote stage, which is known to delay development compared to embryos cultured in vivo for all or part of their development [33, 34]. Our finding that in vitro culture appears to delay epiblast formation is a novel observation which has implications for the use of mouse embryos, commonly collected at the 2-cell stage or later, in the modelling of the development of human embryos which, if cultured in vitro, have been cultured from the zygote stage following in vitro fertilisation. However, the referenced studies [4, 32] used ICR mice which were outbred or hybrid (with B5), whereas we used embryos from inbred C57Bl/6 mice. As such it is possible this observation is the result of strain differences, and further investigation is required for conclusions to be made.
The results of experiment two showed that following plating, embryonic outgrowths from early blastocysts had fewer epiblast cells compared with blastocysts plated on day 6 and that this was not improved by treatment with insulin. A similar result was found for day 5 blastocysts. However, outgrowths from day 6 blastocysts, where the ICM had differentiated to epiblast and primitive endoderm, had more epiblast cells than outgrowths from early blastocysts (day 4) or day 5 blastocysts, generated outgrowths at a higher rate and were more likely to contain an epiblast. Furthermore, when day 6 blastocysts had been cultured in insulin, outgrowth formation rate, the likelihood of these containing an epiblast and epiblast cell number were increased compared with the controls.
These results demonstrate that culture of embryos in the presence of insulin only improves the quality of embryonic outgrowths when embryos are cultured to day 6 prior to plating. The finding that full lineage commitment is important for generating quality outgrowths is consistent with that for human blastocysts, where ESC derivation is highest when embryos are plated after Oct4 has become restricted to the ICM [35]. Furthermore, as well as demonstrating the usefulness of culturing embryos with insulin for increasing the yield of outgrowths with the potential to give rise to an ESC colony, our model demonstrates that the influence of insulin on the blastocysts is not transitory –– as an increased number of epiblast cells in the day 6 blastocyst results in an increased number of epiblast cells in an outgrowth it generates, it is possible that the inclusion of insulin during embryo culture could have long lasting effects on the health and characteristics of ESC lines derived. Little work has previously been done on the effect of embryo culture on outgrowth formation.
The results of experiment three demonstrated that the efficiency with which primary ESC colonies can be generated is increased by culture of embryos with insulin. This effect was also seen when we restricted our analysis to morphologically poor quality embryos; a highly relevant finding as human embryos donated for ESC derivation are, as has been noted, of reduced quality. Interestingly, as well as increasing the proportion of highest quality blastocysts (hatched) at the point of plating, insulin increased the proportion of hatched blastocysts which gave rise to an ESC colony compared with control blastocysts of similar quality. As insulin cultured embryos of an equivalently high morphological quality as control cultured embryos gave rise to ESC colonies with greater efficiency the improvement shown in ESC generation efficiency for culture with insulin is likely to be a consequence of the increase in epiblast cell number seen and not due to a more generalised improvement in embryo quality.
In conclusion, the present study has demonstrated that insulin can improve the number of ESC progenitor cells in the epiblast of day 6 blastocysts and embryonic outgrowths from these. This is a novel finding which results in an improved efficiency with which primary ESC colonies can be generated. These findings may have important implications for the derivation of human ESC lines from donated embryos. These are usually fresh embryos of poor quality or embryos deemed suitable for transfer and cryopreserved at the cleavage stage up to 5 to 10 years previously after culture in relatively simple media known to affect their viability [15,16,17]. Additionally, if human recombinant insulin were used the introduction of xenogenic factors would be avoided and therefore the clinical utility of the resultant lines would not be compromised. Further work is required to determine the efficiency with which cell lines can be established using this approach.
Acknowledgment
The authors acknowledge the support of the NHMRC program grant for funding. Dr M Lane is a recipient of NHMRC Senior Research Fellowship. The authors thank A Filby, SL Wakefield and N Palmer for their technical assistance.
References
- 1.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci USA 1997; 94: 5709–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 1981; 78: 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981; 292: 154–156 [DOI] [PubMed] [Google Scholar]
- 4.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell 2006; 10: 615–624 [DOI] [PubMed] [Google Scholar]
- 5.Debeb BG, Galat V, Epple-Farmer J, Iannaccone S, Woodward WA, Bader M, Iannaccone P, Binas B. Isolation of Oct4-expressing extraembryonic endoderm precursor cell lines. PLoS One 2009; 4: e7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP, Wang S, Morton CC, McMahon AP, Powers D, Melton DA. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med 2004; 350: 1353–1356 [DOI] [PubMed] [Google Scholar]
- 7.Bongso A, Lee EH. Stem Cells: From Bench to Bedside. Singapore: World Sc Press; 2005
- 8.Bryja V, Bonilla S, Arenas E. Derivation of mouse embryonic stem cells. Nat Protoc 2006; 1: 2082–2087 [DOI] [PubMed] [Google Scholar]
- 9.Batlle-Morera L, Smith A, Nichols J. Parameters influencing derivation of embryonic stem cells from murine embryos. Genesis 2008; 46: 758–767 [DOI] [PubMed] [Google Scholar]
- 10.Klock SC. Embryo disposition: the forgotten “child” of in vitro fertilization. Int J Fertil Womens Med 2004; 49: 19–23 [PubMed] [Google Scholar]
- 11.Gardner DK. Dissection of culture media for embryos: the most important and less important components and characteristics. Reprod Fertil Dev 2008; 20: 9–18 [DOI] [PubMed] [Google Scholar]
- 12.Laverge H, De Sutter P, Desmet R, Van der Elst J, Dhont M. Prospective randomized study comparing human serum albumin with fetal cord serum as protein supplement in culture medium for in-vitro fertilization. Hum Reprod 1997; 12: 2263–2266 [DOI] [PubMed] [Google Scholar]
- 13.Gardner DK, Lane M. Towards a single embryo transfer. Reprod Biomed Online 2003; 6: 470–481 [DOI] [PubMed] [Google Scholar]
- 14.Jin XL, Chandrakanthan V, Morgan HD, O'Neill C. Preimplantation embryo development in the mouse requires the latency of TRP53 expression, which is induced by a ligand-activated PI3 kinase/AKT/MDM2-mediated signaling pathway. Biol Reprod 2009; 80: 286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Elst J, Van den Abbeel E, Camus M, Smitz J, Devroey P, Van Steirteghem A. Long-term evaluation of implantation of fresh and cryopreserved human embryos following ovarian stimulation with buserelin acetate-human menopausal gonadotrophin (HMG) or clomiphene citrate-HMG. Hum Reprod 1996; 11: 2097–2106 [DOI] [PubMed] [Google Scholar]
- 16.Oehninger S, Mayer J, Muasher S. Impact of different clinical variables on pregnancy outcome following embryo cryopreservation. Mol Cell Endocrinol 2000; 169: 73–77 [DOI] [PubMed] [Google Scholar]
- 17.Wang YA, Chambers GM, Dieng M, Sullivan EA. Assisted reproductive technology in Australia and New Zealand 2007. Assisted Reproduction Technology Series 2009; 13: 1–56 [Google Scholar]
- 18.Campbell JM, Nottle M, Vassiliev I, Mitchell M, Lane M. Insulin increases epiblast cell number of in vitro cultured mouse embryos via the PI3K/GSK3/p53 pathway. Stem Cells Dev 2012; 21: 2430–2441 [DOI] [PubMed] [Google Scholar]
- 19.Lane M, Mitchell M, Cashman KS, Feil D, Wakefield S, Zander-Fox DL. To QC or not to QC: the key to a consistent laboratory? Reprod Fertil Dev 2008; 20: 23–32 [DOI] [PubMed] [Google Scholar]
- 20.Mitchell M, Cashman KS, Gardner DK, Thompson JG, Lane M. Disruption of mitochondrial malate-aspartate shuttle activity in mouse blastocysts impairs viability and fetal growth. Biol Reprod 2009; 80: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane M, Gardner DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod 1992; 7: 558–562 [DOI] [PubMed] [Google Scholar]
- 22.Bankowski BJ, Lyerly AD, Faden RR, Wallach EE. The social implications of embryo cryopreservation. Fertil Steril 2005; 84: 823–832 [DOI] [PubMed] [Google Scholar]
- 23.Lerou PH, Yabuuchi A, Huo H, Takeuchi A, Shea J, Cimini T, Ince TA, Ginsburg E, Racowsky C, Daley GQ. Human embryonic stem cell derivation from poor-quality embryos. Nat Biotechnol 2008; 26: 212–214 [DOI] [PubMed] [Google Scholar]
- 24.Mitalipova M, Calhoun J, Shin S, Wininger D, Schulz T, Noggle S, Venable A, Lyons I, Robins A, Stice S. Human embryonic stem cell lines derived from discarded embryos. Stem Cells 2003; 21: 521–526 [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Yin Y, Long X, Luo Y, Jiang Y, Zhang W, Du H, Li S, Zheng Y, Li Q, Chen X, Liao B, Xiao G, Wang W, Sun X. Derivation and characterization of human embryonic stem cell lines from poor quality embryos. J Genet Genomics 2009; 36: 229–239 [DOI] [PubMed] [Google Scholar]
- 26.O'Leary T, Heindryckx B, Lierman S, Van der Jeught M, Menten B, Deforce D, Cornelissen R, de Sousa Lopes SC, De Sutter P. The influence of early embryo traits on human embryonic stem cell derivation efficiency. Stem Cells Dev 2011; 20: 785–793 [DOI] [PubMed] [Google Scholar]
- 27.Harvey MB, Kaye PL. Insulin increases the cell number of the inner cell mass and stimulates morphological development of mouse blastocysts in vitro. Development 1990; 110: 963–967 [DOI] [PubMed] [Google Scholar]
- 28.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol 2002; 172: 221–236 [DOI] [PubMed] [Google Scholar]
- 29.Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest 2010; 120: 995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development 2007; 134: 4219–4231 [DOI] [PubMed] [Google Scholar]
- 31.Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development 2008; 135: 3081–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 2010; 137: 715–724 [DOI] [PubMed] [Google Scholar]
- 33.O'Neill C. Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol Reprod 1997; 56: 229–237 [DOI] [PubMed] [Google Scholar]
- 34.Harlow GM, Quinn P. Development of preimplantation mouse embryos in vivo and in vitro. Aust J Biol Sci 1982; 35: 187–193 [DOI] [PubMed] [Google Scholar]
- 35.Chen AE, Egli D, Niakan K, Deng J, Akutsu H, Yamaki M, Cowan C, Fitz-Gerald C, Zhang K, Melton DA, Eggan K. Optimal timing of inner cell mass isolation increases the efficiency of human embryonic stem cell derivation and allows generation of sibling cell lines. Cell Stem Cell 2009; 4: 103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]