Abstract
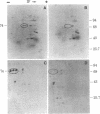
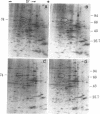
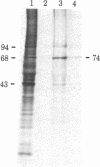
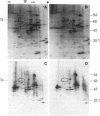
A physiologically relevant increase in body temperature from 39.7 to 42.5 degrees C, which was generated after the intravenous injection of D-lysergic acid diethylamide (LSD), caused the induction of synthesis of a 74,000-dalton heat shock protein in the brain, heart, and kidney of the young adult rabbit. A marked increase in the relative labeling of a 74,000-dalton protein was noted after analysis of both in vivo labeled proteins and cell-free translation products of isolated polysomes. A temporal decrease in the synthesis of this protein was noted as LSD-induced hyperthermia subsided. The 74,000-dalton protein, which is induced in various organs of the intact animal at a body temperature similar to that attained during fever reactions, may play a role in homeostatic control mechanisms.
Full text
PDF

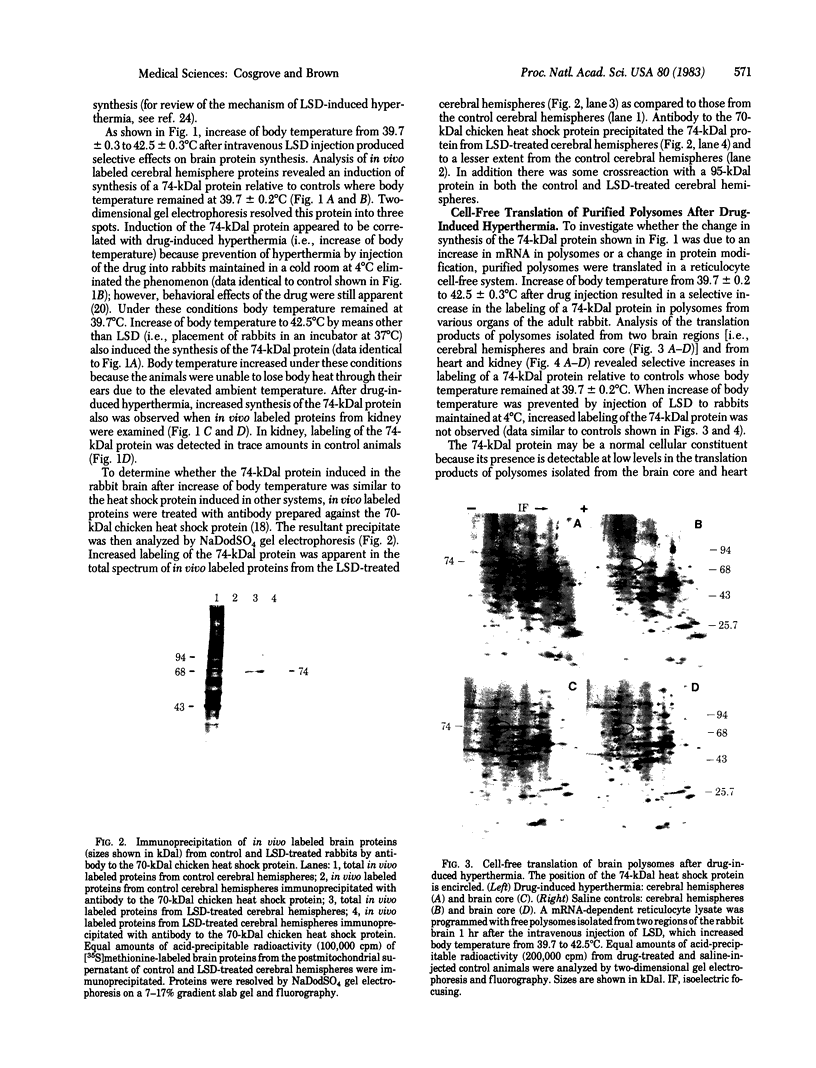

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G. Synthesis of heat-shock proteins by cells undergoing myogenesis. J Cell Biol. 1981 Jun;89(3):666–673. doi: 10.1083/jcb.89.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clark B. D., Brown I. R. Protein synthesis in the mammalian retina following the intravenous administration of LSD. Brain Res. 1982 Sep 9;247(1):97–104. doi: 10.1016/0006-8993(82)91031-9. [DOI] [PubMed] [Google Scholar]
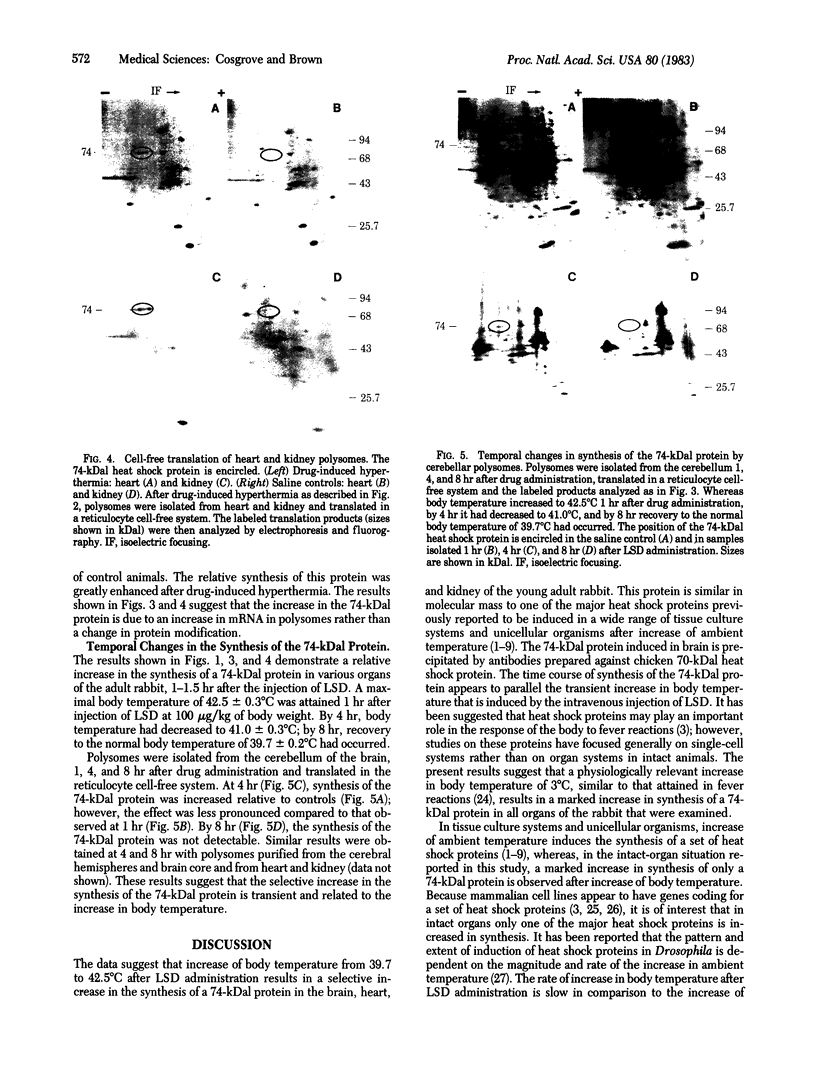
- Cosgrove J. W., Clark B. D., Brown I. R. Effect of intravenous administration of d-lysergic acid diethylamide on subsequent protein synthesis in a cell-free system derived from brain. J Neurochem. 1981 Mar;36(3):1037–1045. doi: 10.1111/j.1471-4159.1981.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Currie R. W., White F. P. Trauma-induced protein in rat tissues: a physiological role for a "heat shock" protein? Science. 1981 Oct 2;214(4516):72–73. doi: 10.1126/science.7280681. [DOI] [PubMed] [Google Scholar]
- Findly R. C., Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. doi: 10.1083/jcb.88.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. S., Clark B. D., Cruz T. F., Gurd J. W., Brown I. R. Selective effects of LSD and hyperthermia on the synthesis of synaptic proteins and glycoproteins. Brain Res. 1981 Feb 23;207(1):129–145. doi: 10.1016/0006-8993(81)90683-1. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Brown I. R. Disaggregation of brain polysomes after LSD in vivo. Involvement of LSD-induced hyperthermia. Neurochem Res. 1979 Dec;4(6):763–776. doi: 10.1007/BF00964473. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Cosgrove J. W., Brown I. R. Cell-free translation of free and membrane-bound polysomes and polyadenylated mRNA from rabbit brain following administration of d-lysergic acid diethylamide in vivo. J Neurochem. 1981 Mar;36(3):1229–1238. doi: 10.1111/j.1471-4159.1981.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Holbrook L., Brown I. R. Disaggregation of polysomes in fetal organs and maternal brain after administration of d-lysergic acid diethylamide in vivo. J Neurochem. 1979 Jun;32(6):1793–1799. doi: 10.1111/j.1471-4159.1979.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., White F. P. Cellular responses to stress: comparison of a family of 71--73-kilodalton proteins rapidly synthesized in rat tissue slices and canavanine-treated cells in culture. J Cell Physiol. 1981 Aug;108(2):261–275. doi: 10.1002/jcp.1041080216. [DOI] [PubMed] [Google Scholar]
- Holbrook L., Brown I. R. Disaggregation of brain polysomes after administration of d-lysergic acid diethylamide (LSD) in vivo. J Neurochem. 1976 Jul;27(1):77–82. doi: 10.1111/j.1471-4159.1976.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Inasi B. S., Brown I. R. Synthesis of a heat shock protein in the microvascular system of the rabbit brain following elevation of body temperature. Biochem Biophys Res Commun. 1982 Jun 15;106(3):881–887. doi: 10.1016/0006-291x(82)91793-4. [DOI] [PubMed] [Google Scholar]
- Johnston D., Oppermann H., Jackson J., Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980 Jul 25;255(14):6975–6980. [PubMed] [Google Scholar]
- Kelley P. M., Aliperti G., Schlesinger M. J. In vitro synthesis of heat-shock proteins by mRNAs from chicken embryo fibroblasts. J Biol Chem. 1980 Apr 25;255(8):3230–3233. [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Kluger M. J., Gonzalez R. R., Stolwijk J. A. Temperature regulation in the exercising rabbit. Am J Physiol. 1973 Jan;224(1):130–135. doi: 10.1152/ajplegacy.1973.224.1.130. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Heat-shock proteins of Drosophila are associated with nuclease-resistant, high-salt-resistant nuclear structures. J Cell Biol. 1981 Sep;90(3):793–796. doi: 10.1083/jcb.90.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Mikelens P., Oppermann H., Jackson J. Effect of antabuse (disulfiram) on Rous sarcoma virus and on eukaryotic cells. Biochim Biophys Acta. 1978 Jun 22;519(1):65–75. doi: 10.1016/0005-2787(78)90062-x. [DOI] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Induction of four proteins in eukaryotic cells by kethoxal bis(thiosemicarbazone). Biochim Biophys Acta. 1978 May 23;518(3):401–412. doi: 10.1016/0005-2787(78)90159-4. [DOI] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980 Jun 15;77(2):463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. A response of protein synthesis to temperature shift in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5222–5225. doi: 10.1073/pnas.76.10.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: preparation of efficient protein synthesis lysates and the purification and characterization of the heme-regulated translational inhibitory protein kinase. Methods Enzymol. 1979;60:459–484. doi: 10.1016/s0076-6879(79)60045-9. [DOI] [PubMed] [Google Scholar]
- Sinibaldi R. M., Morris P. W. Putative function of Drosophila melanogaster heat shock proteins in the nucleoskeleton. J Biol Chem. 1981 Nov 10;256(21):10735–10738. [PubMed] [Google Scholar]
- Walsh C. Appearance of heat shock proteins during the induction of multiple flagella in Naegleria gruberi. J Biol Chem. 1980 Apr 10;255(7):2629–2632. [PubMed] [Google Scholar]
- Wang C., Gomer R. H., Lazarides E. Heat shock proteins are methylated in avian and mammalian cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3531–3535. doi: 10.1073/pnas.78.6.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. P. Differences in protein synthesized in vivo and in vitro by cells associated with the cerebral microvasculature. A protein synthesized in response to trauma? Neuroscience. 1980;5(10):1793–1799. doi: 10.1016/0306-4522(80)90096-2. [DOI] [PubMed] [Google Scholar]
- White F. P. The induction of "stress" proteins in organ slices from brain, heart, and lung as a function of postnatal development. J Neurosci. 1981 Nov;1(11):1312–1319. doi: 10.1523/JNEUROSCI.01-11-01312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]