Abstract
Social and genetic factors can influence smoking behavior. Using olfactogustatory stimuli as the sensory cue for intravenous nicotine self-administration (SA), we previously showed that social learning of nicotine contingent odor cue prevented rats from developing conditioned taste aversion and allowed them to instead establish stable nicotine self-administration. We hypothesized that genetic factors influenced socially acquired nicotine SA. A heterogenous stock (HS; N/NIH) of outbred rats was trained to self-administer nicotine using the social learning protocol. Both male and female HS rats acquired nicotine self-administration, but females self-administered more nicotine than males. After extinction, the context previously paired with nicotine self-administration, in conjunction with socially-transmitted drug cues, were sufficient to cause reinstatement of drug-seeking behavior. Wide variation in both nicotine intake and reinstatement were observed. Using multiple regression analysis, we found measures of social interaction were significant predictors of nicotine intake and reinstatement of drug-seeking in both males and females. Furthermore, measures of depression were predictors of nicotine intake in both males and females while anxiety was a predictor only in males and response to novelty was a predictor only in females. In males, measures of both depression and anxiety predicted nicotine reinstatement. Together, these data supported the ideas that genetically determined propensities for emotional and social phenotypes are significant determinants for nicotine reinforced behavior, and that the heterogeneous stock rat is a suitable tool for dissecting genetic mechanisms that may underlie the interaction between social behavior, anxiety, depression and smoking.
Keywords: Nicotine, outbred, rats, self-administration, social learning
Introduction
Cigarette smoking is one of the leading preventable causes of death worldwide; smoking results in approximately five million deaths per year (Mathers & Loncar, 2006). Many aspects of smoking behavior are influenced by genetic factors (Carmelli et al., 1992, Pergadia et al., 2006). Overall, the heritability of smoking is estimated to be 0.4–0.6 (Hall et al., 2002, Li et al., 2003). Genome-wide studies have found that variants of many genes, such as the CHRNA5-CHRNA3-CHRNB4 cluster (Chen et al., 2012b, Saccone et al., 2009, Thorgeirsson et al., 2008, 2010), CYP2A6 (Siedlinski et al., 2011, Thorgeirsson et al., 2010), and brain-derived neurotrophic factor (2010) contribute to smoking behavior (e.g. cigarettes per day, nicotine dependence, cessation). However, these polymorphisms account for a small amount of the heritable variance in smoking behaviors (<1%), suggesting that most of the genetic factors determining smoking behavior are still unknown (Thorgeirsson et al., 2008, 2010).
Compared to human studies, rodent models can better control both environmental and genetic factors. Many aspects of cigarette smoking have been modeled using nicotine self-administration (SA) in rats, such as blood nicotine levels, dose responses, drug dependence, withdrawal, relapse, and response to pharmacological intervention, etc. (Le Foll & Goldberg, 2006, Matta et al., 2007, O'dell & Khroyan, 2009). The effect of genetic factors on nicotine SA has also been shown. For example, large variation in nicotine intake was found across twelve isogenic strains of rats, with heritability estimated to be 0.64 (Chen et al., 2012a), supporting the idea that rats are suitable tools for studying the genetics of smoking-related behaviors.
Social environment is also critical for smoking. In fact, peer smoking is one of the strongest predictors of smoking initiation (Greenlund et al., 1997, White et al., 2008). Using olfactogustatory stimuli as the contingent sensory cue for intravenous nicotine delivery, we demonstrated the role of social learning in nicotine SA (Chen et al., 2011). In this model, a rat self-administered nicotine when accompanied by a demonstrator rat. These two rats were separated by a divider that allowed orofacial interaction. Licking was used as the operant behavior to contingently deliver olfactogustatory cue and nicotine. Stable nicotine SA was established when the demonstrator rat had access to the same olfactogustatory cue as the SA rat. In contrast, conditioned taste aversion was developed when the olfactogustatory cue was withheld from the demonstrator rat.
Our long-term goal is to study the hypothesis that genetically determined propensity for social interaction predicts nicotine reinforced behaviors. In an effort to lay the groundwork for such studies, we examined the nicotine self-administration of a population of heterogeneous stock (HS) rats (official designation: N/NIH) using the social learning protocol. The HS rat was derived from eight inbred founder strains (Hansen & Spuhler, 1984). After more than 60 generations of rotational outbreeding, each HS rat represents a unique random mosaic of the founders (Mott et al., 2000); these rats represent the most highly recombinant rat intercross available. We also assessed novelty exploration, anxiety, depression, and social interaction phenotypes in each rat and used multiple regression to identify phenotypes that predicted nicotine intake and reinstatement of drug-seeking behavior.
Materials and Methods
Animals
Heterogeneous stock (HS) rats (52 males and 48 females) were transferred from Medical College of Wisconsin (NMcwi:HS) to University of Tennessee Health Science Center. After quarantine, these rats were given at least 7 days of acclimation to a reversed 12:12 h light–dark cycle (lights off at 8:30 AM). Eight adolescent Sprague-Dawley rats (4 for each sex) (Harlan Laboratories, Madison, WI) were used as the stimulus rats in the social interaction test. An additional 30 female and 30 male Sprague-Dawley rats were used as demonstrators in the nicotine self-administration experiment. Standard rat chow and water were provided ad libitum. All rats were group housed with 2–4 same sex peers throughout the experiments to avoid social isolation. All procedures were conducted in accordance with the NIH Guidelines concerning the Care and Use of Laboratory Animals, as approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center.
Study Design
All HS rats were young adults (55–65 days old) when tests began. Each HS rat was first tested in a series of behavioral tests, one test per day, conducted in the dark-phase of the light cycle (9AM – 4PM). These tests were conducted in the following sequence: open field test, novel object interaction test, social interaction test, tail suspension test 1 and tail suspension test 2. Average body weights at the beginning of the experiment were 201.4 ± 3.4 g for females and 312.4 ± 4.7 g for males. The first three tests were conducted in the same open field and recorded using the same video capture system. The sequence and procedures of these tests were adapted from two protocols (Green et al., 2012, Vetter-O'hagen & Spear, 2012).
Open field test
Two open field test chambers constructed using black acrylic glass, measuring 100 cm (l) × 100 cm (w) × 50 cm (h), were placed side by side. The floors were painted with black or white acrylic paint (ART-Alternatives, ASTM D-4236, Emeryville, CA, USA) to contrast the coat of the animals. The test chambers were illuminated by a long-range, 850-nm infrared illuminator (LIR850-70, LDP LLC, Carlstadt, NJ) located 160 cm above the center of the two test chambers. No source of visible light was present during behavioral testing with the exception of a flat panel monitor (Dell 1908FP). A digital camera (Panasonic WV-BP334) fitted with an 830 nm infrared filter (X-Nite830-M37, LTP LLC, Carlstadt, NJ) located next to the infrared light source was used to record the behavior of the rats. All rats were released at the same corner of the test chamber and data were collected for 1 h on day 1 and 20 min on day 2 (novel object) and day 3 (social interaction). Ethovision® XT video tracking system (Version 4.0, Noldus Information Technology, The Netherlands) was used to obtain the total distance traveled and the duration the animal was present in the center of the test field (a circular region with a diameter of 20 cm). Distance traveled in the center zone was determined by analyzing exported data using the R statistical language.
Novel object test
The novel object was a cylindrical cage constructed using 24 aluminum rods (30 cm in length) that were spaced 1.7 cm apart. The bottom and top of the cage (15 cm in diameter) were manufactured using Makerbot Replicator 2 (Makerbot Industries, Brooklyn, NY). The design can be downloaded from http://www.thingiverse.com/haochen/designs. One novel object was placed into the center of each open field before testing. The duration and distance traveled by the HS rat in the center zone, where the novel object was located, were used as an index of the novelty seeking phenotype. Other aspects of the test were the same as open field test with the exception that behavior was only recorded for 20 min.
Social interaction test
A randomly selected stimulus Sprague-Dawley rat of the same sex as the HS test rat was placed into the cylindrical cage (described above) before the test rat was placed into the open field. The stimulus and test rats were never housed together and thus were unfamiliar to each other. No social isolation was conducted on either rat. Each stimulus rat was used once per day. The test duration was 20 min. The duration and distance traveled in the center zone by the HS rats, where the stimulus rat was located, were used as an index of social interaction. Other aspects of the test were the same as the open field test.
Elevated plus maze test
The elevated plus-maze was constructed using black acrylic glass. The platform was 60 cm above the floor, with all four arms measuring 12 cm (W) × 50 cm (L). The two opposing closed arms had walls measuring 30 cm (H). Rats were placed into the center of the maze facing the closed arm. The behavior of the rat was recorded for 6 min using the digital video system described above. Ethovision was used to extract the time rats spent in each arm. The percent of time spent on the two open arms was then obtained for each rat (Carobrez & Bertoglio, 2005) and were used as a measure of anxiety.
Tail suspension test
Each rat was hung upside-down by an adhesive tape, approximately 2 cm from the tip of the tail, to a vertical surface. The position was adjusted so that the rat’s forepaws could lightly touch a sturdy platform and thus minimize the weight sustained by its tail (Hinojosa et al., 2006). The rat was illuminated by backscatter infrared light. The behavior of the rat was recorded for 6 min. Ethovision was used to obtain the time the rat was immobile, by setting the threshold at 5% pixel change. Data obtained using this threshold was in agreement with those provided by a trained technician. Immobile time was used as an index of depression. Rats were tested twice on two consecutive days.
Nicotine self-administration in social context
The nicotine self-administration procedure in social context was conducted according to our published protocol (Chen et al., 2011). An illustration of the operant chamber setup was provided in Fig. 1. Briefly, jugular catheters (Micro-Renathane® tubing, Braintree Scientific Inc., Braintree, MA) were implanted under isoflurane anesthesia. Ketoprofen (2 mg/kg, s.c.) was given for postoperative analgesia. After 3 days of recovery, rats were given access to nicotine SA 3 h/d for 12 days in the dark-phase of the light cycle. Each operant chamber (Med Associates, St Albans, VT) contained a perforated divider located in the middle that separated the SA rat and a randomly selected demonstrator to each side. The demonstrator and the SA rat were always the same sex. Social interaction by orofacial contact through six holes in the divider was monitored by two sets of infrared sensors embedded in the divider, one for each rat. Two drinking spouts were fitted on the same wall of the operant chamber for the SA rat. Each spout connected to a contact lickometer controller allowing the number and timing of licks to be recorded. A solution of saccharin (0.4%) and unsweetened grape Kool-Aid (0.1%) was used as olfactogustatory (OG) cue. SA was conducted using a fixed-ratio 10 (FR 10) schedule with 20 s timeout. Thus, 10 licks on the active spouts elicited a computer driven injection of 60 µl OG cue to the active spout and nicotine (30 µg/kg, free base) via the jugular vein. Using a syringe pump to deliver the OG cue ensured the contingency of the OG cue with nicotine. The inactive spout did not contain any liquid and licks on the inactive spout produced no programmed consequence. No prior food or water deprivation, operant training or priming nicotine injections, which could potentially affect the motivation for operant behaviors, were conducted. The patency of the jugular catheters was tested using a fast acting anesthetic Methohexital (0.2 ml, 10 mg/ml) at the end of 12 SA sessions for each rat. Three female rats had blocked jugular catheter. Data from these rats were excluded from the analysis. Our previous data showed that in the absence of the demonstrators, rats developed conditioned aversion to the appetitive OG cue, but SA was established with the presence of the demonstrators who consumed the same OG cue (Chen et al., 2011). Therefore, OG cue was also provided to the demonstrator rats used in this experiment. Demonstrators were not subjected to nicotine infusion.
Fig. 1.
Illustration of the operant chamber. Each operant chamber contained a perforated divider that separated the self-administration and demonstrator rat to each side. Social interaction by orofacial contact through the divider was monitored by embedded sets of infrared sensors. Two drinking spouts on the self-administration side were connected to lickometer controllers that recorded the licks. Licks on the active spout meeting fixed-ratio 10 schedule triggered concurrent delivery of olfactogustatory cue and intravenous nicotine. The demonstrator had unrestricted access to the same olfactogustatory cue but did not receive nicotine.
Context induced reinstatement of drug-seeking
Context extinction and reinstatement procedures were performed after the SA sessions. Context extinction chambers were different from SA operant chamber in many aspects, including the absence of the divider, flat floor, distinct audiovisual cues and novel odor (apple cinnamon) (Xie et al., 2012). There were two clean dry spouts in the chamber and the number of licks were recorded but had no programmed consequence. The self-administering rats were placed in the extinction environment for 1 h/d, until the lick on the “active” spout was reduced to less than 50 for two consecutive sessions.
Reinstatement of drug-seeking behavior was tested the day after the last context extinction session. During the 1 h reinstatement tests, rats were placed in the original nicotine SA chamber. The demonstrator rats were present and were provided with the OG cue because social environment is one of the strongest components of the complex contextual cues that induces craving for cigarettes (Garcia-Rodriguez et al., 2011). Licking on neither spout had programmed consequence. A demonstrator rat was provided with the OG cue during reinstatement.
Lick microstructure analysis
The microstructure of the licks on the active spout was analyzed to examine potential sex difference in the appetiveness of the OG cue. We focused on the size of lick clusters because previous studies showed it corresponded to the appetitiveness of the solution being consumed (Boughter et al., 2007, Dwyer, 2008) and was not affected by satiety (De Araujo et al., 2006). We only analyzed the data from the first day of SA because the licks were less likely affected by nicotine. Licks with interlick intervals smaller than 0.5 s were treated as one cluster. The size of the cluster was defined as the number of licks it contained. Clusters with less than 2 licks were excluded from the analysis.
Statistical analysis
Data were presented as mean ± SD. Number of licks, infusions and nose pokes were analyzed by repeated-measures ANOVA with spout and day treated as within-subject variables. Paired t-tests were used to compare the number of licks on the two spouts during reinstatement. T-tests were used to compare the sex differences on the means shown in the Supplemental Table. Statistical significance was assigned when p < 0.05. All statistical analyses were conducted using R statistical package. Because the large number of behavioral phenotypes we measured and the correlation between some of these phenotypes, we used principal component analysis (PCA) to obtain orthogonal components, where were then tested as predictors for nicotine intake in a multiple regression analysis. The “principal” function available from the psych package was used for the PCA. The components were rotated using the varimax function. The “lm” and “step” functions were used for multiple regression. Procedures used were described in the results section.
Results
Socially-acquired nicotine self-administration
HS rats were subjected to daily 3-h nicotine SA sessions for 12 days. Licking on the active spout triggered the concurrent delivery of i.v. nicotine (30 µg/kg/infusion, free base) and 60 µl OG cue controlled by a FR10 schedule. Each SA rat was accompanied by a demonstrator rat randomly selected before each session. The demonstrator had unrestricted access to the OG cue but did not receive nicotine. Female demonstrators consumed significantly more OG cue than the male demonstrators (8571.0 ± 240.1 vs. 2966.0 ± 88.4 licks per session, respectively, p = 4.3e-29). The number of nose pokes emitted by the demonstrator and the SA rats were moderately correlated (Fig. 2, r = 0.53 for females and 0.65 for males, ps = 2.2e-16), suggesting the social nature of the nose poke behavior. Male demonstrators emitted more nose pokes than females did (Supplemental Table. 473.0 ± 45.4 vs. 302.6 ± 24.4 per session, respectively, F1,93=10.5, p = 0.002). Both female and male HS rats preferred the active spout compared to the inactive spout (Fig. 3, n = 45, F1,43 = 27.0, p = 5.3e-06 for females and n = 52, F1,50 = 6.2, p = 0.02 for males). The ratio of active/inactive licks during the last three SA days was 20.5 ± 2.0 and 2.1 ± 0.1 for females and males, respectively. The stronger preference to the active spout in the females was mostly attributable to their higher active licks (201.7 ± 42.1 by females vs. 39.8 ± 8.1 by males, F1,93 = 31.2, p = 2.3e-07), although the amount of inactive licks was also higher (F1,93 = 7.1, p = 0.01) in females (43.0 ± 9.1) compared to males (27.0 ± 5.2). Consequently, females also self-administered significantly more nicotine than males (F1,93 = 33.0, p = 1.2e-07). The sex difference in nicotine intake was not a result of differential subjective experience (i.e. the appetitiveness) of the OG cue because the size of lick cluster on day 1 (when responding was mostly for the OG) were not statistically different (p > 0.05) between males (6.3 ± 0.6) and females (7.8 ± 0.8). Within each sex, there was also large variation in nicotine intake (Fig. 4). The range of total nicotine infusions were (10 – 214) and (1 – 94) for females and males, respectively. The coefficient of variation was 0.67 and 0.85 for females and males, respectively.
Fig. 2.
The social nature of nose poking into the divider. Heterogenous stock rats were subjected to 12 sessions of nicotine self-administration with concurrent olfactogustatory cue in the presence of demonstrator rats that had access to the same olfactogustatory cue. The number of nose pokes emitted by the dyads were significantly correlated in both females (r=0.53, p < 0.001) and males (r=0.65, p < 0.001), suggesting nose poking is a social behavior in this setting.
Fig. 3.
Socially-acquired nicotine self-administration in heterogenous stock rats. Both males and females preferred the active spout compared to the inactive spout. The ratio of active / inactive licks during the last three self-administration sessions was 20.5 ± 2.0 for females and 2.1 ± 0.1 for males. Females self-administered significantly more nicotine than males (p < 0.001). ***: p<0.001, *: p<0.05.
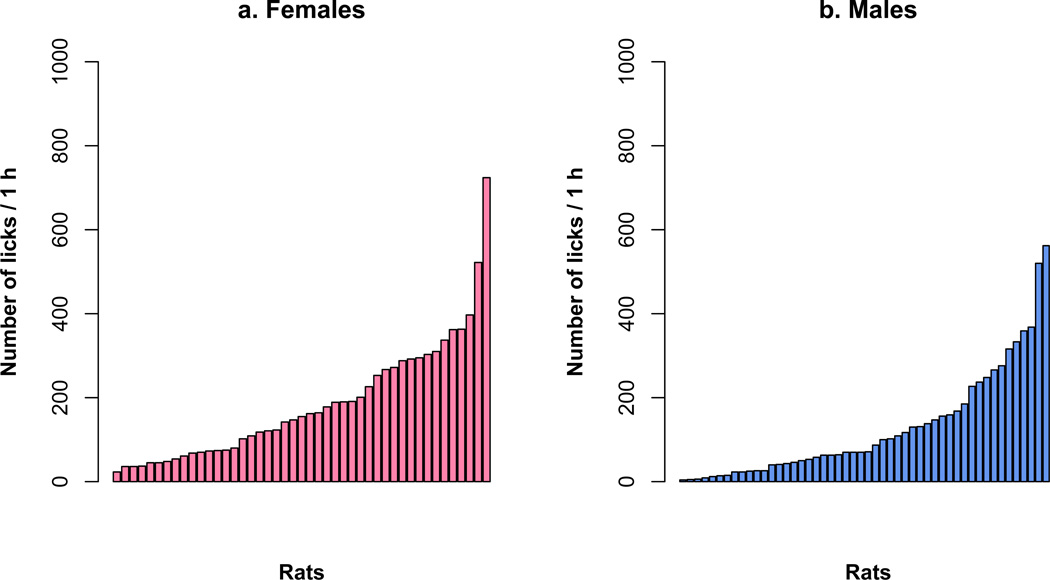
Fig. 4.
Variation of nicotine intake in heterogenous stock rats. The number of total nicotine infusions self-administered during the 12 sessions by individual rats was plotted in ascending order. The range was (10 – 214) for females and (1 – 94) for males.
Multiple regression for nicotine intake
We measured several behaviors in each rat that could potentially contribute to their SA behavior. The mean and SEM of these variables for males and females were provided in the Supplemental Table. Three of these tests, open field exploration, novel object interaction, and social interaction were tested in the same open field. For each of these tests, we obtained data on total distance traveled, distance traveled in the center of the arena, and time spent in the arena center. We also calculated percent time spent in the open arms of an elevated plus maze and immobile time during two daily tail suspension tests. Other data collected during the SA sessions included the number of licks emitted by the demonstrators and the number of nose pokes into the center divider by both the demonstrator and SA rats (during the first 3 sessions and during the 12 sessions). The results of these measurements were also shown in the Supplemental Table. Because some of these variables were highly correlated with each other, we applied principal component analysis and selected the top 8 principal components (PC) that accounted for > 80% of the variance contained among these 18 variables. These 8 PCs were derived by linear combination of the standardized original variables. The weights of the standardized original variables (i.e. loading) were shown in Fig. 5A. All PCs had strong loading (> 0.8) with one or several variables, and biologically related variables had similar loadings on the PCs. For example, nose pokes by the demonstrators and self-administrators both had strong loading on PC1, while open field center duration and center distance both had strong loading on PC2. As a result, the biological meaning of these PCs all could be readily interpreted.
Fig. 5.
Principle component analysis of the predictor variables. The top eight principal components accounting for ~80% of the variance in the dataset was selected as predictors in subsequent multiple linear regression. The loading of the PCs with the original variables were shown for (A) self-administration and (B) reinstatement. Most of the variables used for reinstatement were the same as self-administration, with the exception that data for the first 3 days, critical for self-administration, was replaced by data obtained during reinstatement. The size of the circles corresponded to the loading.
We then used multiple linear regression to analyze the relationship between these PCs with the number of nicotine infusions. Because curvilinear trends were observed, quadratic terms were included. A backward stepwise regression procedure was used to examine the relationships between the PCs and nicotine intake. Because the large sex difference in nicotine intake, males and females were analyzed separately. The final models were shown in Table 1. The relationships between nicotine intake and PC1, PC3, PC5, PC7 and PC8 were statistically significant in males. PC 1 had strong loading with social interaction in the operant chamber, while PC 3 and PC8 had high loading on social interaction in the open field; PC5 loaded with immobile time in the tail suspension test, an index of depression, and PC7 loaded with percent time spent in the open arms in the elevated plus maze and total distance traveled in the open field, both related to anxiety (Fig. 5A). Together, these relationships accounted for 63.0% of the variance in nicotine intake in males. The relationships between nicotine intake and PC3, PC4, PC5, and PC6 were significant in females. These PCs corresponded to social interaction in the open field (PC3), the strength of the socially transmitted cue (i.e. the amount of OG cue consumed by the demonstrators) (PC4), depression (PC5), novel object interaction (PC6). Together, these relationships account for 35.6% of the variance in nicotine intake in females.
Table 1.
Multiple linear regression model of nicotine intake in males and females.
| Loading | Coefficient | p | |
|---|---|---|---|
| Total infusion, male | |||
| PC1 | Social interaction during SA | 13.124 | 0.0003 |
| PC1^2 | −4.126 | 0.0260 | |
| PC3 | Social interaction in open field | 10.702 | 0.0001 |
| PC3^2 | 7.445 | 0.0012 | |
| PC5 | Depression | −8.296 | 0.0016 |
| PC7 | Anxiety | −5.472 | 0.0154 |
| PC8 | 10.194 | 0.0007 | |
| Total infusion, female | |||
| PC3^2 | Social interaction in open field | −9.111 | 0.0397 |
| PC4 | Socially transmitted nicotine cue | −100.307 | 0.0095 |
| PC4^2 | 47.238 | 0.0029 | |
| PC5^2 | Depression | −9.733 | 0.0200 |
| PC6^2 | Novelty | −10.289 | 0.0352 |
The top eight principal components accounting for > 80% of the variance in 18 predictor variables were used for regression. These relationships accounted for 63.0% and 35.6% of the variance in nicotine intake in males and females, respectively.
Context induced reinstatement
After 12 days of nicotine SA, rats were placed in a different environment (located in a different room, had distinct floor, audiovisual cue, and odor) 1 h per day to extinguish their licking on the active spout. Rats reached extinction criteria when they emitted less than 50 licks during two consecutive extinction sessions. Most rats met these criteria in less than 5 sessions. Nicotine seeking behavior was then tested in the original nicotine SA environment for 1 h, with the presence of a demonstrator rat that had access to the OG cue. The number of licks on the previously active spout (referred to as active spout) during the reinstatement session was significantly higher than that emitted during extinction (Fig. 6, p = 2.7e-10 for females and p = 5.5e-8 for males), indicating exposure to a drug taking environment (containing social cue) induced reinstatement of drug seeking behavior. The licks were also specific for the active spout compared to the inactive spout (p = 5.0e-11 for females and p = 4.0e-7 for males). Similar to SA, females also licked more on the active spout than males did during reinstatement (p = 0.049). The range of licks on the active spout were (23–724) for females and (4–562) for males (Fig. 7). The coefficient of variation for the number of active licks was 0.78 for females and 1.1 for males.
Fig. 6.
Context induced reinstatement of drug-seeking behavior. After 12 days of nicotine self-administration, rats were placed in a new environment to extinguish their operant licking behavior. Nicotine seeking behavior was then tested in the original nicotine SA environment, with the presence of a demonstrator rat that carried nicotine associated olfactogustatory cue. The number of licks on the previously active spout during reinstatement was significantly higher than that emitted during extinction in both sexes. Females emitted more licks than males during reinstatement (p<0.05). Multiple regression analysis found social interaction during reinstatement was a significant predictor of drug seeking behavior (see Table 2). ***p<0.001 for active vs. inactive licks; ###: p<0.001, active licks, extinction vs. reinstatement.
Fig. 7.
Variation of context induced reinstatement of drug-seeking behavior. The number of licks on the previously active spout during reinstatement emitted by each rat was plotted in ascending order. The range was (23–724) for females and (4–562) for males.
Multiple regression for Context induced reinstatement
We reapplied the principal component analysis of the predictors by including data obtained from the demonstrators during the reinstatement session and removed the corresponding summary data for the first 3 sessions. The new PCs and their loadings were shown in Fig. 5B. The top eight PCs accounted for > 80% of the variance were used in subsequent multiple regression analysis. The relationship between the number of active licks and PC2, PC3, PC5, PC7 and PC8 were significant in males (Table 3). These PCs corresponded to social interaction in open field (PC2), during SA (PC8) and reinstatement (PC5) as well as anxiety (PC7) and depression (PC3). These relationships account for 45.4% of the variance in the reinstatement data in males. In females, the number of licks on active spout during reinstatement was only related to PC5, which had strong loading with social interaction during reinstatement. This relationship accounted for 10.5% of the variance in the reinstatement data.
Discussion
We studied socially-acquired nicotine SA in outbred HS rats. Both males and females acquired nicotine SA; females self-administered more nicotine than males. After drug-seeking behavior was extinguished in a new environment, the original drug taking environment, which contained drug-related social cues, triggered reinstatement of drug-seeking behavior. Large variation in both nicotine intake and reinstatement were shown; these differences may be due to the genetic heterogeneity of the rats. Multiple regression analysis found measures corresponding to social interaction were significant predictors of nicotine intake and reinstatement of drug-seeking; measures of depression (i.e. immobile time) were predictors of nicotine intake in both males and females while measures of anxiety (i.e. time spent in the open arms and total distance traveled in the open field) was a predictor only in males and response to novelty was a predictor only in females. In males, both depression and anxiety related measures also predicted nicotine reinstatement. Large portions (10.5 – 63.0%) of the variations in nicotine intake and drug-seeking were explained by these phenotypes.
The progression from initial experimentation with cigarettes to regular smoking is perplexing because most first time smokers experience little positive experience. Instead, negative effects, such as nausea, dizziness, coughing, and headache are much more common (Eissenberg & Balster, 2000, Hahn et al., 1990). In spite of these negative effects, ~40% of teenagers that experiment with cigarettes become regular smokers (Hofstetter et al., 2007). One critical factor that has large influence on smoking initiation is social environment (Hu et al., 2006, Schepis & Rao, 2005, White et al., 2008). In the socially-acquired nicotine SA model, social transmission of the nicotine-associated odor cue enabled the transition from nicotine conditioned taste aversion to stable self-administration (Chen et al., 2011). This model thus captured two critical aspects of human smoking behavior: the initial aversive response to nicotine and the critical role of the social environment and thus offered a unique opportunity to identity the complex interaction between aversion and reward (both nicotine and social) in smoking behavior.
Genetic factors also strongly influence smoking behavior (Hall et al., 2002). This study used HS rats to investigate the potential effect of genetic factors on socially-acquired nicotine SA. The HS rats are the result of > 60 generations of intercrossing of 8 inbred rat strains. The large number of accumulated recombinations has enabled many successful genetic studies (Baud et al., 2013, Solberg Woods et al., 2010, Solberg Woods et al., 2012). HS rats acquired stable nicotine SA in the presence of a demonstrator rat carrying nicotine-associated odor cue. This was shown by significant preference to the active spout over the inactive spout (Fig. 3). We have previously shown that rats developed conditioned aversion to the OG cue in the absence of socially-transmitted nicotine-associated odor cue. One indication of this aversion was that SA rats avoided the active spout and thus more licks were emitted on the inactive spout (Chen et al., 2011). Thus, significantly higher number of licks on the active spout suggested the reversal of conditioned nicotine aversion after social learning and the establishment of nicotine SA.
Our previous data provided several lines of evidence that the active licks were specific for nicotine, rather than for the OG cue. For example, we reported an inverted U-shaped nicotine dose response curve in Sprague-Dawley rats (Chen et al., 2011). We also reported that the amount of nicotine intake was not affected by removing the OG cue in rats that had already acquired nicotine SA, but mecamylamine, a nicotinic receptor antagonist, reduced nicotine self-administration (Chen et al., 2011). Further, replacing saccharin (sweet taste) with quinine (bitter) did not affect the amount of nicotine intake (Chen, 2012). Last, we found that the number of active licks increased by a factor of 2 when nicotine dose was decreased by a factor of 2 (Chen, unpublished observations); this observation was made using some of the same inbred strains that are the founders for the HS. Taken together, these data strongly suggest that the active licks that we observed were specific for nicotine.
Social interaction is also a reward, which can interact with drug reward (Yates et al., 2013). Therefore, social interaction per se might support nicotine SA. For example, low dose of nicotine interacted with social reward to enhance conditioned place preference (Thiel et al., 2009). However, in our model, interaction with a demonstrator rat consuming the taste solution without the odor cue was not sufficient to support nicotine SA (Chen et al., 2011). This potentially could be due to less reward is provided by social interaction when a divider is in place than when the social interaction is not restricted by a divider. However, our previous data showed that providing the demonstrator rats with nicotine associated OG cue supported stable nicotine intake using the same setup (Chen et al., 2011), indicating that social learning, rather than social reward, is critical for inducing nicotine SA in our model.
We found female HS rats self-administered more nicotine than males. One potential source of sex difference in nicotine intake was the appetitiveness of the nicotine associated sensory cue. Rats emit licks in clusters (i.e. bouts of licks with less than 0.5 second between licks). The number of licks contained in these clusters reflects the subjective value of the taste (Boughter et al., 2007, Dwyer, 2008), with appetitive tastes associated with larger lick clusters. We analyzed the licks emitted by the HS rats on their first day of SA and found the size of the clusters produced by males and females were not statistically different. Thus, the affective value of the sensory cue was unlikely a source of differential nicotine intake. Another potential source of sex difference was differential social learning. Similar to studies reported by others (Valenstein et al., 1967), female demonstrators consumed more saccharine than males did. As a result, stronger social cues could have been presented to the female SA rats. On the other hand, male demonstrators initiated more social interaction by nose poking into the divider and spent more time in the center zone of the open field, where the stimulus rat was placed (Supplemental Table). The social nature of these nose pokes was shown by the correlation between the demonstrators and the SA rats (Fig. 2). This could potentially enhance social learning in males. Therefore, it was possible that different aspects of the demonstrator rats’ behavior (i.e. consuming the OG cue vs. initiating social contact) could contribute to the sex difference. Lastly, the interaction between age and sex could also contribute to the sex difference in nicotine intake. We have previously shown in Sprague-Dawley adolescent rats that males and females self-administered same amount of nicotine (Chen et al., 2011). It is thus possible that the sex difference we found in HS rats was due to reduced social learning capability in adult male HS rats compared to females, assuming HS rats and Sprague-Dawley rats were similar.
We identified many relationships between nicotine intake and phenotypes related to social interaction, novelty exploration, anxiety, and depression (Table 1). Genetic links between smoking and these phenotypes have been suggested by many clinical studies. For example, polymorphism in DRD4 was associated with both novelty seeking (Munafo et al., 2008) and nicotine dependence (Munafo et al., 2004), and OPRM1 A118G polymorphism was associated with response to depression treatment (Cooper et al., 2013) and smoking behavior (Verhagen et al., 2012). Our analysis work flow first used principal component analysis to obtain orthogonal PCs from the predictor variables, which then were used for regression analysis. This avoided the correlation between the predictor variables from causing erratic estimates of the regression coefficients. We measured two social behaviors, the number of nose poke through the divider during nicotine SA and time spent in proximity to the stimulus rat in an open field. These two variables were loaded onto different PCs. This was potentially because different aspects of social interaction were involved during these tests. For example, the divider only allows orofacial contact, while anogenital sniffing was also allowed in the open field. These analytical methods are more widely used in genomics studies, where having numerous measures with complex interactions are more common. We believe this is a more efficient method for analyzing large data sets compared to studying one correlation at a time.
Relapse, the most challenging part of addiction therapy, is often modeled using reinstatement of drug-seeking tests (Shaham et al., 2003). It has been well established that extinguished nicotine-seeking behavior can be reinstated by nicotine associated discrete (Carroll et al., 2009) or contextual (Diergaarde et al., 2008) cues. After SA training, we placed rats in a new environment to extinguish their operant licking behavior. Rats were then placed in the original drug-taking environment, which contained the socially-transmitted nicotine cue. Because neither nicotine nor the OG cue was provided during this test, the spout specific licking behavior most likely represented drug-seeking. Our data showed that the drug-taking context induced robust nicotine-seeking in both males and females. Anxiety and depression related measures were significant predictors of drug-seeking in males but not in females. This could potentially be due to higher level of anxiety and depression in males than females in the HS rats (Lopez-Aumatell et al., 2008) and shown in the Supplemental Table. Regression analysis showed that social interaction during reinstatement was a significant factor determining reinstatement in both males and females (Table 2). Future studies will aim to isolate the effect of social cue from the overall environmental cues.
Table 2.
Multiple linear regression model of reinstatement of drug-seeking behavior in males and females
| Loading | Coefficient | p | |
|---|---|---|---|
| Drug seeking during reinstatement, male | |||
| PC2 | Social interaction in open field | 35.132 | 0.0477 |
| PC3 | Depression | −33.895 | 0.0483 |
| PC5 | Socially transmitted nicotine cue | 65.009 | 0.0082 |
| PC5^2 | −22.781 | 0.0196 | |
| PC7 | Anxiety | −38.888 | 0.0097 |
| PC8 | Social interaction during SA | 63.732 | 0.0013 |
| Drug seeking during reinstatement, female | |||
| PC5 | Socially transmitted nicotine cue | 45.17 | 0.0704 |
| PC5^2 | −23.51 | 0.0498 |
The top eight principal components accounting for > 80% of the variance in 18 predictor variables were used for regression. These relationships accounted for 45.4% and 10.5% of the variance in nicotine intake in males and females, respectively.
Our data showed that social phenotypes were predictors of nicotine SA and nicotine-seeking behavior in HS rats. Rodents primarily use olfactory signals for social interaction. Human social interactions are accomplished by much higher cognitive capacities. Despite the vast difference in the signals used, the central processing of social signal is, to some extent, conserved. One example is the neuropeptide oxytocin, which mediates a variety of social behaviors in both humans and rodents, including social learning (Hurlemann et al., 2010, Popik & Van Ree, 1993). We reported that a specific oxytocin receptor antagonist (desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT), when injected into the lateral ventricle, reduced the amount of nicotine intake in the social learning model (Chen & Wu, 2011). These data suggest that our model has the potential to reveal other neurobiological mechanisms of social learning that underlies smoking behavior.
Because these studies were conducted in a genetically diverse population, it is reasonable to assume that the variation in nicotine intake were at least partially genetically determined. A compelling postulate that one can formulate from the above data is that genetically determined propensity for social learning contributes to the vulnerability to nicotine use and abuse. This is supported by some clinical data. For example, White et al. (White et al., 2008) conducted a prospective study on smoking involving 1409 pairs of twins and concluded that the effect of genetic factors, if invoked, may be due to its indirect effects acting through the social environment. Potential candidate genes that fit this view are also available. For example, the A118G polymorphism of the opioid receptor mu 1 (OPRM1) is associated with sensitivity to social influence (Way & Lieberman, 2010). It is also implicated in smoking initiation (Falcone et al., 2011, Verhagen et al., 2012). Based on these results we anticipate that future studies in larger cohorts of HS rats will provide powerful tool for identifying specific genetic polymorphisms that underlie smoking related social and emotional phenotypes (Parker et al., 2013).
Supplementary Material
Supplemental Table 1. Behavioral measures potentially contributing to nicotine intake and drug-seeking behavior. Open field exploration, novel object interaction, social interaction, elevated plus maze and tail suspension tests were conducted on each rat before self-administration. Data collected during the SA sessions included the number of licks emitted by the demonstrators and the number of nose pokes into the center divider by both the demonstrator and self-administration rats (for the first 3 sessions, for the 12 sessions, and during reinstatement). All data were expressed as mean ± SEM. Statistical significance of the sex difference were calculated using independent t-tests or repeated measures ANOVA (indicated by #). P values smaller than 0.05 were highlighted in bold and italicized font.
Acknowledgements
We thank Ms. Kaitlyn Hill for analyzing the lick cluster data. Funding was provided by NIDA grant DA-026894 (HC), DA021336 (AAP); and the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust, the Conte Center for Computational Neuropsychiatric Genomics (NIH P50MH94267). The maintenance of the HS rat colony is supported on grant DK-088975 (LSW).
Footnotes
Author contribution
HC, AP, and LSW were responsible for the study concept and design. TW, WH, BW and QJ contributed to the acquisition of animal data. HC, TW and WH analyzed the data and interpreted the findings. TW and WH drafted the manuscript. HC, AP and LSW provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Baud A, Hermsen R, Guryev V, Stridh P, Graham D, McBride MW, Foroud T, Calderari S, Diez M, Ockinger J, Beyeen AD, Gillett A, Abdelmagid N, Guerreiro-Cacais AO, Jagodic M, Tuncel J, Norin U, Beattie E, Huynh N, Miller WH, Koller DL, Alam I, Falak S, Osborne-Pellegrin M, Martinez-Membrives E, Canete T, Blazquez G, Vicens-Costa E, Mont-Cardona C, Diaz-Moran S, Tobena A, Hummel O, Zelenika D, Saar K, Patone G, Bauerfeind A, Bihoreau MT, Heinig M, Lee YA, Rintisch C, Schulz H, Wheeler DA, Worley KC, Muzny DM, Gibbs RA, Lathrop M, Lansu N, Toonen P, Ruzius FP, de Bruijn E, Hauser H, Adams DJ, Keane T, Atanur SS, Aitman TJ, Flicek P, Malinauskas T, Jones EY, Ekman D, Lopez-Aumatell R, Dominiczak AF, Johannesson M, Holmdahl R, Olsson T, Gauguier D, Hubner N, Fernandez-Teruel A, Cuppen E, Mott R, Flint J. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nature genetics. 2013;45:767–775. doi: 10.1038/ng.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD, Jr, Baird JP, Bryant J, St John SJ, Heck D. C57BL/6J and DBA/2J mice vary in lick rate and ingestive microstructure. Genes, brain, and behavior. 2007;6:619–627. doi: 10.1111/j.1601-183X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. The New England journal of medicine. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience and biobehavioral reviews. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug and alcohol dependence. 2009;104(Suppl 1):S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Chen H. Nicotine self-administration with an aversive olfactogustatory cue in a permissive social environment in adolescent rats. Society for neuroscience annual meeting. 2012:777.715. [Google Scholar]
- Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM. Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PloS one. 2012a;7:e44234. doi: 10.1371/journal.pone.0044234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wu Q. The effect of a permissive social environment on nicotine self-administration requires oxytocin in adolescent rats. Society for neuroscience annual meeting. 2011:686.619. [Google Scholar]
- Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, Gogarten SM, Johnson EO, Saccone NL, Wang JC, Weiss RB, Goate AM, Bierut LJ. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. The American journal of psychiatry. 2012b;169:735–742. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Rickels K, Lohoff FW. Association analysis between the A118G polymorphism in the OPRM1 gene and treatment response to venlafaxine XR in generalized anxiety disorder. Human psychopharmacology. 2013;28:258–262. doi: 10.1002/hup.2317. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, Simon SA. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, de Vries W, Raaso H, Schoffelmeer AN, De Vries TJ. Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A) Neuropharmacology. 2008;55:712–716. doi: 10.1016/j.neuropharm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Microstructural analysis of conditioned and unconditioned responses to maltodextrin. Learning & behavior. 2008;36:149–158. doi: 10.3758/lb.36.2.149. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug and alcohol dependence. 2000;59(Suppl 1):S41–S60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Falcone M, Jepson C, Sanborn P, Cappella JN, Lerman C, Strasser AA. Association of BDNF and COMT genotypes with cognitive processing of anti-smoking PSAs. Genes, brain, and behavior. 2011;10:862–867. doi: 10.1111/j.1601-183X.2011.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodriguez O, Ferrer-Garcia M, Pericot-Valverde I, Gutierrez-Maldonado J, Secades-Villa R, Carballo JL. Identifying specific cues and contexts related to smoking craving for the development of effective virtual environments. Cyberpsychology, behavior and social networking. 2011;14:91–97. doi: 10.1089/cyber.2010.0012. [DOI] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Developmental psychobiology. 2012 doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Greenlund KJ, Johnson CC, Webber LS, Berenson GS. Cigarette smoking attitudes and first use among third-through sixth-grade students: the Bogalusa Heart Study. American journal of public health. 1997;87:1345–1348. doi: 10.2105/ajph.87.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G, Charlin VL, Sussman S, Dent CW, Manzi J, Stacy AW, Flay B, Hansen WB, Burton D. Adolescents' first and most recent use situations of smokeless tobacco and cigarettes: similarities and differences. Addictive behaviors. 1990;15:439–448. doi: 10.1016/0306-4603(90)90030-2. [DOI] [PubMed] [Google Scholar]
- Hall W, Madden P, Lynskey M. The genetics of tobacco use: methods, findings and policy implications. Tobacco control. 2002;11:119–124. doi: 10.1136/tc.11.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcoholism, clinical and experimental research. 1984;8:477–479. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- Hinojosa FR, Spricigo L, Jr, Izidio GS, Bruske GR, Lopes DM, Ramos A. Evaluation of two genetic animal models in behavioral tests of anxiety and depression. Behavioural brain research. 2006;168:127–136. doi: 10.1016/j.bbr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Hofstetter CR, Hovell MF, Jung KR, Raman R, Irvin V, Ni R. The first puff: forces in smoking initiation among Californians of Korean descent. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2007;9:1277–1286. doi: 10.1080/14622200701704863. [DOI] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. American journal of public health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction (Abingdon, England) 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Aumatell R, Guitart-Masip M, Vicens-Costa E, Gimenez-Llort L, Valdar W, Johannesson M, Flint J, Tobena A, Fernandez-Teruel A. Fearfulness in a large N/Nih genetically heterogeneous rat stock: differential profiles of timidity and defensive flight in males and females. Behavioural brain research. 2008;188:41–55. doi: 10.1016/j.bbr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Mott R, Talbot CJ, Turri MG, Collins AC, Flint J. A method for fine mapping quantitative trait loci in outbred animal stocks. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12649–12654. doi: 10.1073/pnas.230304397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Yalcin B, Willis-Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biological psychiatry. 2008;63:197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacology, biochemistry, and behavior. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, Solberg Woods LC, Palmer AA. Rats are the smart choice: Rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML, Heath AC, Martin NG, Madden PA. Genetic analyses of DSM-IV nicotine withdrawal in adult twins. Psychological medicine. 2006;36:963–972. doi: 10.1017/S0033291706007495. [DOI] [PubMed] [Google Scholar]
- Popik P, Van Ree JM. Social transmission of flavored tea preferences: facilitation by a vasopressin analog and oxytocin. Behavioral and neural biology. 1993;59:63–68. doi: 10.1016/0163-1047(93)91173-k. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM, Bierut LJ. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer research. 2009;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Rao U. Epidemiology and etiology of adolescent smoking. Current opinion in pediatrics. 2005;17:607–612. doi: 10.1097/01.mop.0000176442.49743.31. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Siedlinski M, Cho MH, Bakke P, Gulsvik A, Lomas DA, Anderson W, Kong X, Rennard SI, Beaty TH, Hokanson JE, Crapo JD, Silverman EK. Genome-wide association study of smoking behaviours in patients with COPD. Thorax. 2011;66:894–902. doi: 10.1136/thoraxjnl-2011-200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl K, Tschannen M, Valdar W. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiological genomics. 2010;41:102–108. doi: 10.1152/physiolgenomics.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl KL, Oreper D, Xie Y, Tsaih SW, Valdar W. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiological genomics. 2012;44:1013–1026. doi: 10.1152/physiolgenomics.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KK, de Vegt F, Mulders PF, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den Heijer M, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De Diego V, Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nature genetics. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature genetics. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science (New York, N.Y.) 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Verhagen M, Kleinjan M, Engels RC. A systematic review of the A118G (Asn40Asp) variant of OPRM1 in relation to smoking initiation, nicotine dependence and smoking cessation. Pharmacogenomics. 2012;13:917–933. doi: 10.2217/pgs.12.76. [DOI] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behavioural brain research. 2012;227:224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Lieberman MD. Is there a genetic contribution to cultural differences? Collectivism, individualism and genetic markers of social sensitivity. Social cognitive and affective neuroscience. 2010;5:203–211. doi: 10.1093/scan/nsq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White VM, Byrnes GB, Webster B, Hopper JL. Does smoking among friends explain apparent genetic effects on current smoking in adolescence and young adulthood? British journal of cancer. 2008;98:1475–1481. doi: 10.1038/sj.bjc.6604250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Arguello AA, Reittinger AM, Wells AM, Fuchs RA. Role of nicotinic acetylcholine receptors in the effects of cocaine-paired contextual stimuli on impulsive decision making in rats. Psychopharmacology. 2012;223:271–279. doi: 10.1007/s00213-012-2715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Beckmann JS, Meyer AC, Bardo MT. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug and alcohol dependence. 2013;129:240–246. doi: 10.1016/j.drugalcdep.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Behavioral measures potentially contributing to nicotine intake and drug-seeking behavior. Open field exploration, novel object interaction, social interaction, elevated plus maze and tail suspension tests were conducted on each rat before self-administration. Data collected during the SA sessions included the number of licks emitted by the demonstrators and the number of nose pokes into the center divider by both the demonstrator and self-administration rats (for the first 3 sessions, for the 12 sessions, and during reinstatement). All data were expressed as mean ± SEM. Statistical significance of the sex difference were calculated using independent t-tests or repeated measures ANOVA (indicated by #). P values smaller than 0.05 were highlighted in bold and italicized font.