Abstract
Objectives
Estrogen and progesterone improve neurologic outcomes in experimental models of cardiac arrest and stroke. Our objective was to determine whether women of child-bearing age are more likely than men to survive to hospital discharge following in-hospital cardiac arrest.
Design
Prospective, observational study
Setting
519 hospitals in the National Registry of CPR database
Patients
95,852 men and women 15-44 years and ≥56 years with pulseless cardiac arrests from 01/01/00 through 07/31/08
Measurements and Main Results
Patients were stratified a priori by sex and age groups (15-44 years and ≥56 years). Fixed effects regression conditioning on hospital was used to examine the relationship between age, sex and survival outcomes. The unadjusted survival to discharge rate for younger women of child bearing age (15-44 years of age) was 19% (940/4887) versus 17% (1203/7025) for younger men (p=.013). The adjusted hospital discharge difference between these younger women and men was 2.8% (95% CI, 1.0%-4.6%; p=.002) and these younger women also had a 2.6% (95% CI, 0.9% - 4.3%; p=.002) absolute increase in favorable neurologic outcome. For older women compared with men (≥56 years), there were no demonstrable differences in discharge rates (18% versus 18%, adjusted difference -0.1%; 95% CI, -0.9% - +0.6%; p=.68) or favorable neurologic outcome (14% versus 14%, adjusted difference -0.1%; 95% CI, -0.7% - +0.5%; p=.74).
Conclusions
Women of child-bearing age were more likely than comparably aged men to survive to hospital discharge following in-hospital cardiac arrest, even after controlling for etiology of arrest and other important variables.
Keywords: heart arrest, cardiopulmonary resuscitation, sex, women, outcome
Introduction
During 2005, more than 20,000 in-hospital cardiac arrests were reported in the American Heart Association's (AHA) National Registry of Cardiopulmonary Resuscitation (NRCPR), a database representing approximately 10% of the hospitals in the United States. According to several studies, only 18% of adults with in-hospital arrests survived to hospital discharge.(1-3)
Survival rates may be related to sex-associated differences and possible hormonal factors.(4-11) Estrogen and progesterone have been extensively studied as neuro-protective agents in animal models of global hypoxic-ischemic injury, stroke, and traumatic brain injury.(12-19) In a pilot controlled clinical trial of progesterone administration in humans following traumatic brain injury, patients treated with progesterone were much more likely to survive to hospital discharge.(20) Accordingly, some experts have recently proposed the administration of estrogen and/or progesterone to improve outcomes from cerebral ischemic insults and cardiac arrest (CA). (21-22)
To further substantiate the postulate that female sex hormones may provide a survival advantage in CA, we compared outcomes between women and men following in-hospital CA. Considering the average onset of menarche in the United States is 13 years old(23) and the average age of menopause is 50 years old,(24) we stratified in-hospital CA victims into younger and older age categories based on a presumed differential of “female sex hormone exposure” in the younger population. It was presumed that women of child bearing age, or, specifically, those 15 to 44 years of age would have a high concentration of female sex hormone exposure relative to men. In turn, it was hypothesized that this younger cohort of women would be more likely to survive to hospital discharge than men of the same age. Conversely, we also hypothesized that survival differences between women and men would not be present for in-hospital CA victims who are ≥56 years of age considering that high concentrations of sex hormone exposure generally would no longer be an important contributing factor.
Materials and Methods
The study was conducted using the AHA NRCPR database. The study design of the NRCPR has been described in detail.(2) The NRCPR is a multicenter observational registry of in-hospital CAs using pre-defined, standardized nomenclature and dataset definitions. Data accuracy is achieved by trained data abstractors at each institution, case-study methodology, and detailed periodic re-abstraction with mean error rates for all of the data of 2.4% +/- 2.7%. The resultant database contains precisely defined variables based on Utstein-style data-reporting guidelines for CA.(25) Explicit operational definitions have been generated for every data element.
This study was approved by the institutional review board of The Children's Hospital of Philadelphia and the analysis included patients from 519 hospitals that provided data from January 1, 2000 through July 31, 2008.
All patients who experienced a pulseless CA requiring chest compressions, defibrillation, or both and eliciting a hospital-wide emergency resuscitation response resulting in the generation of a resuscitation record were eligible for inclusion. CA was defined as cessation of cardiac mechanical activity, determined by absence of central pulse, unresponsiveness and apnea. Only the index pulseless event was included for each patient. An index event was defined as the first CA event for a patient during a hospitalization.
Events were excluded if they began out-of-hospital, occurred in obstetric patients, or included defibrillation by an implantable cardioverter-defibrillator. Patients who had “do not attempt resuscitation” orders before their in-hospital CA were excluded from the registry. Patients without age, sex or survival data were excluded. If a hospital had more than 90% of CAs occurring in one sex (women or men), the patients and hospital were excluded to minimize hospital level bias on sex. This resulted in exclusion of 33 hospitals.
To minimize the risk of misclassification bias with respect to potential female sex hormone exposure, patients less than 15 years of age and 45-55 years of age were excluded from the analysis. It was judged that women in this latter age group may have variable levels of hormone exposure. Therefore, the defined exposure groups used for comparison were women 15-44 years of age, a cohort with presumed high concentrations of endogenous female sex hormones and three cohorts presumed to be without high concentrations of female sex hormones, men 15-44 years, women ≥56 years, and men ≥56 years.
The prospectively-defined primary outcome measurement was survival to hospital discharge.(25) Secondary outcomes included return of spontaneous circulation (ROSC) lasting more than 20 minutes and favorable neurologic outcome. Favorable neurologic outcome was determined using the cerebral performance category (CPC) score for patients ≥ 18 years old and the pediatric cerebral performance category (PCPC) score for patients < 18 years old.(26) Neurologic outcome was categorized as a binomial outcome: favorable (CPC 1, 2 or unchanged from admission) and unfavorable outcome (CPC 3, 4, 5 or worse than admission).(2, 27)
Unadjusted analyses were used to evaluate baseline differences between the four patient groups. Covariates and potential confounders were compared across the four populations to describe differences in distribution clustered within hospitals.
Summary statistics and analyses for the number of epinephrine doses and duration of CA were stratified by age and sex groups and are reported as median and interquartile ranges. The variables were analyzed using Cox regression (number of epinephrine doses) or linear regression (duration of arrest). Process-of-care variables were not included in the multivariable model because they occurred after the exposure of interest.
Fixed effects linear regression (conditioning on hospital) was used to examine the relationship between age and sex groups and survival outcomes to control for potential confounding by hospital. Outcomes varied across hospitals as did the age-sex groups. We used linear rather than logistic regression because the large number of observations per hospital exceeded the capabilities of conventional conditional logistic algorithms and because the expected probabilities (of outcome) were neither close to 0.0 nor 1.0. Using conditional regression, the resulting estimates of the association of age-sex categories and outcome are “within hospital” estimates, unconfounded by differences across hospitals in the age and sex of patients.(28) This methodology also controls for any other unobserved hospital characteristics that might confound the observed results by influencing the hospital-level outcome rates.
Patient-level potential confounders including a priori covariates based upon prior NRCPR publications were: race, Hispanic ethnicity, arrest time of day, arrest day of week (weekend versus weekday), first documented ECG rhythm, discovery status (witnessed and/or monitored), illness category, and event location.(29) Hispanic ethnicity was a patient self-assigned categorization obtained from chart abstraction. Illness category was assigned to describe the primary reason for hospitalization: medical vs. surgical and cardiac vs. non-cardiac. A continuous variable of the difference between a subject's age and the mean for his or her age/sex category was included in the regression model to control for any residual age confounding not already captured by category.
The association between age and sex categories and outcomes were assessed, adjusting in all cases for a priori potential confounders of interest. Additional potential confounders were introduced sequentially into each multivariable model to detect whether they influenced the coefficients of interest. If the coefficients did not change and the factor did not have a significant association with outcome, that covariate was not included in the final model. If the covariate was not a confounder but was statistically significant, we included it in the model to improve the efficiency of our estimates of association of the factors of interest and outcome. Additional covariates added to the final model were pre-existing conditions, therapies already in place prior to arrest and causes of arrest and are listed in Tables 1 and 2.
Table 1. Patient Characteristics by Age and Sex Categories.
| No. (%) | |||||
|---|---|---|---|---|---|
| Women | Men | Women | Men | ||
| 14-44 years | 14-44 years | ≥56 years | ≥56 years | ||
| (n= 4887) | (n=7025) | (n=37326) | (n=46614) | P value | |
| Race | |||||
| White | 2629 (54) | 3763 (54) | 26055 (70) | 34591 (74) | <.001 |
| Black | 1659 (34) | 2267 (32) | 7573 (20) | 7097 (15) | <.001 |
| Unknown | 321 (7) | 489 (7) | 2164 (6) | 2981 (6) | .001 |
| Hispanic | 402 (8) | 815 (12) | 1945 (5) | 2575 (6) | <.001 |
| Illness Category | |||||
| Medical, cardiac | 990 (20) | 1495 (21) | 13831 (37) | 17702 (38) | <.001 |
| Medical, non-cardiac | 2890 (59) | 3230 (46) | 16242 (44) | 18555 (40) | <.001 |
| Surgical, cardiac | 150 (3) | 259 (4) | 2532 (7) | 3816 (8) | <.001 |
| Surgical, non-cardiac | 507 (10) | 678 (10) | 4237 (11) | 5527(12) | <.001 |
| Trauma | 337 (7) | 1349 (19) | 426 (1) | 920 (2) | <.001 |
| Pre-Existing Conditions | |||||
| Congestive heart failure (this admission) | 439 (9) | 601 (9) | 7081 (19) | 8767 (19) | <.001 |
| Acute myocardial infarction | 325 (7) | 510 (7) | 6658 (18) | 9465 (20) | <.001 |
| Arrhythmia | 1085 (22) | 1462 (21) | 12372 (33) | 15873 (34) | <.001 |
| Hypotension | 1554 (32) | 2145 (31) | 9801 (26) | 12070 (26) | <.001 |
| Respiratory insufficiency | 2128 (44) | 2875 (41) | 14661 (39) | 18120 (39) | <.001 |
| Renal insufficiency | 1359 (28) | 1814 (26) | 10774 (29) | 14852 (32) | <.001 |
| Hepatic insufficiency | 500 (10) | 781 (11) | 1661 (4) | 2619 (6) | <.001 |
| Metabolic/electrolyte abnormality | 1138 (23) | 1444 (21) | 6115 (16) | 6857 (15) | <.001 |
| Diabetes mellitus | 819 (17) | 954 (14) | 11444 (31) | 13523 (29) | <.001 |
| Septicemia | 961 (20) | 1146 (16) | 5156 (14) | 5788 (12) | <.001 |
| Major trauma | 361(7) | 1284 (18) | 743 (2) | 1250 (3) | <.001 |
| Malignancy | 584 (12) | 567 (8) | 3930 (11) | 5660 (12) | <.001 |
| Interventions in Place | |||||
| Vascular access | 4456 (91) | 6411 (91) | 34269 (92) | 42949 (92) | .09 |
| Invasive airway | 2127 (44) | 3293 (47) | 10214 (27) | 12531 (27) | <.001 |
| Mechanical ventilation | 2146 (44) | 3285 (47) | 10323 (28) | 12805 (27) | <.001 |
| Vasoactive infusion | 1488 (30) | 2041 (29) | 9177 (25) | 11838 (25) | <.001 |
| Antiarrhythmic infusion | 187 (4) | 268 (4) | 2837 (8) | 2873(6) | <.001 |
P -values refer to the differences across the four populations clustered within hospitals by Wald test.
Factors modeled but not included were: Race: asian/pacific islander, Eskimo and other, Pre-existing conditions: Congestive heart failure (pre-admission), Baseline CNS depression, Acute Stroke, Acute CNS non-stroke event, none, Interventions in Place: pulse oximeter, apnea bradycardia monitor, intra-aortic balloon pump, PA catheter, none.
Table 2. Patient Arrest Characteristics by Age and Sex Categories.
| No. (%) | |||||
|---|---|---|---|---|---|
| Women | Men | Women | Men | ||
| 14-44 years | 14-44 years | ≥56 years | ≥56 years | ||
| (n= 4887) | (n=7025) | (n=37326) | (n=46614) | P value | |
| Immediate Cause of Event | |||||
| Hypotension/Hypoperfusion | 2153 (44) | 3240 (46) | 14322 (38) | 17650 (38) | .002 |
| Active Myocardial Infarction | 208 (4) | 349 (5) | 3493 (9) | 4777 (10) | <.001 |
| Acute respiratory insufficiency | 1862 (38) | 2639 (38) | 14116 (38) | 16992 (36) | .01 |
| Metabolic electrolyte abnormality | 840 (17) | 1155 (16) | 3899 (10) | 4590 (10) | <.001 |
| Acute stroke | 31 (1) | 30(1) | 263 (1) | 239 (1) | .05 |
| Status epilepticus | 40 (1) | 44 (1) | 57 (0) | 105 (1) | .29 |
| Unknown | 480 (10) | 605 (9) | 4139 (11) | 5033 (11) | <.001 |
|
| |||||
| Initial Rhythm | |||||
| Asystole | 1692 (35) | 2556(36) | 13656 (37) | 16006 (34) | <.001 |
| PEA | 1922(39) | 2726 (39) | 14044 (38) | 17139 (37) | <.001 |
| VF | 528 (11) | 852 (12) | 4688 (13) | 6824 (15) | <.001 |
| Pulseless VT | 336 (7) | 443 (6) | 2452 (7) | 3791 (8) | <.001 |
| unknown | 327(7) | 431(6) | 2354 (6) | 2683 (6) | <.001 |
|
| |||||
| Time of Cardiac Arrest | |||||
| After hours | 1524(31) | 2319 (33) | 11749 (31) | 14980 (32) | .02 |
| Weekend | 1551 (32) | 2353 (33) | 11373 (30) | 14276 (31) | <.001 |
|
| |||||
| Event Location | |||||
| ICU | 2621 (54) | 3774(54) | 16309 (44) | 21054 (45) | <.001 |
| procedural area | 306 (6) | 494 (7) | 2318 (6) | 2868 (6) | .21 |
| ED | 696 (14) | 1229 (17) | 3857(10) | 4696 (10) | <.001 |
| inpatient with monitoring | 463 (9) | 592 (8) | 7417(20) | 9306 (20) | <.001 |
| inpatient without monitoring | 686 (14) | 800(11) | 6372 (17) | 7463 (16) | <.001 |
| outpatient | 30 (1) | 25 (0) | 269(1) | 313 (1) | .02 |
| PACU | 19 (0) | 36 (1) | 248 (1) | 299 (1) | .22 |
P values refer to the differences across the four populations clustered within hospitals by Wald test. PEA: pulseless electrical activity, VF: ventricular fibrillation, VT: ventricular tachycardia. Factors modeled but not included were: Immediate Cause of Event: pulmonary embolism, pulmonary edema, and Mode of event discovery
Although, fixed effects (conditional) regression controlled for all hospital-level factors, they did not consider whether the adjusted associations of interest (age/sex and outcome) might vary across hospitals. For that reason, we resampled hospitals using 999 bootstrap samples and calculated bias-corrected (30) confidence intervals to ensure that measure of uncertainty accounted for the possibility of variation in risk differences across the hospitals. These confidence bounds differed only slightly from regression-based confidence intervals, and are more conservative. Results are thus reported as absolute risk reduction and bootstrap analysis confidence intervals. Because initial rhythm is a well-known potential confounder we performed as a sensitivity analysis separate regression for: asystole/PEA and VF/pulseless VT.
All p-values represent two-sided hypothesis tests. The significance level for all tests was α = .05. Statistical analysis was performed using Stata 10.1 (College Station, TX).
The NRCPR data from previous studies indicate that 17% of men 15-44 years of age survive to hospital discharge. Accordingly, it was determined that a sample size of 11,782 would be required using an alpha value = .05 and a power of .8 to detect an absolute survival difference of 2% between men and women 15-44 years of age, translating into a relative survival difference of more than 10%.
Results
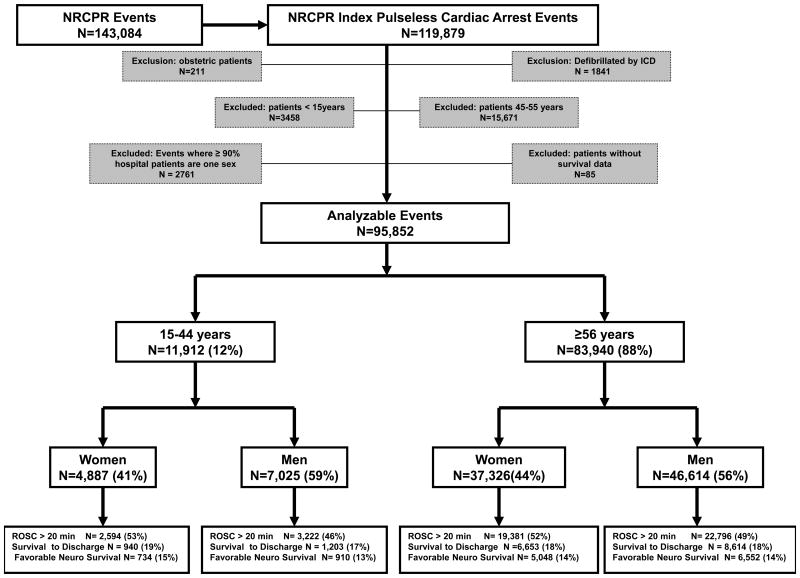
During the study period, 143,084 consecutive CA events were reported from 519 hospitals. There were 119,879 index CA events. After applying the inclusion and exclusion criteria, 95,852 analyzable subjects remained. These subjects were stratified by age and sex (Figure 1).
Figure 1. The distribution of NRCPR cardiac arrest events. The age and sex groups are women 15-44 years old, Men 15-44 years old, Women ≥ 56 years old and Men ≥ 56 years old. ROSC: return of spontaneous circulation.
There were large differences in covariates stratified by age and sex groups (Table 1). Subjects ≥56 years of age had higher percentages of white race, whereas subjects 15-44 years old had higher percentages of black race. Both women and men ≥56 years old had higher prevalences of congestive heart failure, myocardial infarction and diabetes mellitus than patients 15-44 years old. The younger patients (men and women 15-44 years) had a higher prevalence of mechanical ventilation prior to arrest. The younger males had a higher prevalence of preexisting trauma than the subjects in the other three age/sex categories.
Patient arrest characteristics also differed significantly across all four age groups except for stroke and status epilepticus as described in Table 2. Patients < 45 years (9%) were less likely to have an arrest on a monitored inpatient unit than patients ≥56 years (20%) (p<.001). Seventy percent of patients had a monitored and witnessed event and there was no difference between age and gender groups (p=0.77) There was no difference in the number of epinephrine dose administered to survivors (Table 3).
Table 3. Cardiopulmonary Resuscitation Interventions.
| Women | Men | Women | Men | |||||
|---|---|---|---|---|---|---|---|---|
| Interventions | 14-44years | P value | 14-44 years | ≥56 years | P value | ≥56 years | P value | |
| Duration of CPR, min | ||||||||
| Median (IQR) | 17 (8-30) | .39 | 18 (9-30) | REF | 16 (9-27) | <.001 | 17 (9-27) | <.001 |
| Duration of CPR: survivors, min | ||||||||
| Median (IQR) | 8 (4-17) | .99 | 9 (4-18) | REF | 9(4-17) | .5 | 9(4-18) | .17 |
| Duration of CPR: non-survivors, min | ||||||||
| Median (IQR) | 20 (10-33) | .1 | 20 (10-31) | REF | 18 (10-28) | <.001 | 18(11-28) | <.001 |
| No. of epinephrine doses | ||||||||
| Median (IQR) | 3 (1-4) | .1 | 3 (1-4) | REF | 2 (1-4) | <.001 | 2 (1-4) | <.001 |
| No. of epinephrine dose: survivors | ||||||||
| Median (IQR) | 1 (0-2) | .99 | 1 (0-2) | REF | 1(0-2) | .09 | 1(0-2) | .09 |
| No. of epinephrine doses: non-survivors | ||||||||
| Median (IQR) | 3 (2-5) | .14 | 3 (2-5) | REF | 3(1-4) | <.001 | 3(2-4) | <.001 |
P-values are comparing each group to the reference group of 14-44 year old men. CPR: cardiopulmonary resuscitation, IQR: interquartile range
Of all 95,852 patients, 47,993 (50%) survived the event, 17,410 (18%) survived to discharge, and 13,244 (14%) had a favorable neurologic outcome. Unadjusted outcome data are shown in Figure 1 and adjusted outcome data are summarized in Table 4. After conditioning on hospital and adjusting for patient and arrest characteristics (Tables 1 and 2), younger women had an absolute 2.8% (95% CI, 1%-4.6%; p=.002) higher rate of survival to hospital discharge than younger men. In contrast, there was no demonstrable difference in survival to discharge rates between older women and men (-0.1%; 95% CI, -0.9% - +0.6%; p = .68). Women 15-44 years of age had an absolute 2.6% higher rate of survival with a favorable neurological outcome (95% CI, 0.9% - 4.3%; p=.002) compared to men of the same age, whereas there was again no demonstrable difference in survival with a favorable neurologic outcome when comparing the older women and men (-0.l %; 95% CI, -0.7% - +0.5%, p =.74).
Table 4. Adjusted Outcomes of In-Hospital Cardiac Arrests between Women and Men within Age Categories.
| No. patients/Total (%) | No. patients/Total (%) | |||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||
| 14-44years | 14-44 years | adjusted difference % (95% CI) | ≥56 years | ≥56 years | adjusted difference % (95% CI) | |
| ROSC | 2596/4887 (53) | 3222/7025(46) | 7 (4.5-9) | 19381/37326(52) | 22796/46614(49) | 3.9 (2.9-4.8) |
| Survival to discharge | 940/4887(19) | 1203/7025(17) | 2.8 (1 - 4.6) | 6652/37326(18) | 8614/46614(18) | -0.1 (-0.9 - 0.6) |
| Favorable neurologic outcome | 734/4887(15) | 910/7025(13) | 2.6 (0.9-4.3) | 5048/37326(14) | 6552/46614(14) | -0.1 (-0.7 - 0.5) |
Adjusted difference refers to the adjusted absolute difference (95% CI) of women versus men within each age group. Men are the reference population. ROSC: Return of spontaneous circulation
A sensitivity analysis was performed by initial rhythm of PEA/ asystole and VF/pulseless VT and showed no difference in the point estimates for survival to discharge compared to the regression model controlling for initial pulseless rhythm.
Discussion
These data establish that following in-hospital CA women of child-bearing age (15-44 years old) were more likely to survive to hospital discharge than men in the same age range. In addition, the 15-44 year old women were more likely to survive with favorable neurologic outcome. In contrast, there were no demonstrable differences in these outcomes between the cohort of older women and men ≥56 years of age.
Our data indicate that the survival advantage of 15-44 year old women versus 15-44 year old men is substantial. Our estimated absolute difference in outcomes translates to a 17% relative increase in survival to discharge and a 21% relative increase in survival with favorable neurologic outcome for women 15-44 years old. These improvements exceed the absolute and relative improvements in survival from many highly publicized major advances in health care, such as adherence to acute myocardial infarction guidelines and interventions to decrease infections associated with central venous catheters.(31-33) Because more than 20,000 in-hospital CAs are reported to the NRCPR each year from ∼10% of United States hospitals, these absolute and relative outcome differences affect a substantial number of patients. If this difference is due to female sex hormones and administration of female sex hormones post-injury could improve outcomes to this extent or better, tens of thousands of lives may be affected positively.
Both experimental and clinical investigations support the biologic plausibility of the concept that female hormones can improve outcomes from global cerebral hypoxic-ischemic insults.(12-13, 15-20, 34-36) Although several diverse mechanisms of action have been proposed, it is clear that the protective effects of female hormones, such as estrogen, are not receptor-site specific, but rather have impact across multiple cellular lines, thus affording benefit for both males and females.(22) Recent human interventional trials suggest that post-injury exogenous progesterone treatment may improve outcome after traumatic brain injury.(20, 36) Thus far no human intervention trials evaluating the administration of estrogen or progesterone following CA have been published.
Both prospective and retrospective observational clinical studies suggest that women are more likely to survive to hospital admission than men following out-of-hospital CAs. (4, 10-11, 35) This difference persists especially when controlling for age, and despite the increased prevalence of asystole (a less favorable rhythm) in women.(10) Despite this apparent improvement in rates of successful initial resuscitation, these studies were unable to demonstrate improvement in survival to discharge, either because of study design or inadequate power. Importantly, the epidemiology and outcomes following out-of-hospital CA differ from in-hospital CA.
In a recent study showing no improvement in survival from 1992-2005 among >400,000 in-hospital CAs in a Medicare administrative database (>65 years old), men had a lower survival rate than women.(37) While these data may appear to conflict with our results, that study did not adjust for potential confounders of the relationship between sex and outcome including: initial rhythm, event location, and associated acute medical conditions. In contrast, we specifically controlled for known confounders and interactions between age and sex that modify the covariate relationship with outcome.
With the large data set afforded by the NRCPR, the current study was adequately powered to detect a difference in the rate of hospital discharge (our prospectively designated primary outcome). In addition, this study was adequately powered to demonstrate an improved rate of favorable neurological outcome for the women 15-44 years of age (our prospectively designated secondary outcome).
Nevertheless, the basis for this association is limited and cannot be fully confirmed by this observational database. Although we hypothesized that such differences would occur because of protection from high concentrations of female hormones, the increased survival among younger women might be due to other factors, such as pre-arrest risk-taking behaviors, sex-related differences in anatomy, or sex-related differences in the care patients receive before, during and after a CA. For example, chest wall compliance and recoil during chest compression may be different for men and women. Conversely, women and blacks are less likely to receive certain interventions following acute myocardial infarction.(38-39) It is possible that sex-related process-of-care differences that are not reported to the NRCPR also occur during CPR and/or post-resuscitation. Nevertheless, it is notable that the outcome differences in our study were only demonstrated for women of child bearing age, 15-44 years old, but not for women >56 years of age.
As with all multicenter registries, the database used in this study has potential limitations related to integrity and validity of the data. However, the rigorous abstractor-training process, uniform data collection, consistent definitions, detailed periodic reabstraction process and the large sample were implemented prospectively to address the issues of validity and integrity.
The variability in resuscitative and post-resuscitative processes of care between multiple institutions raises the concern that a true treatment effect may be confounded by institutional practice.(40) To help address this potential limitation, hospitals that treated >90% men or women were excluded. Most importantly, hospital level factors were controlled for in our analysis using fixed effects regression.
We categorized our patients' age on the presumed high concentrations of sex hormones. Menarche and menopause have onsets at different times for each woman. In the United States, the average onset of menstruation is 13 years old(23) and the average onset of menopause is 50 years old.(24) Therefore, to minimize the risk of misclassification bias we specifically excluded patients less than 15 years old and those between 45 and 55 years old from the analysis. History regarding oral contraceptive use, exogenous hormonal supplementation, tamoxifen use, and the onset of premature menopause was not available. In addition, estrogen and progesterone levels were not measured in these patients.
To account for our findings, future studies to assess the role of sex-specific process of care variables and hormonal effects on the association between sex and CA survival are necessary. To address the lack of patient-specific hormonal data in this study a prospective evaluation of estrogen and progesterone levels in patients who suffer from CA could be performed. Conditions that affect endogenous hormonal levels (e.g., anorexia, bulimia, polycystic ovarian syndrome), exogenous hormone levels (e.g. contraceptives, tamoxifen) and measured hormonal levels accounting for the effects of one specific hormone (estrogen or progesterone) or their combination should be accounted for to evaluate their impact of survival.
Conclusion
In this multi-center registry of in-hospital CAs, women of childbearing age were more likely to survive to hospital discharge and survive with favorable neurologic outcome compared with age-matched men, even after controlling for etiology of arrest and important measurable patient, event, and hospital variables. This survival benefit was not present for women ≥56 years old (presumed post-menopausal) compared to their age-matched men. Delineation of the mechanisms of younger women's survival advantage may result in new strategies to improve outcomes for victims of CAs.
Acknowledgments
Funding Sources: The Edna G. Kynett Foundation FOCUS Junior Faculty Investigator Award provided funding for design of the study and data analysis.
Dr. Becker has received grant/research support from Philips Medical Systems, Laerdal Medical, NIH, Cardiac Science, and BeneChill. He consulted for Philips Medical Systems, NIH Data Safety Monitoring Board and Protocol Review Committee, and Gaymar Industries. He received patents from Hypothermia Induction and Reperfusion Therapies. He is a special government employee. He has received ownership, equity, and royalties from Cold Core Therapeutics, Inc. He is a member of the American Heart Association. He received speaker honoraria from Zoll Medical, Medtronics, and multiple universities.
Appendix.
National Registry of Cardiopulmonary Resuscitation Investigators: Mary Mancini RN PhD, Emilie Allen BSN, Elizabeth Hunt MD, Joseph Ornato MD, Scott Braithwaite MD, Graham Nichol MD, John Gosbee MD, Greg Mears MD, Kathy Duncan RN, William Kaye MD, Tanya Lane Truitt RN MS, Jerry PottsPhD, Brian Eigel PhD, Paul Chan MD, Tim Mader MD, David Magid, Karl Kern MD, Sam Warren MD, Thomas Noel MD, Jane Wigginton MD, Scott Carey
Footnotes
All other authors have not disclosed any potential conflicts of interest.
References
- 1.Arrich J, Sterz F, Fleischhackl R, et al. Gender modifies the influence of age on outcome after successfully resuscitated cardiac arrest: a retrospective cohort study. Medicine (Baltimore) 2006;85:288–294. doi: 10.1097/01.md.0000236954.72342.20. [DOI] [PubMed] [Google Scholar]
- 2.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. Jama. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 3.de Vos R, Koster RW, De Haan RJ, et al. In-hospital cardiopulmonary resuscitation: prearrest morbidity and outcome. Arch Intern Med. 1999;159:845–850. doi: 10.1001/archinte.159.8.845. [DOI] [PubMed] [Google Scholar]
- 4.Herlitz J, Engdahl J, Svensson L, et al. Is female sex associated with increased survival after out-of-hospital cardiac arrest? Resuscitation. 2004;60:197–203. doi: 10.1016/j.resuscitation.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Fahrenbruch CE, Cobb LA, et al. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104:2699–2703. doi: 10.1161/hc4701.099784. [DOI] [PubMed] [Google Scholar]
- 6.Pell JP, Sirel J, Marsden AK, et al. Sex differences in outcome following community-based cardiopulmonary arrest. Eur Heart J. 2000;21:239–244. doi: 10.1053/euhj.1999.1629. [DOI] [PubMed] [Google Scholar]
- 7.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 8.Tunstall-Pedoe H, Morrison C, Woodward M, et al. Sex differences in myocardial infarction and coronary deaths in the Scottish MONICA population of Glasgow 1985 to 1991. Presentation, diagnosis, treatment, and 28-day case fatality of 3991 events in men and 1551 events in women. Circulation. 1996;93:1981–1992. doi: 10.1161/01.cir.93.11.1981. [DOI] [PubMed] [Google Scholar]
- 9.Vukmir RB. Prehospital cardiac arrest and the adverse effect of male gender, but not age, on outcome. J Womens Health (Larchmt) 2003;12:667–673. doi: 10.1089/154099903322404311. [DOI] [PubMed] [Google Scholar]
- 10.Wigginton JG, Pepe PE, Bedolla JP, et al. Sex-related differences in the presentation and outcome of out-of-hospital cardiopulmonary arrest: a multiyear, prospective, population-based study. Crit Care Med. 2002;30:S131–136. doi: 10.1097/00003246-200204001-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mahapatra S, Bunch TJ, White RD, et al. Sex differences in outcome after ventricular fibrillation in out-of-hospital cardiac arrest. Resuscitation. 2005;65:197–202. doi: 10.1016/j.resuscitation.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Cervantes M, Gonzalez-Vidal MD, Ruelas R, et al. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33:6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 13.Ishrat T, Sayeed I, Atif F, et al. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2008 doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roof RL, Duvdevani R, Braswell L, et al. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- 15.Jover T, Tanaka H, Calderone A, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noppens RR, Kofler J, Grafe MR, et al. Estradiol after cardiac arrest and cardiopulmonary resuscitation is neuroprotective and mediated through estrogen receptor-beta. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkayed NJ, Murphy SJ, Traystman RJ, et al. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 18.Alkayed NJ, Harukuni I, Kimes AS, et al. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YQ, Shi J, Rajakumar G, et al. Effects of gender and estradiol treatment on focal brain ischemia. Brain Res. 1998;784:321–324. doi: 10.1016/s0006-8993(97)00502-7. [DOI] [PubMed] [Google Scholar]
- 20.Xiao G, Wei J, Yan W, et al. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullough LD, Alkayed NJ, Traystman RJ, et al. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 22.Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111:844–850. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- 24.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa) Resuscitation. 2004;63:233–249. doi: 10.1016/j.resuscitation.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 27.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the pediatric Utstein style. A statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Pediatrics. 1995;96:765–779. [PubMed] [Google Scholar]
- 28.Localio AR, Berlin JA, Ten Have TR, et al. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135:112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 29.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. Jama. 2008;299:785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 30.Davison A, Hinkley D. Boostrap Methods and Their Application. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 31.Peterson ED, Roe MT, Mulgund J, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. Jama. 2006;295:1912–1920. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 32.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 33.Glickman SW, Boulding W, Staelin R, et al. A framework for quality improvement: an analysis of factors responsible for improvement at hospitals participating in the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. 2007;154:1206–1220. doi: 10.1016/j.ahj.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–159. [PubMed] [Google Scholar]
- 35.Perers E, Abrahamsson P, Bang A, et al. There is a difference in characteristics and outcome between women and men who suffer out of hospital cardiac arrest. Resuscitation. 1999;40:133–140. doi: 10.1016/s0300-9572(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 36.Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. 402 e391-392. [DOI] [PubMed] [Google Scholar]
- 37.Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361:22–31. doi: 10.1056/NEJMoa0810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353:671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gan SC, Beaver SK, Houck PM, et al. Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med. 2000;343:8–15. doi: 10.1056/NEJM200007063430102. [DOI] [PubMed] [Google Scholar]
- 40.Carr BG, Kahn JM, Merchant RM, et al. Inter-hospital variability in post-cardiac arrest mortality. Resuscitation. 2009;80:30–34. doi: 10.1016/j.resuscitation.2008.09.001. [DOI] [PubMed] [Google Scholar]