Abstract
Background
Blood donors positive only for anti-HBc may have a resolved hepatitis B virus (HBV) infection, low grade chronic infection or infection with variant strains of HBV. We aimed to assess the significance of this serological pattern after hepatitis B vaccination in such cases.
Materials and methods
Twenty-four anti-HBc only blood donors were vaccinated with the Engerix HBV vaccine and a serological and virological evaluation was performed before HBV vaccination and 7–10 days after each dose. Subjects were classified as non-responders if their anti-HBs levels stayed below 10 IU/L after full vaccination, while the response was considered secondary (anamnestic) if anti-HBs levels rose over 10 IU/L after the first vaccine dose, and primary if anti-HBs levels rose over 10 IU/L only after the second or third vaccine dose.
Results
Of the 21 fully evaluable donors, six had no response, eight showed a primary response and seven had an anamnestic response. One non-responder had transient positivity for HBV-DNA at low levels (12 IU/mL) with persistent negativity for HBsAg.
Discussion
Anti-HBc-only positive blood donors are a heterogeneous population including HBV naïve subjects with a likely false-positive anti-HBc reactivity, subjects with a resolved HBV infection, and subjects with persistent low-level HBV replication.
The analysis of the anti-HBs response after a dose of HBV vaccine may help to distinguish among the different causes of the isolated anti-HBc positivity, thereby enabling proper counselling and potential readmission to blood donation.
Keywords: anti-HBc only, anti-HBs kinetics, blood donors, HBV vaccination, occult hepatitis B virus infection
Background
Blood and blood-product safety is a global issue because transfusion therapy is of vital importance in modern medicine although it is also an efficient route of transmitting blood-borne pathogens. Transmission of hepatitis B virus (HBV) continues to occur despite the implementation of serological screening for hepatitis B surface antigen (HBsAg) by using more sensitive assays and the introduction of nucleic amplification tests (NAT) for HBV-DNA1–4.
The presence of hepatitis B core antibody (anti-HBc) in the absence of HBsAg and hepatitis B surface antibody (anti-HBs) is defined as “isolated anti-HBc”. It is well recognized that anti-HBc may be the only detectable serological marker of HBV infection in blood donors with resolved infection, low grade chronic infection or infection with atypical variant strains of HBV5–9. Moreover, reactivity for anti-HBc may be due to a false positive result10–13.
Routine blood donor screening for anti-HBc has been implemented in the USA2 and in some other large countries such as Japan and Germany, but it is not a mandatory practice in Italy where assays for biological qualification of blood donations include HBsAg, anti-hepatitis C virus (HCV), anti-human immunodeficiency virus (HIV) 1–2, syphilis and NAT testing for HBV-DNA, HCV-RNA and HIV-RNA14,15.
In order to investigate the significance of isolated anti-HBc reactivity among blood donors, we evaluated the response to hepatitis B vaccine and the kinetics of anti-HBs in a cohort of Italian repeat blood donors positive only for anti-HBc with the aim of discriminating non-responders from responders and, among the latter, discriminating those with a primary response to the vaccine from those with a secondary response.
Materials and methods
Selection of subjects
There are 3,992 active blood donors at the Transfusion Service of Chioggia. All of these were tested for anti-HBc and anti-HBs when they were enrolled donors. According to the screening policies adopted in our Hospital, after 01/01/2011, blood donors who are negative for HBsAg and positive for anti-HBc are tested for all other HBV markers and deferred from blood donation. We selected from our records 31 repeat blood donors who had tested positive for anti-HBc and negative for HBV-DNA, HCV-RNA, HIV-RNA, HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HCV, anti-HIV1/2, and anti-HBc IgM. Of these 31 subjects, 21 were male and 10 were female (ratio: 2.1:1) and their ages ranged from 18 to 64 years (mean: 51.4±9.2; median 53). None of these donors was diabetic. These subjects underwent medical counselling to explain the reasons for their deferral from donation. In this study we also proposed, as an alternative to permanent deferral, the administration of hepatitis B vaccine.
Study design
After the baseline assessment, subjects enrolled in this study were given a recombinant HBV vaccine (Engerix, GlaxoSmithKline, Verona, Italy) following the protocol recommended for primary immunisation in people 20 years of age and older: three doses of 1 mL each administered subcutaneously at 0, 1, and 6 months. A full serological study for HBV markers together with NAT testing for HBV-DNA was performed 7–10 days after each vaccine dose. Additionally, anti-HBc IgG avidity was evaluated and a quantitative assay was performed for low titre anti-HBc IgM in each subject after the third dose of vaccine.
Ethical considerations
The study was performed according to the requirements of the Declaration of Helsinki and the protocol was approved by the Ethical Review Board of our hospital (AULSS 14, Veneto region). All the study subjects undersigned two written informed consent form, one for enrolment in the study protocol and the other for administration of the HBV vaccine.
Serological assays
Qualitative assays for HBV serology (HBsAg, HBeAg, anti-HBc, anti-HBe, anti-HBc/IgM) and quantitative assays for anti-HBs, anti-HCV and anti HIV 1/2 were performed using commercial kits on the automated ARCHITECT analyser (Abbott Diagnostics, Wiesbaden, Germany). Anti-HBs levels are expressed in International Units per litre (IU/L) and the assay employed is linear from 2 to 1,000 IU/L. Low level HBcAb IgM antibodies were searched for as previously described16 by using, in addition to ARCHITECT, an automated analyser and commercial kit supplied by Medical Systems (Genoa, Italy). Anti-HBc avidity was evaluated experimentally as described elsewhere16,17.
Virological assays
Qualitative NAT screening for HBV-DNA, HCV-RNA and HIV1-RNA was performed on individual samples by using a TMA-based commercial method (Chiron Novartis, Siena, Italy). For HBV-DNA quantification we used two real time polymerase chain reaction (RT-PCR) commercial methods: the COBAS HBV AmpliPrep/COBAS TaqMan HBV Test, v2.0 (Roche Molecular System, Branchburg, New Jersey, USA) and a real-time HBV assay on the fully automated m2000 System analyser (Abbott Molecular Diagnostics, Wiesbaden, Germany). Both assays have a limit of quantification of 12 IU/mL. HBV genotyping was performed by a reverse hybridisation line probe assay (INNO-LiPA HBV Genotyping assay, Innogenetics NV, Ghent, Belgium). All assays have been previously described and were performed according to the manufacturers’ specifications.
Classification of the anti-HBs response
Samples were considered anti-HBs positive at levels ≥10 IU/L. A subject was classified as a non-responder if, after three vaccine doses, anti-HBs was still negative, whereas a response was considered as primary if the anti-HBs concentration rose over 10 IU/L after the second or third vaccine dose, while a secondary (or anamnestic) response was defined by anti-HBs positivity after the first dose of vaccine18–21.
Statistical evaluation
The differences between median levels of anti-HBs were evaluated by the non-parametric Mann-Whitney test and the differences between positivity rates by the χ2 or Fisher’s exact test. To allow calculation of antibody geometric mean a value of 0 IU/L was assigned to samples with undetectable anti-HBs levels.
Results
Population characteristics and baseline results
Among 3,992 blood donors, 31 (0.8%) anti-HBc only subjects were identified. Seven subjects refused HBV vaccination and three subjects dropped out before completion of the vaccination schedule, so only 21 subjects (68%) were fully evaluated. Of these, 14 were male and 7 were female (ratio: 2:1). The mean age (52.8±9.2 years) and median (56 years) of these subjects were not significantly different from those of the initial cohort.
Anti-HBs response
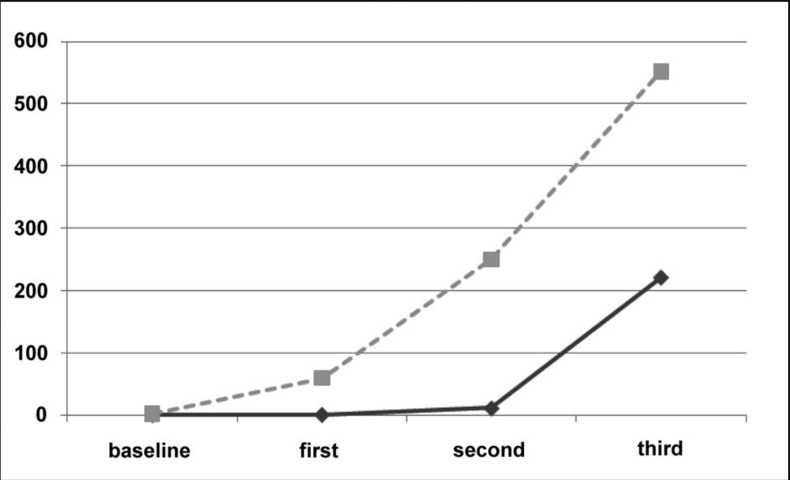
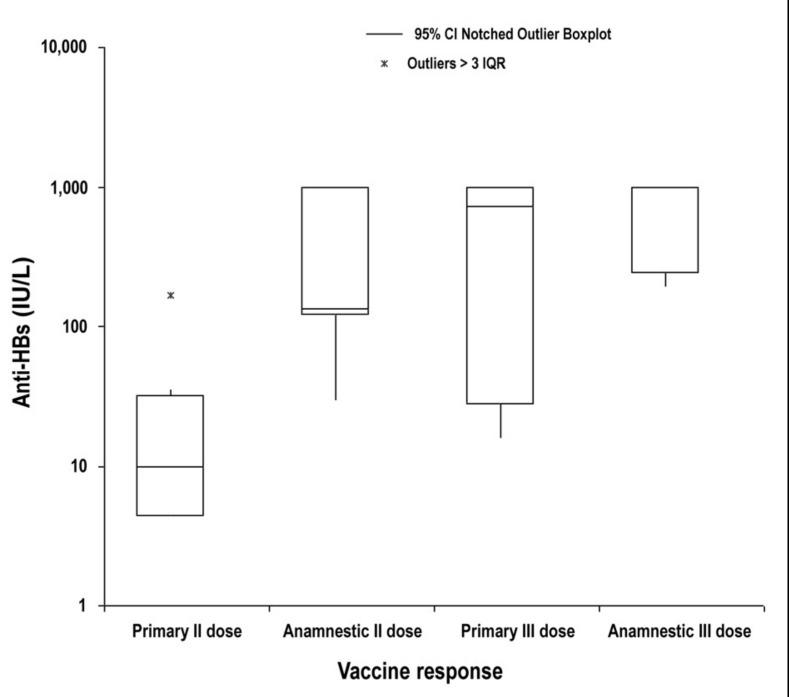
The summary data and anti-HBs response of the 21 fully evaluated subjects are reported in Table I. Overall, there were six (28%) non-responders, eight subjects (38%) showing a primary response and seven subjects (34%) showing a secondary response with a fast rise of anti-HBs titre already after the first vaccine dose. The geometric means of anti-HBs levels in subjects with a primary response were 1.2 IU/L after the first dose, 11.5 IU/L after the second dose and 221.0 IU/L after the third dose, while the geometric means for HBsAb concentration in subjects with a secondary response were 60 IU/L, 250 IU/L and 551 IU/L after the first, second and third doses, respectively (Figure 1). The distribution of anti-HBs levels after the second and third vaccine doses in subjects with primary or secondary responses is shown in Figure 2. The median levels after the second dose were significantly different between those with a primary or secondary response (10 and 134 IU/L; Mann-Whitney non-parametric test P =0.017), while the difference after the third dose (726 and 1,000 IU/mL, respectively) was not statistically significant.
Table I.
Vaccine response and anti-HBs levels (IU/L) before and after HBV vaccination in 21 anti-HBc only blood donors.
| N. | ID | Sex | Age | Anti-HBc AI | Anti-HBs baseline | Anti-HBs after I dose | Anti-HBs after II dose | Anti-HBs after III dose | Response to HBV vaccine |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BGC | M | 64 | 90 | 1 | 2 | 2 | 3 | Non responder |
| 2 | DN | M | 51 | 84 | 0 | 0 | 0 | 4 | Non responder |
| 3 | GG | M | 53 | n.d. | 1 | 2 | 3 | 5 | Non responder |
| 4 | LC | M | 59 | 82 | 0 | 0 | 3 | 1 | Non responder |
| 5 | PF | M | 64 | 77 | 0 | 1 | 4 | 3 | Non responder |
| 6 | TA | M | 57 | 83 | 0 | 0 | 0 | 0 | Non responder |
| 7 | BGR | F | 40 | n.d. | 0 | 0 | 168 | 1,000 | Primary |
| 8 | BMA | M | 58 | n.d. | 0 | 0 | 0 | 16 | Primary |
| 9 | CM | F | 53 | n.d. | 0 | 0 | 28 | 28 | Primary |
| 10 | CL | M | 35 | n.d. | 0 | 0 | 35 | 452 | Primary |
| 11 | DAM | F | 60 | n.d. | 0 | 0 | 5 | 1,000 | Primary |
| 12 | DG | F | 36 | n.d. | 0 | 0 | 4 | 1,000 | Primary |
| 13 | MG | M | 59 | n.d. | 1 | 4 | 7 | 28 | Primary |
| 14 | SO | F | 56 | 86 | 0 | 1 | 13 | 1,000 | Primary |
| 15 | BL | M | 50 | 85 | 0 | 19 | 128 | 1,000 | Secondary |
| 16 | BG | M | 49 | 80 | 7 | 30 | 30 | 366 | Secondary |
| 17 | BMS | M | 48 | n.d. | 2 | 1,000 | 1,000 | 1,000 | Secondary |
| 18 | FNG | F | 60 | 92 | 5 | 33 | 1,000 | 1,000 | Secondary |
| 19 | NM | F | 62 | n.d. | 0 | 66 | 1,000 | 1,000 | Secondary |
| 20 | VE | M | 59 | 75 | 2 | 91 | 134 | 218 | Secondary |
| 21 | VM | M | 36 | 90 | 4 | 24 | 120 | 194 | Secondary |
AI: Avidity Index; n.d.: not determined.
Figure 1.
Evaluation of anti-HBs kinetics after anti-HBV vaccination. Geometric mean titres of HBsAb antibody (IU/L) are reported separately for subjects showing an anamnestic response (dotted grey line) and for subjects showing a primary response (solid black line).
Figure 2.
Distribution of anti-HBs values (IU/L) on a logarithmic scale after the second and third doses of HBV vaccine in subjects showing a primary and an anamnestic response. The median values after the second dose (10 and 134 IU/L, respectively) are significantly different (P =0.017), while those after the third dose (726 and 1,000 IU/L) are not.
Additional serological tests
The anti-HBc IgG avidity index (AI) was not assessed in 10 subjects because after the 1:10 dilution required by this procedure the samples resulted negative for anti-HBc. An AI of 75 or higher was found in all the other 11 subjects tested. Anti-HBc IgM levels were lower than 2 U/L in all subjects.
HBV-DNA testing
HBV-DNA was always negative in all samples from 20 subjects during this study. In one subject from the non-responder group (BGC, Table I) we observed a transient HBV-DNA positivity in the last control performed after the third dose of vaccine. The HBV-DNA load was 12 IU/L and the subject’s genotype was D. The positivity for HBV-DNA lasted for 5 weeks; the donor always remained HBsAg-negative and no modification of the serological profile of HBV was observed.
Discussion
Antibody responses to each of the HBV proteins have been detected in the sera of humans following HBV infection22. After resolution of transient HBV infection, anti-HBc antibodies usually persist longer than other HBV markers and anti-HBc is, therefore, the most universal marker for diagnostic and epidemiological purposes15,16. The anti-HBc only serological profile may be associated with: (i) passive transfer of anti-HBc; (ii) a recent primary HBV infection when HBsAg has disappeared and anti-HBs is not yet detectable; (iii) an HBV infection in which HBsAg is not detectable because of mutant strains; (iv) suppression of HBV replication due to interference with other viral infections, mainly HCV and hepatitis delta virus (HDV) or an impaired host immune response due to HIV infection; (v) non-specific, cross-reacting antibody; (vi) remote infection with loss of detectable anti-HBs; (vii) persistent HBV infection in which HBsAg is not detectable because of a very low viraemia, which is currently classified as an occult HBV infection2,3,6,9,23,24.
Among blood donors, there is evidence that only a small percentage of anti-HBc only subjects are positive for HBV-DNA in serum2,7,8,11,23,25–27. Several reports have indicated that virtually all commercially available anti-HBc assays have a low positive predictive value in a low prevalence population and this problem is most noticeable with low-level anti-HBc-reactive samples10–13 which are often obtained from individuals with no history and no other markers of HBV exposure. Some researchers have found that dilution and treatment with mild reducing agents can selectively eliminate false-positive reactivity and improve specificity of competitive anti-HBc assays17,28,29. On the other hand, there have been reports of post-transfusion hepatitis B due to blood products obtained from HBsAg negative/anti-HBc positive donors, as the implicated blood donors were HBV-DNA positive1,30,31. So a donor with isolated positivity for anti-HBc may be an HBV-naïve subject (false positive test), a person with a past HBV infection, or a low level carrier negative for HBsAg. Hepatitis B vaccination of these individuals has been previously employed to understand the significance of the isolated anti-HBc18,20,27,30,32–34, since immune subjects will show an anamnestic anti-HBs response after a single dose of vaccine.
The results of the current study enabled discrimination among the above-mentioned hypotheses: (i) passive transfer of anti-HBc by a vertical route or by administration of immunoglobulins, which may contain anti-HBc, can be ruled out because all the subjects considered in this study were adults with a negative history of blood product administration35, (ii) recently HBV-infected subjects, in whom the anti-HBc only serological pattern is caused by clearance of HBsAg before the appearance of anti-HBs, are usually positive for anti-HBc IgM, while in our study none of the subjects resulted positive for anti-HBc IgM when tested using two different assays with a sensitivity of 10 IU/L and 2 UI/L16 and, moreover, the anti-HBc avidity was always ≥75, suggesting a long-standing infection; (iii) subjects in whom a negative HBsAg test is the result of a mutant HBV are usually viraemic26,27 while all subjects in our study were HBV-DNA negative when using highly sensitive NAT assays15,36; (iv) interference due to HCV and/or HDV co-infection or to an impaired immunological response linked to HIV infection was excluded by negativity in the specific virological and serological assays37; (v) in this study we adopted a strategy based upon determination of anti-HBc avidity16,17,38,39 which requires a 1:10 sample dilution. In 10/21 subjects the anti-HBc reactivity was no longer detectable after dilution and, furthermore, seven on these subjects showed a primary response after HBV vaccination, indicating the lack of a previous immune status to HBV23. In our opinion these results support the consideration of a low predictive value of anti-HBc reactivity among blood donors40 and these subjects shall be considered naïve to HBV infection; (vi) in subjects with remote HBV infection and loss of anti-HBs and of anti-HBe the persistence of anti-HBc is the only serological marker detectable as a “serological scar” of a previous HBV infection. In these subjects anti-HBc avidity should be high and after HBV vaccination a booster effect for anti-HBs levels is expected after administration of the first vaccine dose18,19,21,22,30,32–34,41. In our study we observed seven subjects with this type of response to HBV vaccine: in five of them anti-HBc avidity was evaluated and was always ≥75, confirming the specificity of the anti-HBc reactivity and suggesting that the HBV infection had been contracted many years previously; (iv) in subjects with an occult HBV infection, anti-HBc positive results should be specific with a high avidity and no response after HBV vaccination should be observed5,16,24,30,32. In our study we observed six non-responders, in five of whom the anti-HBc avidity index was evaluable and was always ≥75: these results confirm the specificity of anti-HBc reactivity and are suggestive of an HBV infection contracted much time before. Of note, one donor in this group showed transient HBV-DNA positivity after completion of the vaccination schedule, with no appearance of HBsAg and no signs of liver damage. The presence of HBV-DNA was confirmed by a repeated positivity by two real-time PCR assays employing different primer sets and by the result of HBV genotyping that demonstrated an infection by genotype D, which is the most prevalent genotype in Italy.
In our opinion data obtained in this study, despite its limitation due to the small numbers of anti-HBc only subjects enrolled, suggest that Italian repeat blood donors with an isolated anti-HBc serological pattern are a heterogeneous group of individuals in which three subgroups are equally represented: (i) subjects naïve for HBV infection in whom anti-HBc reactivity is non-specific and usually at very low levels; (ii) individuals with a previously resolved HBV infection in whom anti-HBc reactivity should be considered a “serological scar”; and (iii) subjects with persistent HBV infection with low levels of virus replication, undetectable serum HBsAg and circulating HBV-DNA usually below the sensitivity threshold of NAT assays. The possibility of evaluating anti-HBc levels28,29,38 and affinity16,38,39 more accurately might contribute to enhancing the specificity of the anti-HBc assays for screening purposes.
Footnotes
Conflict of interest disclosure
Gianluca Gessoni, Samanta Beggio, Paolo Barin, Mosè Favarato, Sara Valverde, Massimo Boscolo Nata, Maria Monica Salvadego, Giorgio Marchiori are employers of Italian National Healts Service. Claudio Galli is chief of Scientific Affairs of Abbott Diagnostics.
References
- 1.Candotti D, Allain J-P. Transfusion transmitted hepatitis B virus infection. J Hepatol. 2009;51:798–809. doi: 10.1016/j.jhep.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman S, Kuhns M, Todd D, et al. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion. 2003;43:696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhns M, Bush M. New strategies for blood donors screening for hepatitis B virus: Nucleic acid testing versus immunoassay methods. Mol Diag Ther. 2006;10:77–91. doi: 10.1007/BF03256447. [DOI] [PubMed] [Google Scholar]
- 4.Velati C, Romanò L, Fomiatti L, et al. Impact of nucleic acid testing for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus on the safety of blood supply in Italy: a 6-year survey. Transfusion. 2008;48:2205–13. doi: 10.1111/j.1537-2995.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 5.Katsurada A, Marusawa H, Uemoto S, et al. Circulating antibody to hepatitis B core antigen not always reflects the latent hepatitis B virus infection in the liver tissue. Hepatol Res. 2003;25:105–14. doi: 10.1016/s1386-6346(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 6.Marusawa H, Uemoto S, Hijikata M, et al. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology. 2000;31:488–95. doi: 10.1002/hep.510310232. [DOI] [PubMed] [Google Scholar]
- 7.Ramia S, Ramalawi F, Kanaan M, et al. Frequency and significance of antibodies against hepatitis B core antigen as the only serological markers for hepatitis B infection in Lebanese blood donors. Epidemiol Infect. 2005;133:695–9. doi: 10.1017/s0950268805003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabor E, Hoofnagle J, Barker L, et al. Antibody to hepatitis B core antigen in blood donors with history of hepatitis. Transfusion. 1981;21:366–71. doi: 10.1046/j.1537-2995.1981.21381201816.x. [DOI] [PubMed] [Google Scholar]
- 9.Weber B, Melchior W, Gehrke R, et al. Hepatitis B virus markers in anti-HBc only positive individuals. J Med Virol. 2001;64:312–9. doi: 10.1002/jmv.1052. [DOI] [PubMed] [Google Scholar]
- 10.Hourfar M, Walch A, Geusendam G, et al. Sensitivity and specificity of Anti-HBc screening assays - which assay is best for blood donor screening? Int J Lab Hematol. 2009;31:649–56. doi: 10.1111/j.1751-553X.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 11.Katz L, Strong D, Tegmeier G, Stramer S. Performance of an algorithm for the reentry of volunteer blood donors deferred due to false positive test results for antibody to hepatitis B core antigen. Transfusion. 2008;48:2315–22. doi: 10.1111/j.1537-2995.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- 12.Ollier L, Laffont C, Kechkekian A, et al. Detection of antibodies to hepatitis B core antigen using the Abbott Architect anti-HBc assay: analysis of borderline reactive sera. J Virol Methods. 2008;154:206–9. doi: 10.1016/j.jviromet.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Schimdt M, Nubling C, Scheiblauer H. Anti-HBc screening of blood donors: a comparison of nine anti-HBc tests. Vox Sang. 2006;914:237–43. doi: 10.1111/j.1423-0410.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 14.Italian Health Ministry. Decreto del Ministro della Salute 3 marzo 2005. Caratteristiche e modalità per la donazione del sangue e degli emocomponenti: Gazzetta Ufficiale Repubblica Italiana n° 85, 13/04/2005.
- 15.Velati C, Formiatti L, Baruffi L, et al. Criteria for hepatitis B virus screening and validation of blood components in Italy: the position of the SIMTI-HBV working group. Blood Transfus. 2011;9:455–61. doi: 10.2450/2011.0014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli C, Orlandini E, Penzo L, et al. What is the role of serology for the study of chronic hepatitis B virus infection in the age of molecular biology? J Med Virol. 2008;80:974–9. doi: 10.1002/jmv.21179. [DOI] [PubMed] [Google Scholar]
- 17.Rodella A, Galli C, Terlenghi L, et al. Quantitative analysis of HBsAg, IgM anti-HBc and HBc avidity in acute and chronic hepatitis B. J Clin Virol. 2006;37:206–12. doi: 10.1016/j.jcv.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Koh H, Kim S, Choi J, et al. A study of immune response to hepatitis B vaccine and HBV-DNA in isolated anti-HBc positive subjects. Prev Med Public Health. 2005;38:170–4. [PubMed] [Google Scholar]
- 19.Odinesen O, Owusu-Ofori S, Dompreh A, et al. Antibody detection and kinetics of antibody production during early stages of immunization with hepatitis B virus vaccine. Clin Vacc Immunol. 2007;14:1623–8. doi: 10.1128/CVI.00158-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim Y, Kim KY, Kim B-H, et al. Anti-hepatitis B core antibody is not required for prevaccination screening in healthcare workers. Vaccine. 2011;29:1721–6. doi: 10.1016/j.vaccine.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Zanetti AR, Mariano A, Romanò L, et al. 2005. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379–84. doi: 10.1016/S0140-6736(05)67568-X. [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Lin S, Chen F, Yang C. The immune response induced by hepatitis B virus principal antigens. Cell Mol Immunol. 2006;3:97–106. [PubMed] [Google Scholar]
- 23.Manzini P, Girotto M, Borsotti R, et al. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007;92:1663–70. doi: 10.3324/haematol.11224. [DOI] [PubMed] [Google Scholar]
- 24.Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652–7. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Altunay H, Kosan E, Birinci I, et al. Are isolated anti-HBc blood donors in high risk group? The detection of HBV DNA in isolated anti-HBc cases with nucleic acid amplification test (NAT) based on transcription-mediated amplification (TMA) and HBV discrimination. Transfus Apher Sci. 2010;43:265–8. doi: 10.1016/j.transci.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Dreier J, Kroger M, Diekmann J, et al. Low level viraemia of hepatitis B virus in an anti-HBc and anti-HBs positive blood donor. Transf Med. 2004;14:97–103. doi: 10.1111/j.0958-7578.2004.0486.x. [DOI] [PubMed] [Google Scholar]
- 27.Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;364:236–47. doi: 10.1056/NEJMoa1007644. [DOI] [PubMed] [Google Scholar]
- 28.Li A, Youan Q, Huang Z, et al. Novel double antigen sandwich immune-assay for human hepatitis core antibody. Clin Vacc Immunol. 2010;17:464–9. doi: 10.1128/CVI.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seiskari T, Lehitisaari A, Haapala A-M, Aittoniemi J. From Abbott ARCHITECT® Anti-HBc to Anti-HBc II--Improved performance in detecting antibodies to hepatitis B core antigen. J Clin Virol. 2010;47:100–1. doi: 10.1016/j.jcv.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Aoki S, Finegold D, Kuramoto I, et al. Significance of antibody to hepatitis B core antigen in blood donors as determined by their serologic response to hepatitis B vaccine. Transfusion. 1993;33:362–7. doi: 10.1046/j.1537-2995.1993.33593255593.x. [DOI] [PubMed] [Google Scholar]
- 31.Zeyenap-Akam F, Demir C, Kocak N. Acute hepatitis B infection due to erythrocyte suspension obtained from anti-HBc alone positive blood donor. Transf Med. 2005;15:61–3. doi: 10.1111/j.1365-3148.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 32.Draelos M, Morgan T, Schifman R, Sampliner R. Significance of isolated antibody to hepatitis B core antigen determined by immune response to hepatitis B vaccination. J Amer Med Ass. 1987;258:1193–5. [PubMed] [Google Scholar]
- 33.Ural O, Findik D. The response of isolated anti-HBc positive subjects to recombinant hepatitis B virus vaccine. J Infect. 2001;43:187–90. doi: 10.1053/jinf.2001.0878. [DOI] [PubMed] [Google Scholar]
- 34.Su F-H, Bai C-H, Chu F-Y, et al. Significance and anamnestic response in isolated hepatitis B core antibody-positive individuals 18 years after neonatal hepatitis B virus vaccination in Taiwan. Vaccine. 2012;30:4034–9. doi: 10.1016/j.vaccine.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Arnold D, Crowther M, Meyer R, et al. Misleading hepatitis B test results due to intravenous immunoglobulin administration: implications for a clinical trial of rituximab in immune thrombocytopenia. Transfusion. 2010;50:2577–81. doi: 10.1111/j.1537-2995.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 36.Pisani G, Cristiano K, Marino F. External quality assessment programs for detection of HCV-RNA, HIV-RNA and HBV-DNA in plasma: improve proficiency of the participants observed after a 2 year period. Vox Sang. 2010;99:319–24. doi: 10.1111/j.1423-0410.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 37.Allain J-P, Cox L. Challenges in hepatitis B detection among blood donors. Curr Opin Hematol. 2011;18:461–6. doi: 10.1097/MOH.0b013e32834bac10. [DOI] [PubMed] [Google Scholar]
- 38.Han Y, Wang B, Liu H. A novel use of a routine quantitative system to analyze the activity, content and affinity of an antibody to hepatitis B core antigen. J Clin Virol. 2011;52:295–9. doi: 10.1016/j.jcv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Thomas H. Relative functional affinity of specific anti core IgG in different categories of hepatitis B virus infection. J Med Virol. 1997;51:189–97. doi: 10.1002/(sici)1096-9071(199703)51:3<189::aid-jmv8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Yang R, Gui X, Chen X, Zhu Y. Interference of replication between hepatitis B and C viruses in patients infected with HIV. J Med Virol. 2011;83:1159–64. doi: 10.1002/jmv.22102. [DOI] [PubMed] [Google Scholar]
- 41.Sünbül M, Leblebicioglu H, Esen S, et al. Response to hepatitis B vaccine in HBsAg/anti-HBs negative and anti-HBc positive subjects. Scand J Infect Dis. 2000;32:315–6. doi: 10.1080/00365540050165983. [DOI] [PubMed] [Google Scholar]