Abstract
Background
Platelet-rich plasma consists of platelets concentrated in a small volume of plasma and constitutes a reservoir of bio-modulators potentially useful in tissue repair. The amounts of bio-modulators detectable in platelet-rich plasma prepared with various commercial or “in house” methods have been reported, but virtually all the analyses described have been performed on platelet-rich plasma derived from healthy donors. Since leucocyte contamination is technically unavoidable, we investigated whether platelet-rich plasma prepared from patients could contain different amounts of bio-modulators because of a possible activated status of the leucocytes.
Materials and methods
We evaluated platelet-rich plasma prepared with three different techniques (the commercial Vivostat and Biomet recover GPS II systems and an “in house” method) starting from whole blood from healthy donors and patients. Specifically, we compared the levels of sHLA-I, sFasL, platelet-derived growth factor, transforming growth factors-beta and vascular endothelial growth factor in the platelet-rich plasma releasates according to the method of preparation and to the immune system activation status of the subjects.
Results
With the exception of sHLA-I levels, no differences were found in the surrogate indices of lymphocyte activation between healthy donors and patients. No significant differences were found in sHLA-I, sFasL, platelet-derived growth factor, transforming growth factors-beta and vascular endothelial growth factor levels detectable in platelet-rich plasma produced with the three different methods in either healthy donors or patients.
Discussion
On the whole our findings indicate that the overall content of bio-modulators in autologous platelet-rich plasma is not influenced by T-lymphocyte activation status, at least in patients with uncomplicated femoral fractures. The amounts of sFasL and sHLA-I detected in all the platelet-rich plasma releasates studied were very small, far below the amounts detectable in all clinically available blood derivatives and absolutely insufficient to induce sHLA-I and/or sFasL mediated immunomodulation.
Keywords: platelet-rich plasma, leucocyte contamination, activation of immune system
Introduction
Platelet-rich plasma (PRP) is a preparation of concentrated platelets in a small volume of plasma and can be produced using various different, relatively simple and low-cost techniques1,2. As an autologous product, PRP is intrinsically free of concerns over transmissible diseases and is, therefore, generally well accepted by patients and surgeons3. On this basis, there is considerable and increasing clinical interest in using PRP, alone or in combination with grafting materials, for topical applications in orthopaedic4, periodontal5,6, and plastic surgery7. The scientific rationale behind the use of these preparations lies in the fact that platelet granules are a reservoir of many growth factors/cytokines that are known to play crucial roles in hard and soft tissue repair8–12. For this reason immediately before local application, PRP is activated by addition of thrombin and calcium, a process that results in the extracorporeal formation of a jelly-like material called platelet gel (PG). Upon activation and gelation, platelets in PRP release α-granules via exocytosis. These granules contain numerous substance, including platelet-derived growth factor (PDGF)13, transforming growth factors-beta (TGFβ)14 and vascular endothelial growth factor (VEGF)15.
Theoretically, the local application of PG might deliver a higher level of growth factors to specific sites to stimulate tissue healing16,17. On this premise, the variable amounts of growth factors/cytokines in different PG preparations should be considered when administering PG for therapeutic purposes. However, there is still no consensus regarding the method of production, characterisation and standardisation of PG, which is delaying the establishment of clinically effective standards18,19. As a result, the efficacy of PG in clinical applications is still controversial.
Any method used to prepare PRP includes centrifugation, which may induce a certain degree of platelet activation20,21. If platelets are activated during the process of producing PRP, the growth factors in the platelets will be released into the plasma, most of which is discarded. This would have the consequence of decreasing the efficacy of PRP when the product is applied to surgical sites. Furthermore, the density of some subgroups of white blood cells is nearly the same as that of platelets. Therefore, almost all preparations of locally applicable PRP or PG contain considerable amounts of leucocytes22–24. Although contaminating white blood cells contribute to the overall growth factor content of locally applied PRP or PG few data are available about the ex vivo effects of contaminating leucocytes in these products. Moreover, what little information there is on this topic in the scientific literature is based on blood derivatives produced from healthy donors, not taking into account the influence of the possible activation of leucocytes circulating in the blood of the real patients for whom PRP may be proposed.
On this background, we compared the levels of PDGF, TGFβ1 and VEGF in the releasates of PRP produced with three different techniques, the commercial Vivostat (Copenaghen, Denmark) and Biomet recover GPS II (Warsaw, Indiana, USA) systems and an “in house” method, starting from the blood of healthy donors and patients with femoral fractures.
Finally, leucocyte contamination involves the release of soluble molecules such as HLA class I (sHLA-I) and Fas ligand (sFasL). Our group has long been concerned about the immunomodulatory role of sHLA-I molecules in all clinically available blood derivatives such as blood components for transfusion, clotting factors and intravenous immunoglobulin25–27. The biological activities of classical and not classical sHLA-I molecules have been demonstrated in in vitro and ex vivo experiments. It has been reported that following the binding of adequate amount of sHLA-I to CD8, ILT and NCR membrane receptors on activated lymphocytes, neutrophils and natural killer cells, respectively, there is a cascade of events: up-regulation of membrane Fas molecules, release of sFasL and transcriptional and post-transcriptional modulation of TGFβ1, which, in turn, have been shown to have functional consequences such as induction of apoptosis, impairment of chemotaxis and antigen responses and loss of cytotoxic competence28–30. In this study we, therefore, also compared sHLA-I and sFasL levels in the releasates of PRP produced from healthy donors and patients with the three different, above-cited methods in order to evaluate a potential modulation of leucocyte activity involved in tissue healing, possibly dependent on activation of the immune system.
Materials and methods
Patients and healthy donors
This study was approved by the local human subjects ethics board. Following information about the nature and the objectives of our study, consent was obtained (according to World Medical Association Helsinki Declaration) for the collection of blood from 15 male, non-smokers, aged 35 to 59 years with fracture of the femur and ten healthy volunteers, whose demographics, including median age and sex distribution, were comparable to those of the patients. The blood was used to prepare PRP with the techniques described below.
Preparation of platelet-rich plasma
“In house” platelet gel
The day prior to surgery, 450 mL of whole blood were collected by venepuncture in the same way from patients and donors. The blood was immediately centrifuged at 1,700 rpm for 7 minutes at 20 °C to obtain packed red blood cells and PRP. The autologous packed red cells were infused back into the patients. The PRP was centrifuged to obtain platelet-poor plasma and platelet concentrate. The platelet-poor plasma was immediately frozen at −80 °C in a mechanical refrigerator and the frozen plasma was then kept at +4 °C for 18 hours to enable spontaneous thawing. Cryodepleted plasma was removed and the residual cryoprecipitate dissolved in 30 mL of plasma. The platelet concentrate was kept at +22 °C under continuous agitation. One platelet aliquot was mixed with one aliquot of cryoprecipitate in a sterile plastic Petri dish. Analyses were conducted on 500 μL of the biological sample. For every 10 mL of platelet concentrate-cryoprecipitate solution, 1 mL of autologous thrombin and 1 mL of calcium gluconate were added and then the contents of the Petri dish were mixed in order to produce a gel-like material in 10–15 minutes. Cryoplatelet gel was kept at room temperature and used within 8 hours. Six millilitres of the collected whole blood were transferred to dry tubes and then placed on a tray to settle for 15 minutes before the cells and platelets were counted.
Vivostat-produced platelet-rich fibrin
Blood samples were taken by venepuncture in the same way for patients and donors. The Vivostat System (ConvaTec, Skillman, New Jersey, USA) has three components: an automatic processor unit, an applicator unit and a single use kit providing the required consumables, including the unique Spraypen for application of the platelet-rich fibrin. Venous blood (120 mL) was drawn into a sterile preparation unit containing citrate buffer and the anti-fibrinolytic agent tranexamic acid. The biochemical process that produces the final product, in aliquots of 6 mL, takes only 25 minutes. The concentrations of platelets and fibrin in Vivostat platelet-rich fibrin, are approximately 10 and 7 times greater, respectively, than those in the donor’s blood. Batroxobin acts on plasma fibrinogen to catalyse the release of fibrin peptide A. The result is a fibrin I polymer soluble in an acid ambient without the activation of factor XIII. The fibrin I polymer is isolated by centrifugation and added to sodium acetate buffer at the concentration of 0.2 mol/L at pH 4. The purified fibrin I solution obtained is transferred to the applicator (500 μL of the biological sample were collected for analysis), including a syringe of 1 mL of carbonate/bicarbonate buffer at the concentration of 0.75 mol/L (pH 10). In the presence of Ca2+ at a neutral pH, the final PG is obtained: in these conditions endogenous prothrombin is converted to thrombin, cleaving the fibrinopeptide B from fibrin I to fibrin II. Six millilitres of the collected whole blood were transferred to dry tubes and placed on a tray to settle for 15 minutes before counting the cells and platelets.
The GPS II system
Blood samples were taken by venepuncture in the same way for patients and donors. For each patient and donor, 52 mL of blood were collected into a 60 mL syringe containing 8 mL of adenosine-citrate-dextrose acid (ACD-A) anticoagulant. A 12 mL syringe containing 1.6 mL of ACD-A was used to collect 10.4 mL of blood in order to provide a base measurement. The PRP, prepared from anti-coagulated blood and contained in the 60 mL syringes, was transferred to the GPS II and centrifuged for 15 minutes at 3,200 rpm (755 VES-230 V, Biomet1 Biologics, LLC). The buffy coat containing the majority of the platelets was then agitated for 30 seconds. The PRP was aspirated into 12 mL syringes (500 μL of the biological sample were collected for analysis).
Determination of TGFβ1, VEGF, PDGF, sHLA-I, sCD8 and sFasL concentrations
Following the preparation of PRP from healthy donors and patients using each of the three different techniques, 500 μL of each biological sample were centrifuged at 2,500 rpm for 15 minutes; the resultant supernatants were collected and the concentrations of sFasL, sHLA-I, PDGF, VEGF, sCD8 and TGF β1 molecules in them were evaluated.
The concentrations of sHLA-I and sFasL molecules were measured by double-determinant immunoassays, as previously described31. The concentrations of TGFβ1, PDGF, VEGF and sCD8 were also evaluated by double-determinant immunoassays using commercially available kits (Quantikine R&D System Inc. Minneapolis, Minnesota, USA and Bender MedSystems, Vienna, Austria, respectively).
Statistical analysis
All variables are expressed in the text and in the figures as median values with interquartile ranges (25th–75th percentiles). One-way analysis with Wilcoxon’s test was used to compare the values of all the parameters between the two groups (healthy donors and patients).
Results
Lymphocyte activation parameters
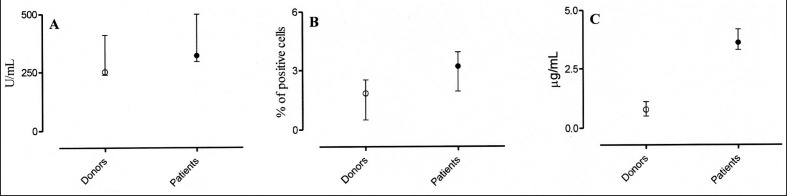
We evaluated the sCD8 levels, an indicator of T-cell activation32,33, in donors’ and patients’ plasma. As shown in Figure 1 (panel A), sCD8 levels in the plasma of healthy subjects (range, 241–355 U/mL) were not statistically different from those in patients (range, 229–531 U/mL). Likewise, the expression of CD25 molecules34 on the T cells did not differ statistically between healthy donors (range, 0.9–2.44%) and patients (range, 1.5–4.4%) (Figure 1, panel B).
Figure 1.
sCD8, CD25 and sHLA-I expression, evaluated as indicators of lymphocyte activation, in healthy donors (○) and in patients (●). Minimal differences were observed in sCD8 levels and CD25 expression, as shown in panels A and B, respectively. In contrast, in all the enrolled patients sHLA-I levels, panel C, were significantly more elevated if compared to healthy subjects (p =0,002). All variables were expressed as median and range interquartile (25°–75° percentile).
In contrast, being a biological response modifier as well as a marker of activation35,36, plasma sHLA-I levels were slightly, but significantly, higher in patients than in healthy donors (range, 0.54–1.12 μg/mL and 3.4–4.6 μg/mL, respectively) (Figure 1, panel C).
Determination of PDGF, VEGF, TGF β1, sFasL and sHLA-I molecules
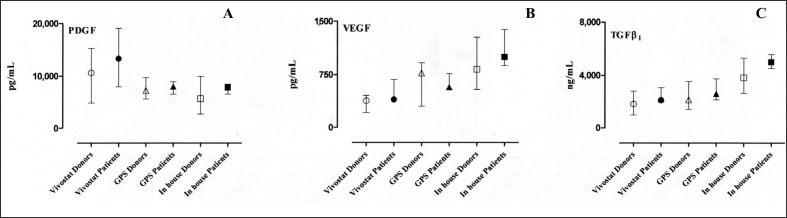
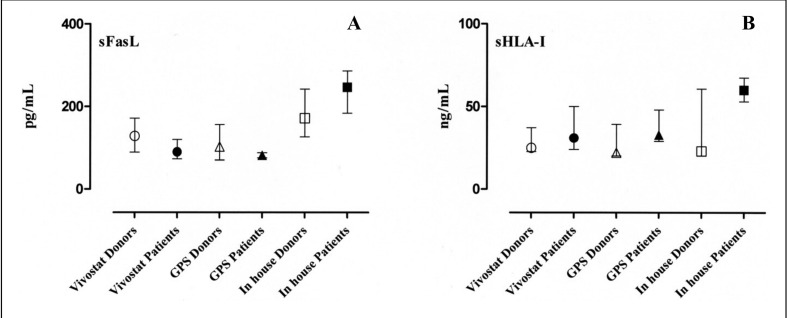
The results of the measurements of PDGF, VEGF and TGFβ1 in the patients’ and healthy subjects’ PRP releasates are shown in Figure 2, panels A, B and C, respectively. Similarly, the levels of sFasL and sHLA-I molecules in the releasates are presented in Figure 3, panels A and B, respectively. All the detected quantities are expressed as median and interquartile range (25th–75th percentiles).
Figure 2.
The concentrations of PDGF, VEGF, and TGFβ1 molecules in the releasates of PRP prepared with three different techniques both from healthy donors and from patients are shown in panels A, B and C, respectively. Wilcoxon’s test did not show statistically significant differences between any of the preparations. All variables are expressed as median and interquartile range (25th–75th percentiles).
Figure 3.
Concentrations of sFasL and sHLA-I molecules in the releasates of PRP prepared with three different techniques both from healthy donors and from patients are shown in panels A and B, respectively. Wilcoxon’s test did not show statistically significant differences between any of the preparations. All variables are expressed as median and interquartile range (25th–75th percentiles).
Platelet-derived growth factor
The median, range, and the lower and upper values of the 95% confidence interval (CI) of the mean of PDGF levels in PRP prepared with the Vivostat system were 10,569 pg/mL (range 3,169–19,417 pg/mL; 95% CI 5947–14311 pg/mL) for healthy donors and 13,288 pg/mL (range 7,922–19,437 pg/mL; 95% CI 6,569–20,350 pg/mL) for patients. The corresponding values for PRP prepared with the Biomet recover GPS II system were 7,246 pg/mL (range 3,945–12,900 pg/mL; 95% CI 5,635–9,472 pg/mL) for healthy donors and 7,988 pg/mL (range 5,849–9,023 pg/mL; 95% CI 6,169–9,375 pg/mL) for patients. The values in PRP prepared with an “in house” system were 5,671 pg/mL (range 1,799–10,134 pg/mL; 95% CI 3,532–8,131 pg/mL) for healthy donors and 7,788 pg/mL (range 6,209–8,791 pg/mL; 95% CI 6,274–8,790 pg/mL) for patients.
Vascular endothelial growth factor
The median, range, and the lower and upper values of the 95% CI of the mean of VEGF levels in PRP prepared with the Vivostat system were 379 pg/mL (range 127–554 pg/mL; 95% CI 252–448 pg/mL) for healthy donors and 398 pg/mL (range 393–734 pg/mL; 95% CI 311–707 pg/mL) for patients. When the Biomet recover GPS II system was used the values were 770 pg/mL (range 195–1,258 pg/mL; 95% CI 412–924 pg/mL) for donors and 573 pg/mL (range 527–919 pg/mL; 95% CI 434–835 pg/mL) for patients, while the PRP prepared with the “in house” system from healthy donors contained 826 pg/mL (range 213–1,403 pg/mL; 95% CI 549–1,153 pg/mL) and that from patients contained 1,000 pg/mL (range 769–1,494 pg/mL; 95% CI 755–1,496 pg/mL).
Transforming growth factor β1
The median, range, and the lower and upper values of the 95% CI of the mean of TGFβ levels in PRP prepared with the Vivostat system were 1,828 ng/mL (range 698–5,742 ng/mL; 95% CI 1,064–3,230 ng/mL) for healthy donors and 2,121 ng/mL (range 1,998–3,086 ng/mL; 95% CI 1,752–3,142 ng/mL) for patients. The corresponding values in PRP prepared with the Biomet recover GPS II system was 2,154 ng/mL (range 1,065–4,387 ng/mL; 95% CI 1,652–3,290 ng/mL) for donors and 2,601 ng/mL (range 2,134–3,999 ng/mL; 95% CI 1,844–3,907 ng/mL) for patients. When the “in house” system was used, the PRP from healthy donors contained 3,828 ng/mL (range 2,217–5,969 ng/mL; 95% CI 3,052–5,003 ng/mL), while that from patients contained 5,021 ng/mL (range 4,190–6,089 ng/mL; 95% CI 4,217–5,903 ng/mL).
Soluble Fas ligand
The median, range, and the lower and upper values of the 95% confidence interval (CI) of the mean of sFasL levels in PRP prepared with the Vivostat system were 128 pg/mL (range 86–225 pg/mL; 95% CI 100–174 pg/mL) for healthy donors and 90 pg/mL (range 56–128 pg/mL; 95% CI 62–129 pg/mL) for patients. The corresponding values for PRP prepared with the Biomet recover GPS II system were 102 pg/mL (range 50–270 pg/mL; 95% CI 74–174 pg/mL) for healthy donors and 82 pg/mL (range 72–88 pg/mL; 95% CI 74–90 pg/mL) for patients, while those for the PRP prepared with the “in house” system were 172 pg/mL (range 114–280 pg/mL; 95% CI 140–224 pg/mL) for healthy donors and 247 pg/mL (range 159–294 pg/mL; 95% CI 170–305 pg/mL) for patients.
Soluble HLA-I
The median, range, and the lower and upper values of the 95% CI of the mean of sHLA-I levels in PRP prepared with the Vivostat system were 25 ng/mL (range 16–100 ng/mL; 95% CI 17–52 ng/mL) for healthy donors and 31 ng/mL (range 21–62 ng/mL; 95% CI 16–56 ng/mL) for patients. The values for PRP prepared with the Biomet recover GPS II system were 22 ng/mL (range 16–100 ng/mL; 95% CI 14–54 ng/mL) for healthy donors and 33 ng/mL (range 29–54 ng/mL; 95% CI 24–51 ng/mL) for patients. The corresponding values in PRP prepared with the “in house” system were 23 ng/mL (range 16–170 ng/mL; 95% CI 9–92 ng/mL) for healthy donors and 60 ng/mL (range 49–72 ng/mL; 95% CI 50–71 ng/mL) for patients.
Finally, the white blood cell and platelets counts in whole blood drawn from healthy donors and patients and the corresponding counts in PRP are summarised in Tables I and II, respectively.
Table I.
White blood cell counts (×109/L) in PRP prepared from healthy donors (left column) and from patients (right column) with three different methods and in whole blood from the donors and patients.
| Vivostat system | 13.89 (range 11.56–17.88; 95% CI 11.75–16.39) | 14.99 (range 9.28–18.65; 95% CI 11.36–17.85) |
| Biomet recover GPS II system | 6.23 (range 5.23–8.20; 95% CI 5.28–7.48) | 5.31 (range 3.5–11.30; 95% CI 3.23–8.93) |
| “In house” system | 5.56 (range 4.10–9.36; 95% CI 3.89–7.97) | 5.22 (range 2.08–9.74; 95% CI 2.96–8.30) |
| Peripheral blood count | 5.85 (range 4.52–7.61; 95% CI 5.84–7.43) | 6.70 (range 3.57–9.20; 95% CI 4.44–8.39) |
Table II.
Platelet counts (×109/L) in PRP prepared from healthy donors (left column) and from patients (right column) with three different methods and in whole blood from the donors and patients.
| Vivostat system | 1,933 (range 1,432–3,092; 95% CI 1,408–2,627) | 1,898 (range 1,655–2,408; 95% CI 1,632–2,213) |
| Biomet recover GPS II system | 2,500 (range 1,821–4,034; 95% CI 1,792–3,737) | 2,934 (range 2,223–3621; 95% CI 2,468–3,535) |
| “In house” system | 2,246 (range 1,462–2760; 95% CI 1,741–2,686) | 2,429 (range 1,867–2,809; 95% CI 1,971–2,728) |
| Peripheral blood count | 227 (range 185–263; 95% CI 200–255) | 258 (range 187–299; 95% CI 216–295) |
Discussion
Despite conflicting results about the clinical efficacy of PRP37, an ever-growing body of evidence indicates that the rationale for the therapeutic use of this product is the release of growth factors/cytokines initiating the processes of inflammation38,39, proliferation40,41 and remodelling42 that result in healing or scarring. On these bases, an increasing number of studies are being focused on the need to standardise production, investigating the equivalence of or differences among the methods and devices available to produce PRP43,44.
As leucocyte contamination appears to be unavoidable during the preparation of PRP, in this study we investigated possible differences in amounts of PDGF, VEGF, TGFβ1, sFasL and sHLA-I in the releasates of PRP prepared with three different systems starting from the whole blood of healthy donors and patients possibly with inflammation whose immune competent cells could, therefore, be circulating in an activated state and possibly make different contributions to the milieu of bio-modulators. Moreover, since the white blood cells that are particularly concentrated in PRP are mononuclear cells, predominantly lymphocytes, we evaluated the percentage of CD25 expression and the plasma levels of sCD8 and sHLA-I, as T-lymphocyte activation markers, immediately before the preparation of the PRP. We found that, compared to T lymphocytes from healthy donors, T lymphocytes from the patients had slightly higher levels of sCD8, CD25 expression and sHLA-I, although the difference was statistically significant (P =0.002) only for sHLA-I, probably because the levels of this last molecule, being a marker of activation, are also dependent on the activation of other types of leucocytes.
With regards to the amounts of PDGF, VEGF, TGFβ1, sFasL and sHLA-I table in the releasates of the PRP analysed, although there was a small tendency to higher values in patients’ preparations than in those of healthy subjects, no statistically significant differences were found depending on the different systems used for preparation of the products.
With the awareness that further analysis and larger studies are desirable, on the whole our findings indicate that the overall content of PDGF, VEGF and TGFβ1 in PRP does not appear to be influenced by T lymphocyte activation status, at least in patients with uncomplicated fracture of the femur. Furthermore, the amounts of sFasL and sHLA-I detected in all the PRP releasates analysed were very limited, far below the amounts in all clinically available blood derivatives and absolutely insufficient to induce sHLA-I and/or sFasL mediated immunomodulation.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–9. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 2.Vassallo RR, Murphy S. A critical comparison of platelet preparation methods. Curr Opin Hematol. 2006;13:323–30. doi: 10.1097/01.moh.0000239703.40297.a5. [DOI] [PubMed] [Google Scholar]
- 3.Mei-Dan O, Lippi G, Sánchez M, et al. Autologous platelet-rich plasma: a revolution in soft tissue sports injury management? Phys Sportsmed. 2010;38:127–35. doi: 10.3810/psm.2010.12.1835. [DOI] [PubMed] [Google Scholar]
- 4.Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 5.Javed F, Al-Askar M, Al-Rasheed A, Al-Hezaimi K. Significance of the platelet-derived growth factor in periodontal tissue regeneration. Arch Oral Biol. 2011;56:1476–84. doi: 10.1016/j.archoralbio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Del Fabbro M, Bortolin M, Taschieri S, Weinstein R. Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J Periodontol. 2011;82:1100–11. doi: 10.1902/jop.2010.100605. [DOI] [PubMed] [Google Scholar]
- 7.Farrior E, Ladner K. Platelet gels and hemostasis in facial plastic surgery. Facial Plast Surg. 2011;27:308–14. doi: 10.1055/s-0031-1283050. [DOI] [PubMed] [Google Scholar]
- 8.O’Shaughnessey KM, Panitch A, Woodell-May JE. Blood-derived anti-inflammatory protein solution blocks the effect of IL-1β on human macrophages in vitro. Inflamm Res. 2011;60:929–36. doi: 10.1007/s00011-011-0353-2. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Chen WH, Zao B, et al. Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. Biomaterials. 2011;32:5847–54. doi: 10.1016/j.biomaterials.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105:13–33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 11.Giovanini AF, Gonzaga CC, Zielak JC, et al. Platelet-rich plasma (PRP) impairs the craniofacial bone repair associated with its elevated TGF-β levels and modulates the co-expression between collagen III and α-smooth muscle actin. J Orthop Res. 2011;29:457–63. doi: 10.1002/jor.21263. [DOI] [PubMed] [Google Scholar]
- 12.He L, Lin Y, Hu X, et al. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–13. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Okuda K, Kawase T, Momose M, et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74:849–57. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]
- 14.Cho HS, Song IH, Park SY, et al. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31:212–8. doi: 10.3343/kjlm.2011.31.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:51–5. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Rodeo SA, Delos D, Williams RJ, et al. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40:1234–41. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 17.Birang R, Torabi A, Shahabooei M, Rismanchian M. Effect of plasma-rich in platelet-derived growth factors on peri-implant bone healing: An experimental study in canines. Dent Res J. 2012;9:93–9. doi: 10.4103/1735-3327.92961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borzini P, Mazzucco L, Giampaolo A, Hassan HJ. Platelet gel - the Italian way: a call for procedure standardization and quality control. Transfus Med. 2006;16:303–4. doi: 10.1111/j.1365-3148.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 19.Su CY, Kuo YP, Nieh HL, et al. Quantitative assessment of the kinetics of growth factors release from platelet gel. Transfusion. 2008;48:2414–20. doi: 10.1111/j.1537-2995.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- 20.Ali SF. Platelet activation of platelet concentrates derived from buffy coat and apheresis methods. Transfus Apher Sci. 2011;44:11–3. doi: 10.1016/j.transci.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Edvardsen L, Taaning E, Dreier B, et al. Extracellular accumulation of bioactive substances during preparation and storage of various platelet concentrates. Am J Hematol. 2001;67:157–62. doi: 10.1002/ajh.1099. [DOI] [PubMed] [Google Scholar]
- 22.Weibrich G, Kleis WK, Kunz-Kostomanolakis M, et al. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants. 2001;16:693–9. [PubMed] [Google Scholar]
- 23.Weibrich G, Kleis WK, Hafner G, et al. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res. 2003;14:357–62. doi: 10.1034/j.1600-0501.2003.00810.x. [DOI] [PubMed] [Google Scholar]
- 24.Cieslik-Bielecka A, Bielecki T, Gazdzik TS, et al. Autologous platelets and leukocytes can improve healing of infected high-energy soft tissue injury. Transfus Apher Sci. 2009;41:9–12. doi: 10.1016/j.transci.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Ghio M, Contini P, Mazzei C, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93:1770–7. [PubMed] [Google Scholar]
- 26.Ghio M, Contini P, Ottonello L, et al. Effect of clotting factors concentrates on lymphocyte and neutrophil function in vitro. Thromb Haemost. 2003;89:365–73. [PubMed] [Google Scholar]
- 27.Ghio M, Contini P, Negrini S, et al. sHLA-I contaminating molecules as novel mechanism of ex vivo/in vitro transcriptional and posttranscriptional modulation of transforming growth factor-beta in CD8+ T lymphocytes and neutrophils after intravenous immunoglobulin treatment. Transfusion. 2010;50:547–55. doi: 10.1111/j.1537-2995.2009.02479.x. [DOI] [PubMed] [Google Scholar]
- 28.Puppo F, Contini P, Ghio M, et al. Soluble human MHC class I molecules induce soluble Fas ligand secretion and trigger apoptosis in activated CD8(+) Fas (CD95)(+) T lymphocytes. Int Immunol. 2000;12:195–203. doi: 10.1093/intimm/12.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Ottonello L, Ghio M, Contini P, et al. Nonleukoreduced red blood cell transfusion induces a sustained inhibition of neutrophil chemotaxis by stimulating in vivo production of transforming growth factor-beta1 by neutrophils: role of the immunoglobulinlike transcript 1, sFasL, and sHLA-I. Transfusion. 2007;47:1395–404. doi: 10.1111/j.1537-2995.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- 30.Spaggiari GM, Contini P, Carosio R, et al. Soluble HLA class I molecules induce natural killer cell apoptosis through the engagement of CD8: evidence for a negative regulation exerted by members of the inhibitory receptor superfamily. Blood. 2002;99:1706–14. doi: 10.1182/blood.v99.5.1706. [DOI] [PubMed] [Google Scholar]
- 31.Ghio M, Contini P, Setti M, et al. sHLA-I Contamination, a novel mechanism to explain ex vivo/in vitro modulation of IL-10 synthesis and release in CD8(+) T lymphocytes and in neutrophils following intravenous immunoglobulin infusion. J Clin Immunol. 2010;30:384–92. doi: 10.1007/s10875-009-9364-y. [DOI] [PubMed] [Google Scholar]
- 32.Osmond DH, Shiboski S, Bacchetti P, et al. Immune activation markers and AIDS prognosis. AIDS. 1991;5:505–11. doi: 10.1097/00002030-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa S, Matsubara T, Tsuji K, et al. Serum soluble CD4 and CD8 levels in Kawasaki disease. Clin Exp Immunol. 1991;86:134–9. doi: 10.1111/j.1365-2249.1991.tb05785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swaak AJ, Hintzen RQ, Huysen V, et al. Serum levels of soluble forms of T cell activation antigens CD27 and CD25 in systemic lupus erythematosus in relation with lymphocytes count and disease course. Clin Rheumatol. 1995;14:293–300. doi: 10.1007/BF02208342. [DOI] [PubMed] [Google Scholar]
- 35.Brieva JA, Villar LM, Leoro G, et al. Soluble HLA class I antigen secretion by normal lymphocytes: relationship with cell activation and effect of interferon-gamma. Clin Exp Immunol. 1990;82:390–5. doi: 10.1111/j.1365-2249.1990.tb05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bresciani A, Pirozzi G, Spera M, et al. Increased level of serum HLA class I antigens in patients with systemic lupus in patients with systemic lupus erythematosus. Correlation with disease activity. Tissue Antigens. 1998;52:44–50. doi: 10.1111/j.1399-0039.1998.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 37.Carless PA, Rubens FD, Anthony DM, et al. Platelet-rich-plasmapheresis for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;3:CD004172. doi: 10.1002/14651858.CD004172. [DOI] [PubMed] [Google Scholar]
- 38.van Buul GM, Koevoet WL, Kops N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362–70. doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 39.Boswell SG, Cole BJ, Sundman EA, et al. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–39. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Demidova-Rice TN, Wolf L, Deckenback J, et al. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS One. 2012;7:e32146. doi: 10.1371/journal.pone.0032146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park HB, Yang JH, Chung KH. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Up-regulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol. 2011;46:265–73. doi: 10.5045/kjh.2011.46.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagata M, Hoshina H, Li M, et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: coordinated activation of bone formation and resorption. Bone. 2012;50:1123–9. doi: 10.1016/j.bone.2012.02.631. [DOI] [PubMed] [Google Scholar]
- 43.Castilloux JF, Moffat KA, Liu Y, et al. A prospective cohort study of light transmission platelet aggregometry for bleeding disorders: is testing native platelet-rich plasma non-inferior to testing platelet count adjusted samples? Thromb Haemost. 2011;106:675–82. doi: 10.1160/TH11-06-0378. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez M, Anitua E, Andia I. Poor standardization in platelet-rich therapies hampers advancement. Arthroscopy. 2010;26:725–6. doi: 10.1016/j.arthro.2010.03.002. [DOI] [PubMed] [Google Scholar]