Abstract
Background
The cause of transfusion-related immunomodulation (TRIM) has proved tantalisingly elusive. An ever-growing body of evidence indicates that the infusion of large amounts of soluble and cell-associated antigens into a recipient can somehow induce TRIM. One soluble molecule that has been implicated in TRIM is soluble human leucocyte antigen I (sHLA-I). However, patients infused with large amounts of sHLA-I do not always and unambiguously experience TRIM. As soluble CD8 (sCD8) molecules have been shown to capable of binding membrane and soluble HLA-I molecules, we focused on sCD8 as a possible modulator of sHLA-I-mediated TRIM.
Material and methods
To this aim we compared the up-regulation of circulating sCD8 in plasma from patients suffering from the same pathology, but chronically transfused with two different blood derivatives: pre- and post-storage leucodepleted red blood cells which contain low and high levels of contaminating sHLA-I, respectively.
Results
Significantly larger amounts of sCD8 circulating molecules were detectable in the plasma of patients transfused with post-storage leucodepleted red blood cells whose supernatants contained significantly larger amounts of sHLA-I contaminating molecules.
Conclusion
With the limitation of indirect evidence, this report introduces a new facet of the bioactivity of sCD8 as a possible modulator of sHLA-I-mediated TRIM.
Keywords: TRIM, sHLA-I, sCD8
Introduction
Thanks to the work of the Transfusion Medicine colleagues, the clinical and therapeutic uses of blood and its derivatives have acquired such a level of safety and simplicity that we tend to forget that transfusions are by far the most frequently performed tissue transplants, and yet there are few certainties regarding their immunological consequences. In fact, intentionally excluding potential iatrogenic infections from such consequence, it has been known for years that blood transfusions can induce immunomodulating effects, more conveniently defined as immunosuppressive, in a syndrome that has been termed transfusion-related immunomodulation (TRIM). Clinical evidence for the existence of TRIM as a biological phenomenon with beneficial clinical effects in humans has been available since 1973, whereas the existence of deleterious clinical effects of TRIM has not been definitively confirmed. In any case, there is an ever-growing body of evidence indicating that the infusion of large amounts of soluble and cell-associated antigens into a recipient can somehow induce TRIM. The various factors that have been implicated in this phenomenon include soluble HLA-I (sHLA-I) molecules1,2.
The biological activities of classical and non-classical sHLA-I molecules have been demonstrated in in vitro and ex vivo experiments. It has been reported that following the binding of adequate amount of sHLA-I to CD8, ILT and NCR membrane receptors on activated lymphocytes, neutrophils and natural killer cells, respectively, there is a cascade of events: up-regulation of membrane Fas molecules, release of sFasL and transcriptional and post-transcriptional modulation of TGFβ1, which, in turn, have been shown to have functional consequences such as induction of apoptosis, impairment of chemotaxis and antigen responses and loss of cytotoxic competence3–5.
Although detectable in all clinically available blood derivatives, the largest amount of sHLA-I molecules has been detected in packed red blood cells (RBC) units prepared without a pre-storage leucodepletion step. As residual leucocytes which undergo membrane damage during storage are the major source of sHLA-I molecules, the duration of storage appears to be a potentially important factor in the total amount of sHLA-I in RBC units6.
On these premises, if a patient with lymphocytes, neutrophils and natural killer cells circulating in activated status is transfused with a large number of non-leucodepleted RBC, possibly stored for a long time, he should experience sHLA-I-mediated TRIM; however this is not always and inescapably the case: why?
One way by which the biological functions of bioactive antigens can be inactivated, besides an antibody response, is the production and/or release of soluble ligands of the protein to be inactivated. Most of these soluble receptors, precisely because of their nature of being soluble, capture their respective ligands without any cellular functional consequences, unlike what would occur following binding by the analogous membrane isoform.
Human soluble CD8 molecules (sCD8) are secreted by activated CD8+ T lymphocytes7. On this basis sCD8 titre has been considered a surrogate index of activation of the immune system; nevertheless, the real immunological role of sCD8 is poorly defined. The few available data on its functional role indicate that sCD8 binding to the alpha3 domain of the heavy chain stabilises the membrane HLA class I molecule interfering with T-cell activation and proliferation by an allosteric block7,8. Moreover, we have previously shown that sCD8 is fully capable of binding sHLA-I as well4. On the whole, these findings point to an immunoregulatory role for sCD8.
As high levels of sCD8 have been found in polytransfused human immunodeficiency virus-negative haemophiliacs9 and polytransfused patients suffering from beta-thalassaemia major have been shown to have higher sCD8 levels than normal controls10, we focused on sCD8 as a possible modulator of sHLA-I-mediated TRIM.
Materials and methods
Patients and controls
Following approval by the local ethical board for studies and in human subjects and informed consent from the participants, 20 patients with beta-thalassaemia major who had been transfused with pre-storage leucodepleted RBC for at least 5 years were enrolled in the study (group A). Another 20 patients with beta-thalassaemia major, but routinely transfused with post-storage leucodepleted RBC, were also enrolled (group B). All the patients entering the study were reportedly free from other diseases. Finally, 20 patients with beta-thalassemia minor who had never been transfused but whose demographics were comparable to the transfused patients, including median age and sex distribution, were enrolled into the study as a control arm.
Blood components and biological samples
Based on information in the patients’ clinical records, the patients were transfused with an average of 27±3 RBC units infused per year. The average period of storage of the RBC leucodepleted before or after storage was 14±2 days in both cases. Immediately before transfusion, samples of RBC supernatants were collected and centrifuged at 12,000 g for 2 minutes in order to evaluate the concentrations of sHLA-I. Plasma samples were drawn from enrolled patients immediately before transfusion, which was performed at least 21 days after the previous one.
Determination of sHLA-I and sCD8 concentrations
The concentrations of sHLA-I molecules were determined by double-determinant immunoassay (DDIA), as previously described4. The concentrations of sCD8 were also determined by a double-determinant immunoassay using a commercially available kit (Bender MedSystems, Vienna, Austria).
Statistical analysis
All values of all variables are expressed in the text and figures as medians with their interquartile range (25th-75th percentile). A comparison between results for the two groups of transfused patients was performed by one-way analysis with Wilcoxon’s test. Correlations between sCD8 and sHLA-I levels were explored by Spearman’s correlation coefficient. r values between 0.8 and 1 and 0.7 to 0.8 were considered to indicate very strong and strong correlation between variables, respectively. Variables with a Spearman’s correlation coefficient <0.6 were considered poorly correlated.
Results
sCD8 plasma levels
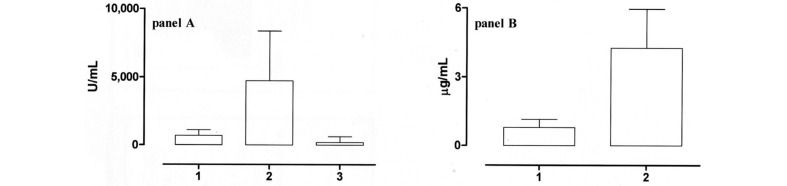
The plasma levels of sCD8 in patients and controls are shown in Figure 1, panel A. The median, range and 95% confidence interval (95% CI) of the means in the plasma of patients transfused with pre-storage leucodepleted RBC (group A, column 1) were 702 U/mL (range 324–1463 U/ml; 95% CI 577–839 U/mL). Group B patients (column 2), who had been transfused with post-storage leucodepleted RBC, had significantly higher levels of sCD8 (P <0.0001); in detail, the median level detected in this group was 4738 U/mL (range 1990–8363 U/mL; 95% CI 4,090–5,756 U/mL). Finally, the median plasma level of sCD8 in 20 patients with beta-thalassaemia who had never been transfused (column 3), who were enrolled as controls, was 205 U/mL (range 122–603 U/mL; 95% CI 188–324 U/mL).
Figure 1.
Panel A: levels of sCD8 in the plasma of patients transfused with pre-storage leucodepleted RBC (column 1), transfused with post-storage leucoreduced RBC (column 2) or never transfused (control, column 3). Panel B: levels of sHLA-I in the supernatant of pre-storage leucodepleted RBC (column 1) and post-storage leucoreduced RBC (column 2). The levels of sHLA-I were significantly higher (P <0.0001) in the post-storage leucoreduced RBC.
sHLA-I levels detectable in red blood cell units
sHLA-I levels detectable in RBC units are shown in Figure 1, panel B. More in detail, column 1 shows the amount in the supernatant of pre-storage leucodepleted RBC:median0.79μg/mL(range0.25–1.15μg/mL;95%CI 0.68–0.92 μg/mL). The post-storage leucodepleted RBC supernatants contained significantly more sHLA-I (P <0.0001, column 2): median 4.2 μg/mL (range 3–5.97 μg/mL; 95% CI 3.77–4.57 μg/mL).
Analysis of Spearman’s correlation coefficient
There was a very strong correlation between sCD8 and sHLA-I levels in all patients, with Spearman’s coefficient being ≥0.80 for all correlations (P ≤0.001 for all correlations).
Clinical follow-up
No clinical differences were observed between the groups of enrolled subjects during the 12-month period of follow-up.
Discussion
The cause of TRIM has proven tantalisingly elusive, although it appears quite clear that TRIM is dependent on multiple factors. Our group has long been concerned with one of these factors: the immunomodulatory role of sHLA-I molecules detectable in all available blood derivatives and hence infused into recipients following their clinical use6,11,12. Since there is a discrepancy between the significant in vitro immunosuppressive bioactivity of the large quantities of sHLA-I detectable in blood derivatives and the ambiguously demonstrable in vivo effects following transfusion, some further control element must be involved in sHLA-I-mediated TRIM.
Focusing on the possibility that this control could be exerted by sCD8 molecules, acting as soluble ligands of the protein to be inactivated, we compared two groups of patients suffering from the same pathology but chronically transfused with two differently prepared blood derivatives: pre- and post-storage leucodepleted RBC, which, by virtue of this difference, had low and high levels of contaminating sHLA-I, respectively. Group A patients, who received smaller amounts of sHLA-I, secreted low levels of sCD8 in the plasma. In contrast, the sCD8 levels in group B patients, who had been infused with large amounts of sHLA-I, were significant higher. Finally, an analogous control group of patients with beta-thalassemia minor, who had not, therefore, been transfused, had almost undetectable sCD8 plasma levels. No differences were found between the groups during the 12 months of clinical follow-up. Finally, there were no records of clinical events able to induce activation of the immune system of the enrolled subjects and thus be possible causes of up-regulation of sCD8 levels.
In our opinion, the simultaneous absence of TRIM, together with the possible cause-effect relationship between the amount of sHLA-I infused and the detected plasma levels of sCD8 endorsed by the Spearman’s correlation coefficient, support our hypothesis that sCD8 binding to sHLA-I could modulate this latter’s immunosuppressive bioactivity involved in the induction of TRIM, at least in polytransfused subjects.
With the limitation of deriving from a single category and a small number of patients, this report introduces a new facet of the possible bioactivity of sCD8; if confirmed, it might also address further studies on TRIM mechanisms. For example, while some authors have concluded that there is an association between transfusion of blood stored for more than 14 days and worse clinical outcomes, others have not found this association13,14. Could these discrepancies be dependent on different pre-existing titres of circulating sCD8 in the evaluated patients? In the present report, we hypothesize that increased amounts of sCD8 could be the result of sHLA-I infusion during transfusion; however as sCD8 can also be secreted following activation of the immune system for whatever reason, the levels of sCD8 could also be elevated independently of and/or prior to transfusions.
Although this latter aspect might appear marginal, in general the clinical conditions necessitating transfusions frequently involve activation of the immune system, thus making it susceptible to sHLA-I-mediated TRIM. Furthermore, there may be large amounts of circulating sCD8, capable of binding the sHLA-I and modulating its effects. While waiting to understand what sets this balance, sCD8 molecules appear to be a possible candidate as a new factor involved in the control of sHLA-I-mediated TRIM.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–95. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 3.Puppo F, Contini P, Ghio M, et al. Soluble human MHC class I molecules induce soluble Fas ligand secretion and trigger apoptosis in activated CD8(+) Fas (CD95)(+) T lymphocytes. Int Immunol. 2000;12:195–203. doi: 10.1093/intimm/12.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Ottonello L, Ghio M, Contini P, et al. Nonleukoreduced red blood cell transfusion induces a sustained inhibition of neutrophil chemotaxis by stimulating in vivo production of transforming growth factor-beta1 by neutrophils: role of the immunoglobulinlike transcript 1, sFasL, and sHLA-I. Transfusion. 2007;47:1395–404. doi: 10.1111/j.1537-2995.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- 5.Spaggiari GM, Contini P, Carosio R, et al. Soluble HLA class I molecules induce natural killer cell apoptosis through the engagement of CD8: evidence for a negative regulation exerted by members of the inhibitory receptor superfamily. Blood. 2002;99:1706–14. doi: 10.1182/blood.v99.5.1706. [DOI] [PubMed] [Google Scholar]
- 6.Ghio M, Contini P, Mazzei C, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93:1770–7. [PubMed] [Google Scholar]
- 7.Morgan CL, Price CP, Cohen SB, et al. Soluble CD8 stabilizes the HLA class I molecule by promoting beta2M exchange: analysis in real-time. Hum Immunol. 1999;60:442–9. doi: 10.1016/s0198-8859(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto N, Watanabe M, Matsuzuka F, et al. Lower concentration of serum soluble CD8 in severe Hashimoto’s disease. Clin Exp Immunol. 2004;137:601–5. doi: 10.1111/j.1365-2249.2004.02576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smid WM, Martens A, van der Meer J, et al. Soluble interleukin 2 receptor and soluble CD8 levels in previously treated human immunodeficiency virus-negative hemophiliacs multiply transfused with a monoclonal antibody-purified factor VIII concentrate. Transfusion. 1997;37:86–9. doi: 10.1046/j.1537-2995.1997.37197176956.x. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi G, Matera R, Minervini MM, et al. Serum levels of cytokines and soluble antigens in polytransfused patients with beta-thalassemia major: relationship to immune status. Haematologica. 1994;79:406–12. [PubMed] [Google Scholar]
- 11.Ghio M, Contini P, Negrini S, et al. sHLA-I contaminating molecules as novel mechanism of ex vivo/in vitro transcriptional and posttranscriptional modulation of transforming growth factor-beta in CD8+ T lymphocytes and neutrophils after intravenous immunoglobulin treatment. Transfusion. 2010;50:547–55. doi: 10.1111/j.1537-2995.2009.02479.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghio M, Contini P, Ottonello L, et al. Effect of clotting factors concentrates on lymphocyte and neutrophil function in vitro. Thromb Haemost. 2003;89:365–73. [PubMed] [Google Scholar]
- 13.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 14.Glynn SA. The red blood cell storage lesion: a method to the madness. Transfusion. 2010;50:1164–9. doi: 10.1111/j.1537-2995.2010.02674.x. [DOI] [PubMed] [Google Scholar]