Dear Sir,
The development of allo-antibodies against exogenous “wild-type” factor VIII (FVIII), known as “inhibitors”, is currently the most serious complication in the management of patients with haemophilia A. Inhibitors neutralise FVIII, leading to treatment failure and are, therefore, usually detected when bleeding episodes fail to respond to appropriate FVIII replacement. Although such inhibitors are most frequently seen in patients with severe haemophilia A, the development of antibodies against FVIII in mild haemophilia A can cause considerable clinical problems because the inhibitors, directed against both endogenous and exogenous FVIII, usually change the bleeding phenotype1. We present the case of a 12-year old male with mild haemophilia A (FVIII activity 7%) caused by a mutation in exon 12p. Arg593Cys within the A2 domain in whom successful 4-year long eradication of high-titre inhibitors was achieved by four standard doses of rituximab.
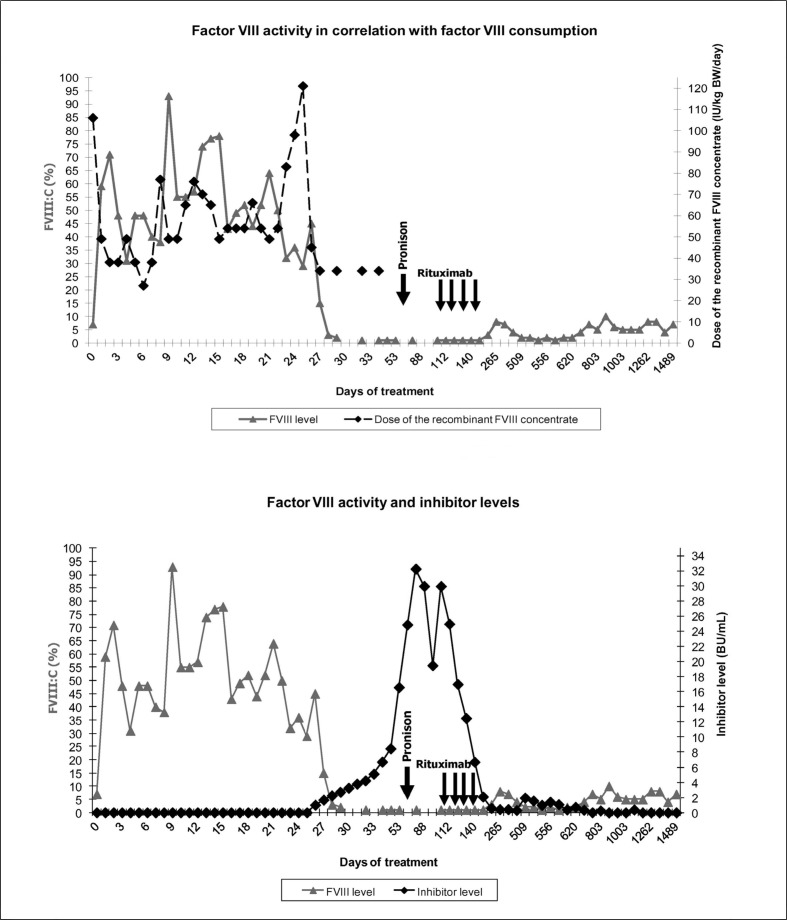
The patient urgently needed abdominal surgery because of acute appendicitis with perforation and peritonitis. Before the operation he was treated three times only with DDAVP and antifibrinolytics. The family and personal history was unremarkable except that the patient’s 3-year older brother was diagnosed with diabetes mellitus type 1 at the age of 5 years. Immediately before the surgery the patient was treated with a bolus of recombinant FVIII concentrate (Advate®, Baxter, Lessines, Belgium) and later with a continuous infusion of the same concentrate. On the 6th post-operative day mild intraperitoneal bleeding occurred. On the 8th post-operative day haematochezia appeared, the patient became haemodynamically unstable and computed tomography of the abdomen and pelvis revealed a pelvic fluid collection of 10×10 cm in size. FVIII activity was 38%: a bolus of FVIII concentrate was added and the FVIII activity increased to 87%. Given the severe intrapelvic bleeding, the treatment with FVIII by continuous infusion was continued for another 2 weeks trying to maintain the FVIII activity at 40–50%. In spite of an increase of FVIII consumption FVIII activity decreased from the 23th post-operative day, but inhibitors were still negative (Figure 1). Inhibitors were clearly positive on the 26th post-operative day, when the haematoma had almost cleared and the continuous infusion of FVIII was discontinued. However, four bolus injections of FVIII (36 U/kg/day) were additionally given on post-operative days 27, 29, 32 and 34 with the intention of introducing low-dose immune tolerance induction. The inhibitor titre increased to its highest level, 32 Bethesda units (BU)/mL on the 74th post-operative day (Figure 1).
Figure 1.
Factor VIII activity in correlation with factor VIII consumption and inhibitor levels.
Corticosteroid therapy at a dose of 1 mg/kg, started on day 79 post-operatively, lowered the inhibitor titre in 17 days from 32 to 20 BU/mL. At that time corticosteroid therapy was tapered and inhibitors titre rose again to 30 BU/mL on day 108 post-operatively. On day 112 rituximab was introduced at the standard dose of 375 mg/m2 per week for four weeks. By 4 months after the beginning of the rituximab therapy the titre of inhibitors was steadily decreasing to the level ×0.6 BU/mL and the concentration of FVIII slowly increasing to 4–9%. However, 14 months after the beginning of the rituximab therapy the inhibitor level increased again -but only up to 1.9 BU/mL- and FVIII activity fell to 2%. This situation lasted less than 4 months. Since then inhibitors have not been detectable and FVIII activity has been at the basal level, around 7% (Figure 1).
Huge and very painful muscle bleeds occurred with minimal trauma from day 42 until day 151 post-operatively, the period when FVIII activity was below 2%. These bleeds were successfully treated with recombinant human coagulation factor VIIa (NovoSeven®, Novo Nordisk A/S, Copenhagen, Denmark) and tranexamic acid (Cyklokapron®, Meda Manufacturing GmbH, Cologne, Germany). We noted that the patient needed this therapy only as long as he had pain. Later complete resorption of bleeding was achieved, independently of further treatment with NovoSeven. When the inhibitors decreased below 2 BU/mL and FVIII activity rose to at least 2% there were only occasional bleeds which were manageable with subcutaneous or intranasal DDAVP with or without the addition of tranexamic acid.
The concentrations of B lymphocytes 2, 7 and 12 months after beginning of the rituximab therapy were decreased (0.0-0.004-0.166×109/L; normal range according to age: 0.2–0.5×109/L). Despite transient B lymphocyte depletion the patient was without infection. At the next B lymphocyte measurement, 44 months after beginning rituximab therapy, their concentration was 0.274×109/L, which is in the normal range for the patient’s age (0.1–0.4×109/L). Despite B-cell recovery an increase in inhibitor concentration was not observed.
The management of patients with mild haemophilia A depends on the basal FVIII activity level, the type of bleeding, the type of surgical intervention or invasive procedure and the presence of inhibitors. The development of inhibitors in patients with mild/moderate haemophilia A is a major complication, because the bleeding phenotype of the patient becomes similar to that of a patient with severe or acquired haemophilia A1.
Some specific missense mutations, mostly located within the C1 and C2 domains of the light chain and within the A2 domain of the heavy chain of the FVIII molecule, are associated with an unexpectedly high incidence of inhibitors1. Eckhardt et al. collected data from 138 patients with mild/moderate haemophilia A over a 28-year period and identified a 10-fold increased risk of developing inhibitors in patients with the Arg593Cys missense mutation2.
In a study by Mauser-Bunschoten et al., age at first exposure, age at peak treatments, number of peak treatments and Arg593Cys mutation were significantly associated with the development of an inhibitor in mild haemophilia A patients, while continuous infusion with FVIII was not3. Our patient had multiple risk factors for developing an inhibitor: type of mutation, major surgery, suggested to facilitate an antibody response against exogenous FVIII, intensive and first exposure to FVIII, and, potentially, continuous infusion of FVIII.
Immune tolerance induction (ITI) is the most common approach used to eliminate inhibitors that develop in patients with haemophilia A. Limited data are available on ITI in patients with mild disease and the treatment seems to be less effective than in severe haemophilia A. Dunkley et al. presented preliminary success with the use of rituximab to treat a patient with mild haemophilia A with inhibitors without the use of ITI. The same group of authors proposed a treatment protocol with rituximab for patients with mild haemophilia A with inhibitors4.
To our knowledge, according to published data, our patient with mild haemophilia A with inhibitors is the youngest to have been treated with rituximab. Successful, long-term inhibitor eradication was achieved through four standard doses of rituximab. The observation period of 4 years is the longest among published cases5.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Franchini M, Favaloro EJ, Lippi G. Mild hemophilia A. J Thromb Haemost. 2010;8:421–32. doi: 10.1111/j.1538-7836.2009.03717.x. [DOI] [PubMed] [Google Scholar]
- 2.Eckhartd CL, Menke LA, van Ommen CH, et al. Intensive peri-operative use of factor VIII and the Arg593×Cys mutation are risk factors for inhibitor development in mild/moderate hemophilia A. J Thromb Haemost. 2009;7:930–7. doi: 10.1111/j.1538-7836.2009.03357.x. [DOI] [PubMed] [Google Scholar]
- 3.Mauser-Bunschoten EP, Den Uijl IEM, Schutgens RE, et al. Risk of inhibitor development in mild haemophilia A increases with age. Haemophilia. 2011;18:263–7. doi: 10.1111/j.1365-2516.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 4.Dunkley S, Kershaw G, Young G, et al. Rituximab treatment of mild haemophilia A with inhibitors: a proposed treatment protocol. Haemophilia. 2006;12:663–7. doi: 10.1111/j.1365-2516.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Mengoli C, Lippi G, et al. Immune tolerance with rituximab in congenital haemophilia with inhibitors: a systematic literature review based on individual patients’ analysis. Haemophilia. 2008;14:903–12. doi: 10.1111/j.1365-2516.2008.01839.x. [DOI] [PubMed] [Google Scholar]