Abstract
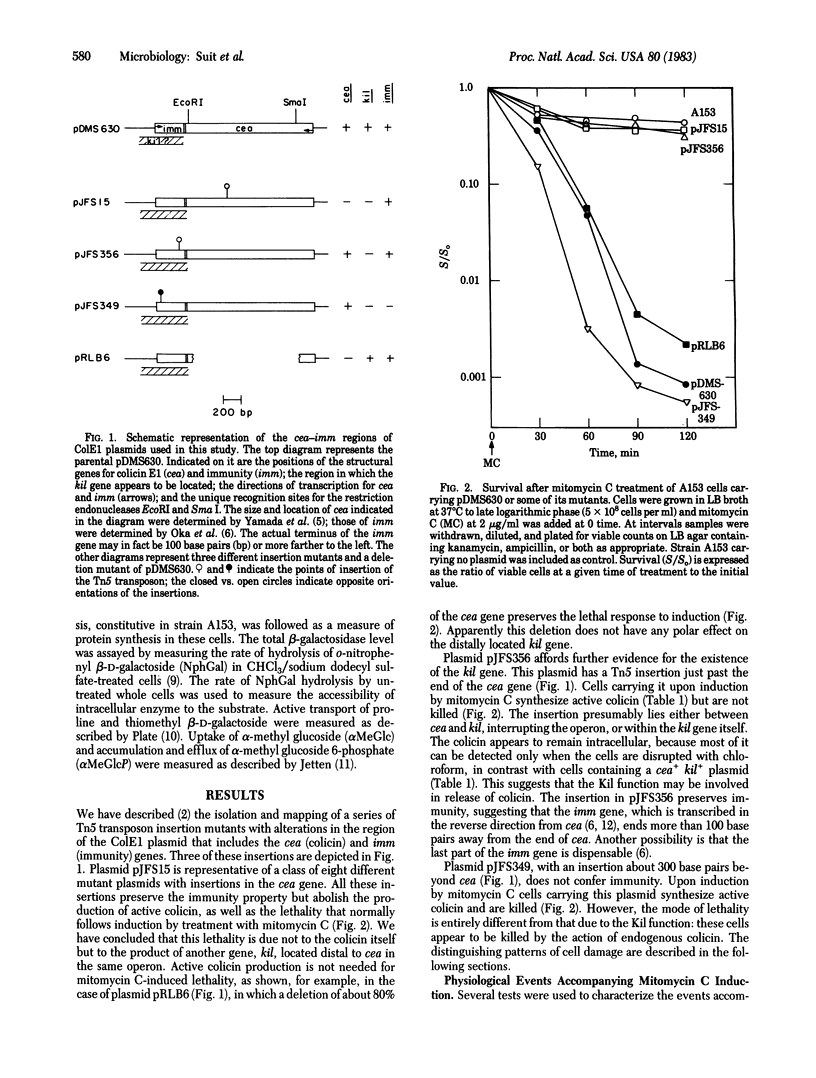
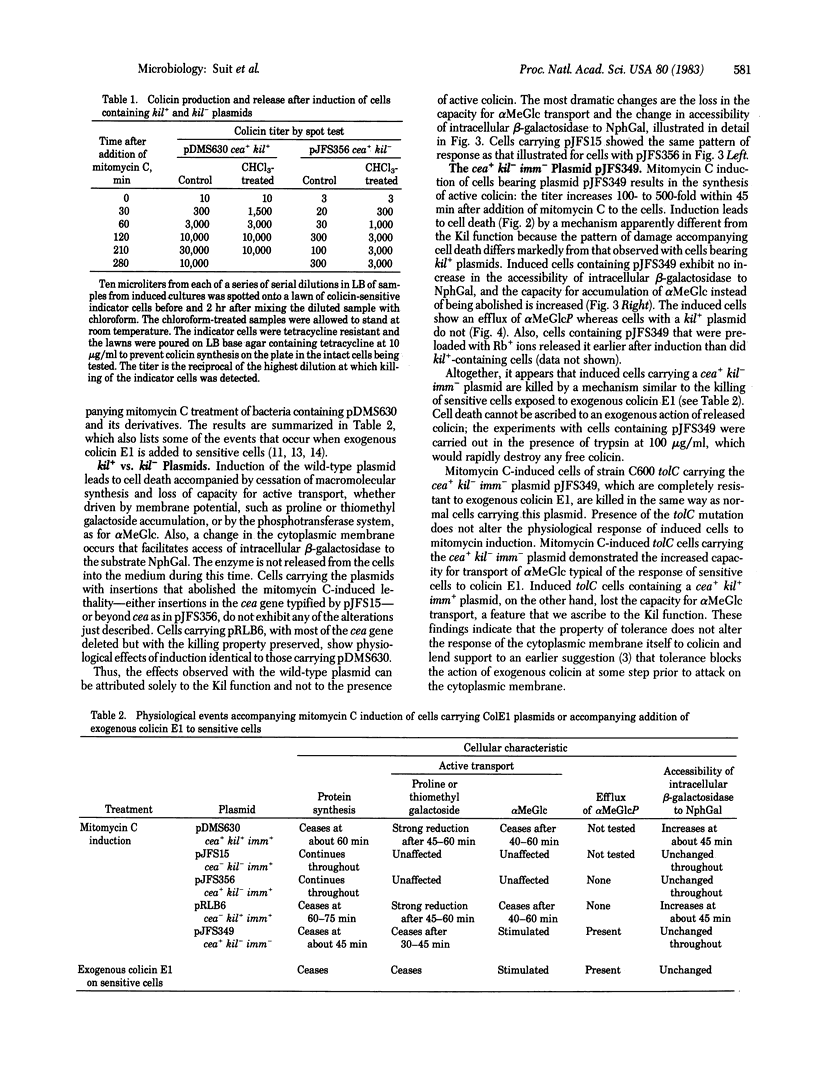
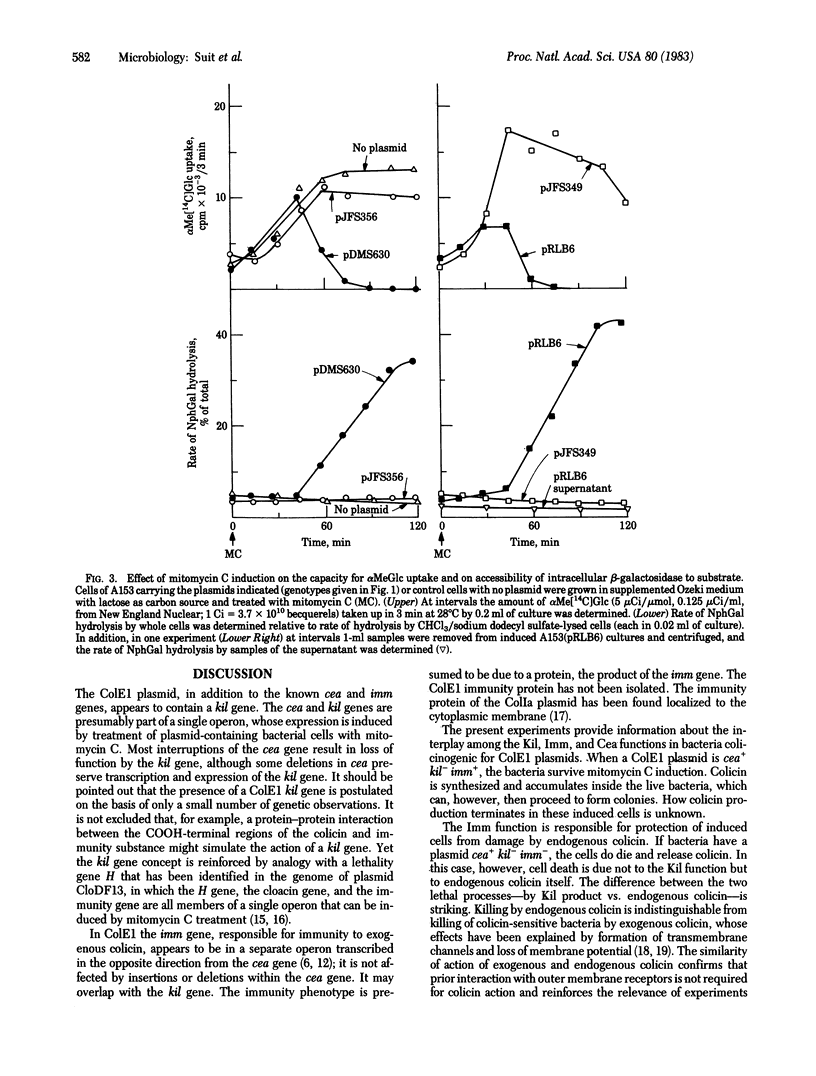
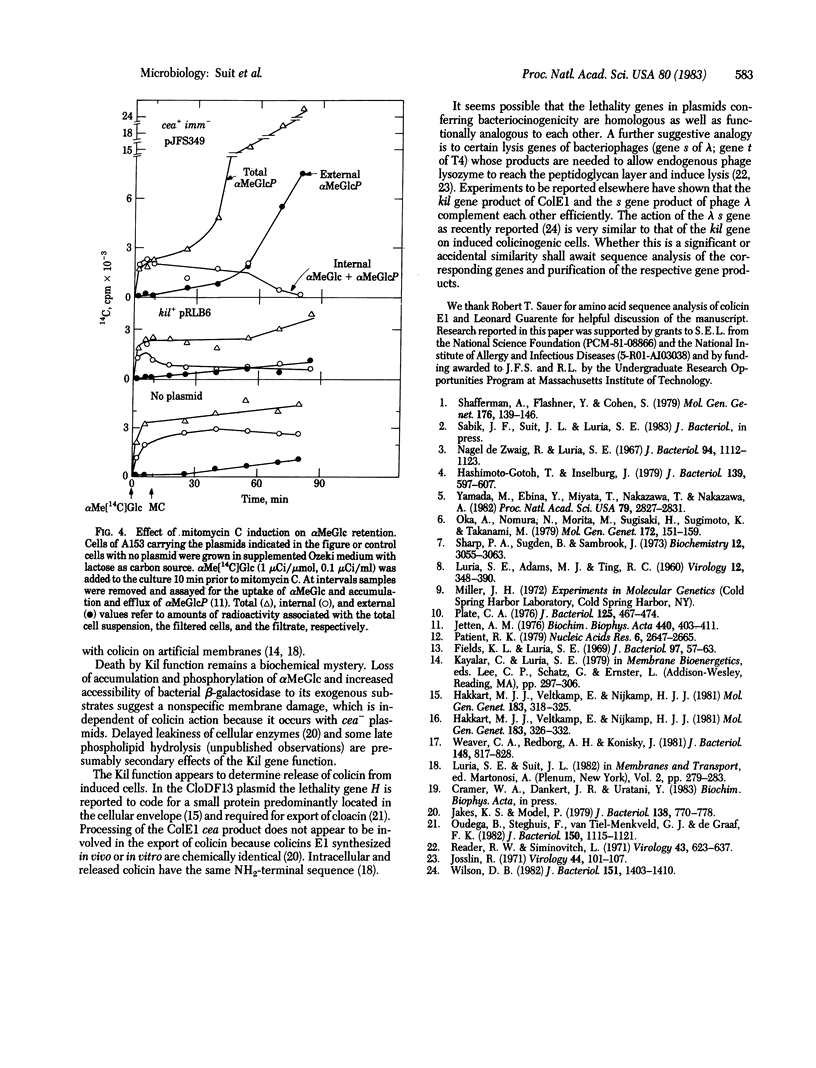
We have studied the physiological effects of mitomycin C induction on cells carrying ColE1 plasmids with differing configurations of three genes: the structural gene coding for colicin (cea), a gene responsible for mitomycin C lethality (kil) that we located as part of an operon with cea, and the immunity (imm) gene, which lies near cea but is not in the same operon. kil is close to or overlaps imm. When cea+ plasmids are present mitomycin C induction results in 100-fold or greater increases in the level of colicin. Within an hour after induction more than 90% of cells carrying cea+kil+ plasmids are killed and macromolecular synthesis stops, capacity for transport of proline, thiomethyl β-D-galactoside, and α-methyl glucoside is lost, and the membrane becomes abnormally permeable as indicated by an increased accessibility of intracellular β-galactosidase to the substrate o-nitrophenyl β-D-galactoside. All of these events occur when a cea-kil+imm+ plasmid is present and none does when the plasmid is cea+kil-imm+, so the damage can be attributed solely to the Kil function and not to the presence of colicin. However, cells carrying a cea+kil-imm- plasmid are killed upon induction, apparently by action of endogenous colicin on the nonimmune cytoplasmic membrane. The pattern of accompanying physiological damage is distinguished from the kil+-associated damage by an enhancement of α-methyl glucoside uptake and accumulation and efflux of α-methyl glucoside 6-phosphate and by an absence of the alteration in membrane permeability for o-nitrophenyl β-D-galactoside. These features are typical of colicin E1 action on the membrane. The induced damage is not prevented by trypsin and occurs in cells of a strain specifically tolerant to exogenous colicin E1, indicating that the attack is from inside the cell.
Keywords: kil gene, Kil function, endogenous colicin E1, cytoplasmic membrane damage
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. I. Localisation of the gene and identification and subcellular localisation of the gene H product. Mol Gen Genet. 1981;183(2):318–325. doi: 10.1007/BF00270635. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. II. Functions and regulation of synthesis of the gene H product. Mol Gen Genet. 1981;183(2):326–332. doi: 10.1007/BF00270636. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Inselburg J. Isolation and characterization of replication-deficient mutants of ColE1 plasmids. J Bacteriol. 1979 Aug;139(2):597–607. doi: 10.1128/jb.139.2.597-607.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K. S., Model P. Mechanism of export of colicin E1 and colicin E3. J Bacteriol. 1979 Jun;138(3):770–778. doi: 10.1128/jb.138.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M. Effects of colicins K and E1 on the glucose phosphotransferase system. Biochim Biophys Acta. 1976 Aug 13;440(2):403–411. doi: 10.1016/0005-2728(76)90074-8. [DOI] [PubMed] [Google Scholar]
- Josslin R. Physiological studies on the t gene defect in T4-infected Escherichia coli. Virology. 1971 Apr;44(1):101–107. doi: 10.1016/0042-6822(71)90157-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Oudega B., Stegehuis F., van Tiel-Menkveld G. J., de Graaf F. K. Protein H encoded by plasmid CloDF13 is involved in excretion of cloacin DF13. J Bacteriol. 1982 Jun;150(3):1115–1121. doi: 10.1128/jb.150.3.1115-1121.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient R. K. Characterization of in vitro transcription initiation and termination sites in Col E1 DNA. Nucleic Acids Res. 1979 Jun 25;6(8):2647–2665. doi: 10.1093/nar/6.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Mutant of Escherichia coli defective in response to colicin K and in active transport. J Bacteriol. 1976 Feb;125(2):467–474. doi: 10.1128/jb.125.2.467-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: on the role of the S function in lysis. Virology. 1971 Mar;43(3):623–637. doi: 10.1016/0042-6822(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Flashner Y., Cohen S. ColE1 DNA sequences interacting in cis, essential for mitomycin-C induced lethality. Mol Gen Genet. 1979 Oct 2;176(1):139–146. doi: 10.1007/BF00334305. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Redborg A. H., Konisky J. Plasmid-determined immunity of Escherichia coli K-12 to colicin Ia Is mediated by a plasmid-encoded membrane protein. J Bacteriol. 1981 Dec;148(3):817–828. doi: 10.1128/jb.148.3.817-828.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Effect of the lambda S gene product on properties of the Escherichia coli inner membrane. J Bacteriol. 1982 Sep;151(3):1403–1410. doi: 10.1128/jb.151.3.1403-1410.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]


