Dear Sir,
Upshaw-Schulman syndrome (USS) is a congenital deficiency of ADMTS13 (a disintegrin-like and metalloprotease with thrombospondin type 1 motifs, 13) activity caused by gene mutations. ADAMTS13 specifically cleaves unusually large von Willebrand factor multimers produced in and released from vascular endothelial cells under high shear stress conditions in the microvasculature1,2. Thus, in the absence of ADAMTS13 activity, the uncleaved unusually large von Willebrand factor multimers are released into the circulation, causing a life-threatening systemic disease termed thrombotic thrombocytopenic purpura (TTP). Most cases of TTP are induced by acquired autoantibodies against this enzyme. USS is an extremely rare disease, and to date approximately 100 affected patients have been reported in the literature, of whom 43 are in Japan3.
According to our experience in Japan, bouts of TTP in USS patients are triggered by various stimuli, including pregnancy, severe infection, administration of 1-deamino-8-D-arginine vasopressin (DDAVP) and drinking large amounts of alcohol. Pregnancy is the single most common trigger in female patients. In fact, in an analysis of the natural history of our 43 USS patients in Japan, we found that 26 (60%) were diagnosed during childhood (early-onset phenotype), and the remaining 17 (40%) were diagnosed after 15 years of age (late-onset phenotype). In the early-onset group, the female:male ratio was 13:13, while it was 14:3 in the late-onset group. These 14 female patients were aged between 15 and 45 years, and nine were diagnosed during pregnancy. In contrast, all three male patients had their first bouts after 45 years of age. With regards to ADAMTS13 activity, 35 patients had extremely low levels (<0.5% of normal), seven had trace amounts (0.5–0.8% of normal), and one male patient (USS-GG2) who had his first bout of TTP at 63 years of age had some activity (2.4–3.6%). Thus, one important determinant of the late-onset phenotype in USS patients is the level of ADAMTS13 activity. However, here we present the late-onset phenotype found in a middle-aged nulliparous USS female with severe deficiency of ADAMTS13 activity (<0.5% of the normal), whose first bout of TTP was triggered by an oral herpes simplex infection at the age of 45.
The proband (USS-Y3), born in Sapporo in 1960, was the first of three siblings born to non-consanguineous parents. Her parents and two brothers have had no episodes of thrombosis or excessive bleeding. Her perinatal medical history was unclear, but she did not have any exchange blood transfusions as a neonate. By the age of 3, she suffered from repeated episodes of thrombocytopenia and was diagnosed with idiopathic thrombocytopenic purpura, for which she received transfusions of fresh whole blood on a few occasions. Further details of her medical history during childhood were unavailable. Since the age of 38, her platelet count has been occasionally evaluated at a nearby hospital. The counts were almost normal (104–175×109/L). However, when she has a cold, her platelet count temporarily drops to less than 50×109/L (minimum 19×109/L), but normalises without any specific medical therapy. At the age of 45, she suffered from an oral herpes simplex infection complicated by thrombocytopenia (11×109/L), for which the antiviral aciclovir (1,000 mg/day) was prescribed. Subsequently, she has had repeated episodes of oral herpes simplex infection; therefore, she received a prescription of acyclovir for 5 months but has not had an appreciable clinical improvement. She was referred to our hospital for analysis of the cause of her thrombocytopenia. Laboratory findings on admission were as follows: thrombocytopenia (9×109/L), haemolytic anaemia (red cell count 1.84×109/L, haemoglobin 6.7 g/dL, reticulocyte 168‰, schistocytes on a peripheral smear [2+], total bilirubin 2.8 mg/dL, lactate dehydrogenase 872 IU/L, and haptoglobin <10 mg/dL), near-normal renal function (blood urea nitrogen 19 mg/dL, creatinine 1.07 mg/dL, and positive occult blood in urine), C-reactive protein 0.1 mg/dL, negative direct and indirect Coombs’ tests, and normal haemostatic tests. She was initially treated with oral prednisolone (50 mg/day) for a diagnosis of Coombs-negative Evans syndrome, but soon thereafter her general condition worsened, and ADAMTS13 analyses were performed for diagnostic purposes.
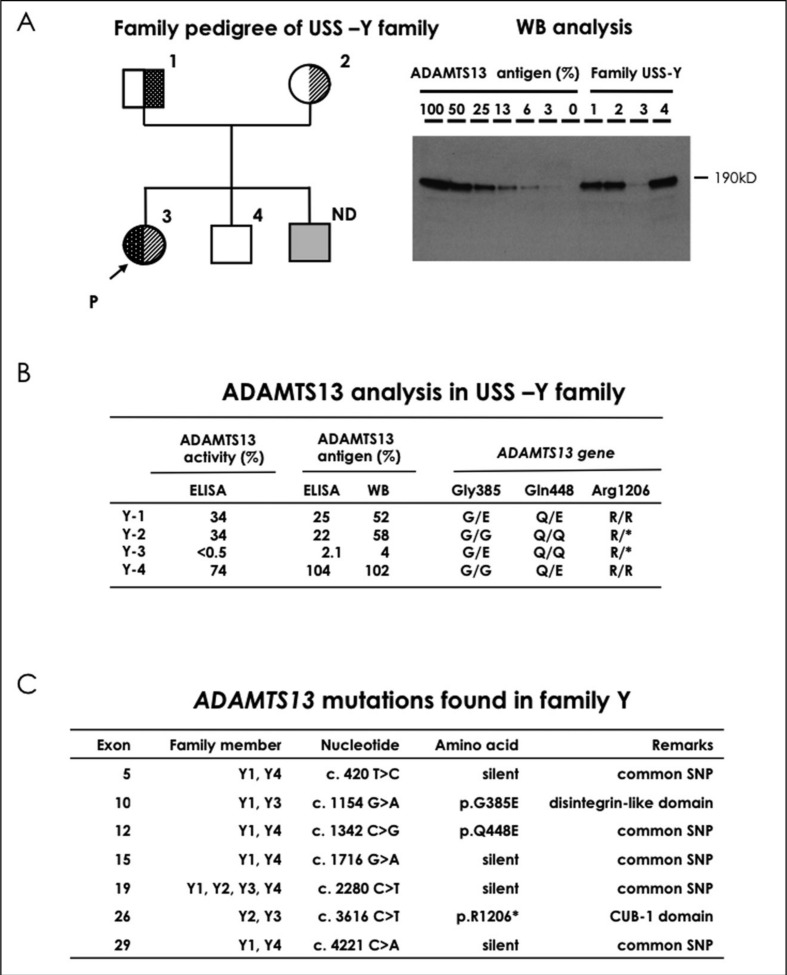
The family pedigree of this patient is shown in Figure 1A (left). The patient had very low ADAMTS13 activity (<0.5% of normal) and an absence of ADAMTS13 inhibitor (<0.5 BU/mL). Both of her parents had mild deficiencies of ADAMTS13 activity (both 34%) without its inhibitor. Plasma levels of ADAMTS13 activity in one of her younger brothers were normal (74%). Plasma levels of ADAMTS13 antigen as analysed by enzyme-linked immunosorbent assay (Figure 1B) were 2.1% of the normal control in the patient, and 25%, 22%, and 104% of normal in her father, mother, and younger brother, respectively. Furthermore, as analysed by western blot, plasma levels of ADAMTS13 antigen (Figure 1A right and B) were 4% of the normal control in the patient, and 52%, 58%, and 102% of normal in the father, mother, and younger brother, respectively. ADAMTS13 gene analysis revealed that the patient was a compound heterozygote for two mutations in ADAMTS13: p.G385E (c.1154G>A, exon 10) from her father and p.R1206* (c.3616 C>T, exon 26) from her mother. Her parents were heterozygous carriers of each of the two mutations (Figure 1B). These two mutations were not found in her younger brother. p.Q448E was reported as a single nucleotide polymorphism causing a missense mutation4. All mutations found in this family are shown in Figure 1C, including common single nucleotide polymorphism without amino acid substitutions. We previously reported the p.R1206X nonsense mutation in a USS-I4 patient5. The p.G385E missense mutation presented here is novel. Our experience indicates that the clinical phenotype of females with USS who have never been pregnant is almost indistinguishable from that of males.
Figure 1.
The family pedigree of USS-Y is shown in Figure 1A (left). Squares and circles indicate males and females, respectively, and the arrow with P indicates the proposita. Filled symbols represent a patient of USS-Y3. The half-filled symbols represent asymptomatic carriers. Figure 1A (right) shows western blot (WB) analysis of ADAMTS13 antigen followed by anti-ADAMTS13 monoclonal antibody detection using plasma samples, according to the previous method. Note a trace amount of ADAMTS13 antigen in USS-Y3 (patient) in lane 3 of Figure 1A (right). ND indicates not determined. ADAMTS13 activity was measured by chromogenic act-enzyme linked immunosorbent assay (ELISA), and the ADAMTS13 antigen was determined by both WB and antigen-ELISA (Figure 1B). The ADAMTS13 gene mutations found in this family are shown as one-letter amino acid abbreviations (Figure 1B). The ADAMTS13 single nucleotide polymorphisms (SNP) are also shown in Figure 1C.
Acknowledgements
This work was supported in part by grants-in-aid from the Ministry of Health, Labour and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Takeda Science Foundation.
Footnotes
Conflict of interest disclosure
Yoshihiro Fujimura is a member of clinical advisory boards for Baxter BioScience.
All other Authors declare no conflicts of interest.
References
- 1.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 2.Lotta LA, Wu HM, Mackie IJ, et al. Residual plasmatic activity of ADAMTS13 is correlated with phenotype severity in congenital thrombotic thrombocytopenic purpura. Blood. 2012;120:440–8. doi: 10.1182/blood-2012-01-403113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimura Y, Matsumoto M, Isonishi A, et al. Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan. J Thromb Haemost. 2011;9(Suppl 1):283–301. doi: 10.1111/j.1538-7836.2011.04341.x. [DOI] [PubMed] [Google Scholar]
- 4.Kokame K, Matsumoto M, Soejima K, et al. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci USA. 2002;99:11902–7. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibagaki Y, Matsumoto M, Kokame K, et al. Novel compound heterozygote mutations (H234Q/R1206X) of the ADAMTS13 gene in an adult patient with Upshaw-Schulman syndrome showing predominant episodes of repeated acute renal failure. Nephrol Dial Transplant. 2006;21:1289–92. doi: 10.1093/ndt/gfk072. [DOI] [PubMed] [Google Scholar]