Introduction
Paroxysmal cold haemoglobinuria, first described by Donath and Landsteiner in 19041,2, is a rare autoimmune haemolytic anaemia, defined by the presence of an IgG biphasic haemolysin. It is generally considered an acute self-limited anaemia caused by an IgG biphasic auto-anti-P antibody (Donath-Landsteiner [DL]-haemolysin). The autoantibody is called biphasic because it sensitises red cells at 0–4 °C and causes complement-mediated haemolysis at higher temperatures.
The generally reported incidence ranges from 1.6 to 5.1% in childhood haemolytic anaemia3, although an incidence of as high as 32% was found in one small series4. Most of the data are probably too old to reflect the true incidence at present; the haemolysis caused by DL antibody could be underestimated since proper testing is rarely performed. In the past years, a positive DL test has characterized a chronic disorder known as cold haemoglobinuria, usually secondary to syphilis.
Currently, most cases of DL-haemolytic anaemia occur as acute transient episodes in children and young adults after and/or concomitant with a variety of viral and bacterial infections including measles, mumps, chickenpox, cytomegalovirus, Epstein-Barr virus, adenovirus type 2, influenza A, Klebsiella spp., Haemophilus influenzae, Mycoplasma and E. Coli infections, or as a complication of vaccinations2–6.
We report here the case of a very young Caucasian child with acute haemolytic anaemia due to a DL red cell autoantibody, as the first sign of leishmaniasis.
Case report
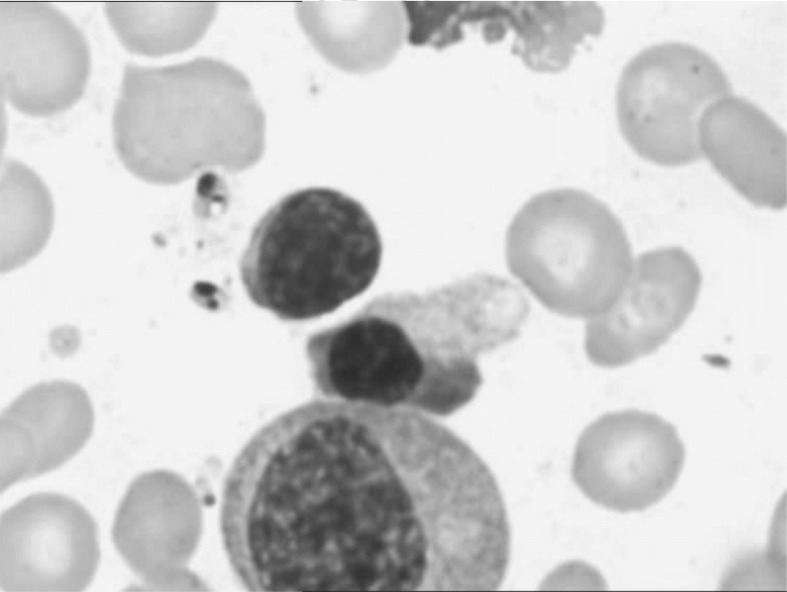
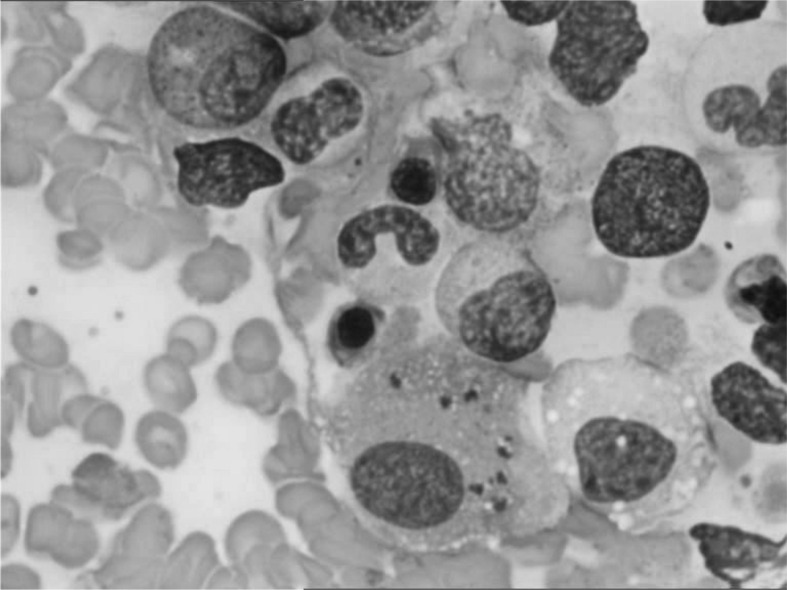
A 18-month old girl was admitted to our paediatric unit with a 2-week history of fatigue, pallor and dark urine. The child’s body temperature was 37.8 °C. There were no past records or laboratory evidence of anaemia; she had been treated with oral amoxicillin because of fever and a positive throat swab for Streptococcus pyogenes, with no clinical improvement. When examined, she was pale and her spleen was palpable 2 cm below the costal margin. A complete blood cell count and chemistry tests revealed microcytic anaemia (haemoglobin 5.7g/dL, mean corpuscular volume 69.4 fL), mild thrombocytopenia (platelet count: 107×109/L), leucopenia (white blood cell count 3.16×109/L, absolute neutrophil count 0.96×109/L, L 1.69×109/L, M 0.25×109/L), high reticulocyte count (256.4×109/L), high lactate dehydrogenase concentration (520 U/L) and raised transaminase levels (SGPT 155 IU/L, SGOT 272 IU/L), low albumin and immunoglobulin levels (albumin 3.3 g/dL; IgG 293 mg/dL; IgM 86 mg/dL; Ig A39 mg/dL). Direct and indirect bilirubin, haptoglobin and all other parameters were within the normal ranges. The examination of a peripheral blood smear showed anisocytosis with spherocytes and anulocytes; no nucleated or fragmented red cells were found. There was no evidence of lung involvement on the chest X ray; ultrasonography of the spleen confirmed the splenomegaly (maximum diameter 9.1 cm) and did not reveal any focal lesions. Considering the signs of haemolysis, an immune-haematological work-up was performed. Polyspecific and monospecific direct antiglobulin tests (DAT) were negative with anti-IgA, -IgG, -IgM, -C3c and -C3d antisera. Tests performed at 4 °C, 20 °C and 37 °C with serum and autologous and homologous red blood cells did not show either haemolysis or agglutination. The DL test indicated the presence of IgG biphasic antibody binding complement components to autologous red blood cells at a cold temperature (4 °C) causing haemolysis of the coated red blood cells at 37 °C. The blood samples for the DL test must be drawn and kept at 37 °C before testing. The classical test with the patient’s serum and untreated red blood cells was performed in parallel with the modified test, employing the patient’s serum, autologous and homologous O P-positive ficin-treated red blood cells. The mixtures (500 μL of serum and 50 μL of 50% in saline erythrocytes for each tube) were incubated in melting ice (0 °C) for 30 minutes and then warmed to 37 °C for 1 hour; this procedure included control tubes incubated only at 37 °C or at 0 °C. DL haemolysin is considered present when haemolysis is found only in the tube undergoing “biphasic” incubation and if haemolysis disappears when serum is inactivated at 56 °C. To enhance the sensitivity of the test we used ficin-treated red blood cells, a treatment that increases the exposure of P-antigen sites on the red blood cells. All the serological tests for cytomegalovirus, parvovirus B19, hepatitis B and C viruses and human immunodeficiency viruses were carried out and found to be negative. The child was treated with folic acid. After a few days the haemoglobin concentration had increased up to 8.8 g/dL. On day 9 of hospitalisation, the child developed a high fever (39 °C), her haemoglobin level dropped to 5.7 g/dL and her clinical condition worsened; transfusion of red blood cells was mandatory. Blood cultures were positive for Citrobacter feundii and Enterobacter cloacae. A 9-day course of ciprofloxacin was administered which improved the fever although there was no reduction in spleen size, determined either clinically or by ultrasonography. Bone marrow aspiration was performed on day 9 and showed normal trilineage haematopoiesis, extracellular leishmania amastigotes and macrophages with parasitisation by protozoa (Figures 1 and 2). Serological and polymerase chain reaction testing confirmed the presence of Leishmania infantum and the diagnosis of DL-haemolytic anaemia secondary to visceral leishmaniasis was made. The child was, therefore, given a 5-day course of liposomal amphotericin B at the dosage of 3 mg/kg/day plus another single dose on the 10th day after the start of the treatment. Red blood cell transfusion increased the haemoglobin to 8.0 g/dL; it gradually increased up to 12 g/dL with anti-fungal therapy. A few days after the end of the treatment, the bone marrow aspiration was repeated; the leishmania amastigotes had disappeared and polymerase chain reaction analysis for leishmania DNA as well as the DL test were negative. On day 24 the child was discharged from hospital with the following peripheral blood parameters: haemoglobin 12.4 g/dL, platelet count 323×109/L, leucocyte count 11.1×109/L. Lactate dehydrogenase level and liver function tests were in the normal range.
Figure 1.
Bone marrow smear: extracellular protozoa.
Figure 2.
Bone marrow smear: histiocyte with parasitization by Leishmania amastigotes.
Discussion
In the past years, a positive DL test was typical of a chronic disorder known as paroxysmal cold haemoglobinuria, which was usually associated with syphilis. Today DL-haemolytic anaemia is mainly present in children, with the median age at presentation being 5 years (range, 1–82 years)7. Gottsche et al. studied a total of 531 adults and 68 children with clinical and serological forms of immune haemolytic anaemia. Among them, a positive DL test was found in 22 of the 68 children (32.4%) but in none of the adults. The incidence of DL-haemolytic anaemia is estimated to be 0.001/100,000 per year in boys and 0.005/100,000 per year in girls. DL-haemolytic anaemia classically occurs 1–2 weeks after a respiratory tract infection; all Gottsche’s cases suffered from acute viral illnesses. Several viral and bacterial infection agents have been implicated in DL- haemolytic anaemia including influenza, measles, mumps, Epstein-Barr virus, cytomegalorivus, varicella-zoster virus, adenovirus, Mycoplasma pneumonia, Haemophilus influenzae and E. Coli3. The clinical characteristics include fever, haemoglobinuria, jaundice and pallor. Abdominal pain and palpable liver and spleen have also been reported in some patients. Polyspecific and monospecific DAT and a careful serological examination are necessary for the diagnosis. The DAT is usually positive because of complement sensitisation of red blood cells. Given that other causes of a complement-positive DAT are relatively uncommon in children, DL-haemolytic anaemia should be suspected and both a simple DL test and modified DL test, adding complement or employing papain or ficin-treated red blood cells, should be performed in a child with acute haemolysis, prominent haemoglobinuria and red cell complement sensitisation. Treatment is not usually necessary; the use of steroids is controversial and red blood cell transfusion is not generally required. The prognosis is good: the condition is usually self-limiting with rapid and complete recovery within a few days.
In our case, visceral leishmaniasis presented with DL haemolysis; the high fever, pancytopenia and splenomegaly strongly suggested visceral leishmaniasis. In addition the child’s blood culture and viral tests failed to demonstrate known pathogens associated with childhood DL-haemolytic anaemia. The treatment of the infection with liposomal amphotericin B increased the haemoglobin level and there was a gradual reduction of DL antibody, until it was no longer detectable. To our knowledge leishmaniasis has not previously been described in association with DL-haemolytic anaemia. Some studies have demonstrated B-cell activation in mice and in human beings during the infection. Polyclonal hypergammaglobulinemia (not present in our patient) is typically found in leishmaniasis. Besides, in mice, leishmaniasis has been associated with CD4 expansion with secretion of interleukin-4, resulting in B-cell activation. In a series of 12 paediatric cases of visceral leishmaniasis identified in France over a 17-year period, three cases had a positive Coombs’ test and anti-platelet antibodies, probably secondary to polyclonal B-cell activation8.
Other authors have confirmed the high incidence of circulating immune complexes in patients with visceral leishmaniasis9,10. Several hypotheses could explain the appearance of autoantibodies during visceral leishmaniasis, such as the release of antigens by parasite-infected host cells, cross-reactivity between parasite and host antigens or polyclonal B-cell activation. The anaemia induced by DL-antibody is generally mild and self-limiting; in our case the very low haemoglobin level could be related to a combination of acute haemolysis and erythrophagocytosis, a common feature in leishmania infection as in other intracellular parasitic infections. Our child had three other infections: a throat infection caused by Streptococcus pyogenes, diagnosed before her admission to our department, and positive blood cultures for Citrobacter feundii and Enterobacter cloacae. Consistent with this finding, Garcés et al. described that recurrent infections can be associated with visceral leishmaniasis. No other infections were diagnosed in our child after her discharge from hospital11.
In conclusion and based on our experience, leishmania infection was the cause of DL-haemolytic anaemia; we suggest that such an infection should be suspected in young patients with DL-haemolytic anaemia associated with neutropenia, thrombocytopenia and hepato-splenomegaly.
Footnotes
The Authors declare no conflicts of interest
References
- 1.Donath J, Landsteiner K. Uber paroxysmale hamoglobinurie. Munch Med Wochenschr. 1904;51:1590–3. [Google Scholar]
- 2.Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22:1–15. doi: 10.1016/j.blre.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Ziman A, His R, Goldfinger D. Donath-Landsteiner antibody-associated hemolytic anemia after Haemophilus influenza infection in a child. Transfusion. 2004;44:1127–8. doi: 10.1111/j.1537-2995.2004.03403.x. [DOI] [PubMed] [Google Scholar]
- 4.Gottsche B, Salama A, Muwller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. A study of 22 cases. Vox Sang. 1990;58:281–6. doi: 10.1111/j.1423-0410.1990.tb05000.x. [DOI] [PubMed] [Google Scholar]
- 5.Papalia MA, Schwarer AP. Paroxysmal cold haemoglobinuria in an adult with chicken pox. Br J Haematol. 2000;109:328–9. doi: 10.1046/j.1365-2141.2000.02010.x. [DOI] [PubMed] [Google Scholar]
- 6.Chambers LA, Rauck AM. Acute transient hemolytic anemia with a positive Donath Landsteiner test following parvovirus B19 infection. J Pediatr Hematol Oncol. 1996;18:178–81. doi: 10.1097/00043426-199605000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Sokol RJ, Booker DJ, Stamps R. Erythropoiesis: paroxysmal cold haemoglobinuria: a clinico-pathological study of patients with a positive Donath-Landsteiner test. Hematology. 1999;4:137–64. doi: 10.1080/10245332.1999.11746439. [DOI] [PubMed] [Google Scholar]
- 8.Gagnaire MH, Galambrun C, Stéphan JL. Hemophagocytic syndrome: a misleading complication of visceral leishmaniasis in children - a series of 12 cases. Pediatrics. 2000;106:e58, 1–6. doi: 10.1542/peds.106.4.e58. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal S, Aikat BK, Pathania AGS. Immune complexes in Indian kala-azar. Bull World Health Organ. 1982;60:945–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Ahlin E, Elshafei A, Nur M, et al. Anti-citrullinated peptide antibodies and rheumatoid factor in Sudanese patients with Leishmania donovani infection. Rev Bras Reumatol. 2011;51:572–86. [PubMed] [Google Scholar]
- 11.Garcés JM, Tomàs S, Rubiés-Prat J, et al. Bacterial infection as a presenting manifestation of visceral leishmaniasis. Rev Infect Dis. 1990;12:518–9. doi: 10.1093/clinids/12.3.518. [DOI] [PubMed] [Google Scholar]