Abstract
Background
Moderate haemophilia is the rarest form of haemophilia. This study aims to assess short- and long-term outcome, including its association with treatment, in patients with moderate haemophilia.
Material and methods
Seventy-five patients with moderate haemophilia (1–5% factor VIII/ factor IX activity), without a history of inhibitors, treated at the van Creveldkliniek, Utrecht (NL) were included in the study. Life-long data on bleeding and treatment were collected. Joints were evaluated using the Haemophilia Joint Health Score. Adults completed questionnaires on activity (HAL) and quality of life (SF-36, EQ5D).
Results
The median age of the patients was 37 years (IQR 23–52 years) and haemophilia A was diagnosed in 89%. Bleeding frequency was low: the median annual bleeding rate was 2.0 bleeds/ year (IQR 0.8–3.7 bleeds/year), including a median of 0 joint bleeds/year (IQR 0.8–3.7 bleeds/year). Joint function was good: 82% scored <10 out of 126 points of the Haemophilia Joint Health Score (HJHS). Nevertheless, 29% of patients with moderate haemophilia had a history of prophylaxis, because of a high bleeding frequency. Median age at first joint bleed was 4.8 years (IQR 3.5–8.5). Use of prophylaxis was more associated with age at first joint bleed (P <0.01) than with baseline factor activity (P =0.12). Most patients (52%) who suffered their first joint bleed before the age of 5 years required prophylaxis later in life.
Discussion
The majority of patients with moderate haemophilia have few bleeds and complications; however, a considerable subset of patients with a more severe bleeding pattern need prophylactic treatment. These latter patients may be identified by the onset of joint bleeding before the age of 5 years.
Keywords: moderate haemophilia, HJHS, physical activity, joint bleed
Introduction
Haemophilia is an inherited, X-chromosome-linked bleeding disorder. Moderate haemophilia is the rarest form of haemophilia; globally about 15% of haemophilia patients have residual factor activity levels of 1–5 IU/dL1. These patients have a milder bleeding pattern than patients with severe haemophilia who completely lack factor VIII (FVIII) or factor IX (FIX) activity. Patients with moderate haemophilia mostly suffer from traumatic bleeds only and generally do not experience spontaneous bleeding2. Consequently, patients with moderate haemophilia are mostly treated on demand3. Nevertheless, these patients do report complications due to haemophilia. Results from the 5th Haemophilia In The Netherlands (HIN) nationwide questionnaire suggested severe complaints in moderate haemophilia; 11% of patients aged 19–40 years and 33% of patients aged 41–64 years experienced severe hindrance of their daily activities due to haemophilia4. Moreover, it has been established that most of these patients are not on home treatment and have limited experience in recognising a bleed and are, therefore, at risk of delay in treatment5. Treatment delay may result in increased and prolonged exposure to intra-articular blood, and eventually increase joint damage. The bleeding pattern of patients with moderate haemophilia is currently unclear and it is not known whether such patients are being undertreated. Moreover, assessing outcome in moderate haemophilia may be used as a benchmark for the results of life-long prophylaxis in patients with severe haemophilia. The aim of this study was to assess short- and long term outcome, including its association with treatment, in patients with moderate haemophilia.
Materials and methods
Patients with moderate haemophilia (1–5% FVIII/ IX activity) born before 2000 and treated at the van Creveldkliniek in Utrecht, The Netherlands were included in the study. Patients who had inhibitors (4 patients), or did not have access to haemophilia care in their childhood (6 patients) were excluded.
Age at entry into the clinic, the onset of bleeding, history of home treatment, orthopaedic surgery and prophylaxis were collected from the medical files. Annual data on bleeding and treatment were collected from 1970 onwards. After signing informed consent, patients underwent a physical examination and the Haemophilic Joint Health Score (HJHS, version 1.0)6 was determined; those above the age of 18 were asked to complete questionnaires on quality of life (SF-367, EQ5D8) and daily activities (HAL9).
The onset of bleeding and bleeding frequency, home treatment, clotting factor consumption, use of prophylaxis, orthopaedic outcomes and quality of life were compared across residual factor activity levels. Clotting factor activity was measured by a one-stage assay10 in a single certified laboratory. If residual factor levels were measured more than once, the lowest factor level was used for classification. Prophylaxis was defined as at least one regular infusion per week for at least 45 weeks per year. Joint bleeds were defined as complaints in ankles, knees, elbows, hips, wrists, or shoulders requiring treatment with FVIII at least once.
Information on bleeding and factor consumption was available from 1970 onwards and had been collected annually from entry at the clinical until 2007.
Statistical testing was performed using Kruskal-Wallis rank-sum test, a non-parametric test for continuous variables and Fisher’s exact test for categorical variables.
Results
A total of 75 patients participated in the study, 60 (80%) underwent a physical examination and 56 (84% of adult patients) returned completed questionnaires. Among the non-responders, nine were under the age of 18 years and were not, therefore, eligible for the questionnaire, four were no longer treated at the centre at the time that the questionnaires were administered and physical examinations were performed and six never responded. The bleeding and treatment data of these 19 patients, extracted from their clinical files, were included in the study.
Table I shows the demographics of the patients with moderate haemophilia. Haemophilia A was more prevalent (67 patients, 89%). The median age at evaluation in 2007 was 34.3 years and the range was from 5.1 to 73.6 years. The median follow-up was 14.8 years (range, 3.1–35.5 years). Patients with moderate haemophilia were diagnosed at a median age of 1.5 years (range, 0 to 48 years). The distributions of current age and age at diagnosis were similar across different factor activity levels (P =0.85 and P =0.84, respectively). Chronic hepatitis C virus infection was present in 17 patients (23%), equally distributed across different factor activity levels. Only one patient was positive for human immunodeficiency virus. None of the patients with moderate haemophilia had ever needed a central venous access device.
Table I.
Characteristics of the patients with moderate haemophilia.
| n=75 | |
|---|---|
| Age in 2007 (years) | 37 (23–52) |
| Follow-up (years) | 14.8 (8.3–22.8) |
| Age at diagnosis (years) | 1.5 (0–5.9) |
| Baseline factor activity (IU/dL) | 3 (2–4) |
| Haemophilia A | 67 (89%) |
| HCV-positive | 17 (23%) |
| HIV-positive | 1 (1%) |
| History of CVAD | 0 |
| History of prophylaxis | 22 (29%) |
| Prophylaxis at last evaluation | 17 (23%) |
| Age at start of prophylaxis (years) | 13.7 (6.5–26) |
| Currently on home treatment | 34 (45%) |
Values are medians (interquartile range) or n (%). HCV, hepatitis C virus; HIV, human immunodeficiency virus; CVAD, central venous access device.
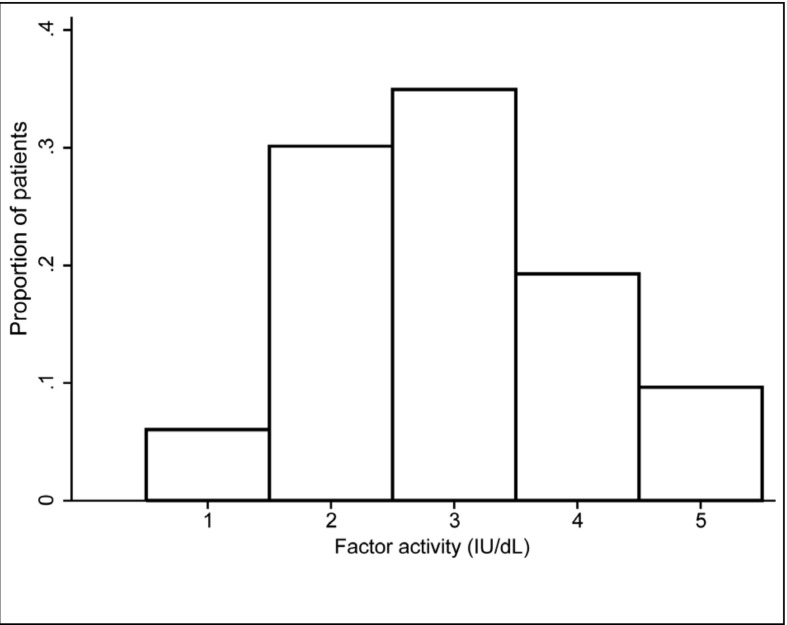
Residual factor activity levels ranged from 1–5 IU/ dL. The median number of factor measurements was five (IQR=3–6); eight patients had only one measurement of baseline clotting factor activity available. Residual FVIII activity was 1 IU/dL in 5 patients (6%), 2 IU/dL in 23 (31%), 3 IU/dL in 26 (34%), 4 IU/dL in 13 (17%) and 5 IU/dL in 8 (11%) (Figure 1).
Figure 1.
Distribution of residual clotting factor activity of patients with moderate haemophilia (n=75).
Twenty-two (29%) patients with moderate haemophilia were treated with prophylaxis at some time in their life. The median age at starting prophylaxis was 14 years (range, 1–59 years). All patients were on secondary prophylaxis; they suffered a median of 4.1 joint bleeds/year (IQR=2.5–6.5) between their first joint bleed and starting prophylaxis. Seven of these patients (32 %) discontinued prophylaxis after a median of 3.2 years, two restarted prophylaxis due to recurrent bleeding (Table I). All patients treated with prophylaxis used home treatment; in total 34 patients (45%) used home treatment at last evaluation.
Outcome according to factor activity level
The prevalence of haemophilia A was similar across factor activity levels (P =0.4).Overall, bleeding frequency was low (Table II) with the median annual bleeding frequency being 2.0 bleeds/year (IQR 0.8–3.7 bleeds/year) and the median annual number of joint bleeds beings 0 (range, 0 to 8.8 joint bleeds/year). Thirty-three patients (44%) had not suffered any joint bleeds during the preceding 5 years.
Table II.
Outcome according to residual factor activity level.
| 1 IU/dL n=5 |
2 IU/dL n=23 |
3 IU/dL n=26 |
4 IU/dL n=13 |
5 IU/dL n=8 |
|
|---|---|---|---|---|---|
| Haemophilia B | 1 (20%) | 3 (13%) | 3 (12%) | 0 | 1 (13%) |
|
| |||||
| Bleeding and treatment | |||||
| Age at 1st joint bleed (years) | 2.8 (2.3–2.8) | 4.5 (3.5–8.3) | 4.9 (4.1–8.5) | 5.7 (4.0–12) | 6.1 (4.3–18) |
| Median annual n. of bleeds | 2.9 (1.9–7.8) | 3.7 (1.8–7.7) | 1.9 (0.8–3.6) | 1.2 (0.5–3.1) | 1.2 (0.2–2.1) |
| Median annual n. of joint bleeds | 1.0 (0.3–3.1) | 1.3 (0.4–2.5) | 0 (0–1.2) | 0 (0–0.96) | 0 (0–0.73) |
| No joint bleeds in last 5 years | 2 (40%) | 7 (32%) | 14 (54%) | 8 (62%) | 3 (38%) |
| History of prophylaxis | 2 (40%) | 11 (44%) | 5 (17%) | 3 (19%) | 1 (13%) |
| Annual factor consumption (IU/kg/year) | 119 (48–1,215) | 264 (119–1,044) | 217 (34–347) | 22 (0–244) | 46 (12–277) |
| Without treatment | 0 | 2 (8%) | 0 | 2 (15%) | 2 (25%) |
|
| |||||
| Orthopaedic outcome | |||||
| HJHS (max. 128 points) | 0 | 5 (2–10) | 4 (0–9) | 1 (0–4) | 2 (0–6) |
| History of orthopaedic surgery | 0 | 5 (22%) | 4 (15%) | 4 (31%) | 0 |
| HAL sum score (max 100 points) | 97 | 96 (81–100) | 96 (85–100) | 92 (79–97) | 86 (70–100) |
| Utility (EQ5D, max 1.0) | 0.80 | 0.90 (0.72–1) | 1 (0.78–1) | 0.80 (0.73–1) | 0.76 (0.62–0.81) |
Values are medians (IQR) or n (%).
Patients with low baseline factor activity had higher bleeding frequencies than those with higher baseline factor activity. Patients with 1–2 IU/dL residual factor activity suffered a median of 2.9 bleeds/year (IQR=1.4–7.2), including a median of 0.5 joint bleeds/year (IQR=0.5–2.5); this was significantly more than patients with a residual factor activity of 3–5 IU/dL who had a median of 1.4 bleeds/year (IQR=0.5–3.4), including 0 joint bleeds/year (IQR=0–1.0) (P =0.02). Prophylaxis was more frequently prescribed in patients with a residual factor activity of less than 3 IU/dL (39% vs 13%). The median dose of prophylaxis was 21 IU/kg (IQR, 14–36 IU/kg; range, 14 to 36 IU/kg). These patients received prophylaxis on average twice weekly (range, one to seven times per week) for a period ranging from 1 to 34 years.
The more severe phenotype in patients receiving prophylaxis was obvious: patients requiring prophylaxis experienced a median of 6.1 bleeds/year (IQR 3.6–10.4), including a median of 2.1 joint bleeds (IQR 1.4–4.5), while those treated on demand suffered a median of 1.6 bleeds/year (IQR 0.8–3.9), including a median of 0.5 joint bleeds (IQR 0.15–1.1) (P <0.001). Although prophylaxis reduced bleeding, these patients continued to suffer joint bleeds, having a median of 1.2 joint bleeds/year (IQR 0.8–3.4) (P <0.01).
The median annual factor consumption was 148 IU/kg (range, 0–2,903 IU/kg). Six patients (11%) had not used any replacement therapy in the preceding 5 years; one patient, with a residual FVIII activity of 4 IU/dL, had never used factor replacement therapy. As for bleeding frequencies and prophylactic use, factor consumption appeared higher in patients with lower residual factor activity (1–2 IU/dL) (P <0.001). Patients treated with prophylaxis used significantly more factor concentrate than patients treated on demand: median 939 IU/kg (IQR=224–1,964) vs 112 IU/kg (IQR=16–248) (P <0.001).
The orthopaedic outcome in patients with moderate haemophilia was good. Most patients (50/60; 82%) had minimal loss of function; less than 10 points on the HJHS scale (Table II). As for the bleeding and treatment parameters, orthopaedic outcome was similar across factor activity levels. Only 13 patients (17%), median age 53 years (range 22–78 years, 23% born before 1965), had a history of orthopaedic surgery (Table II). Five patients had had only minor orthopaedic surgery, such as synovectomy or excision of cysts, three patients had had at least one ankle arthrodesis and six patients had had additional joint replacements. A history of orthopaedic surgery appeared to be independent of treatment intensity; only three patients who had undergone orthopaedic surgery had ever been treated with prophylaxis.
Outcome according to onset of joint bleeding
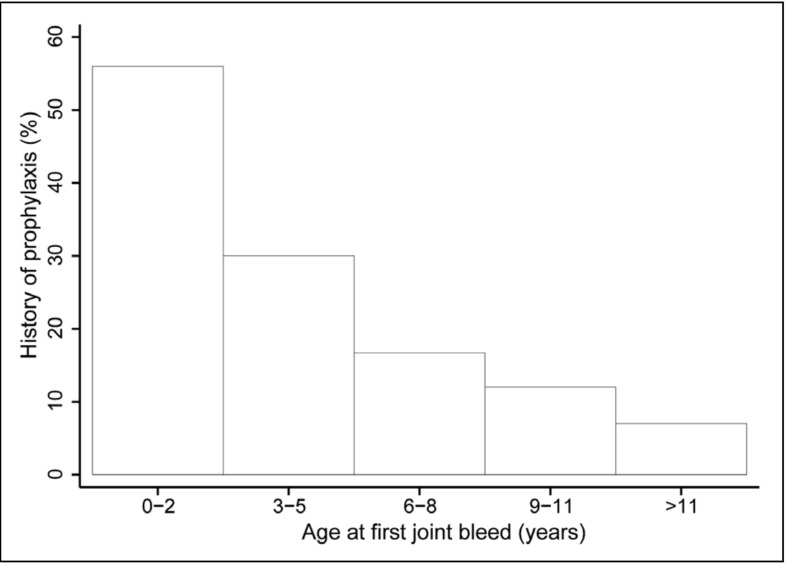
Residual factor activity levels were not clearly associated with the onset of joint bleeding. Age at first joint bleed was similar across residual factor activity levels (P =0.10), except for patients with a residual factor activity of 1 IU/dL who had all experienced their first joint bleed before the age of 3 years (Table II). Age at first joint bleed was, however, associated with the need for prophylaxis. Patients who had had their first joint bleed early, before the age of 5 years, more often required prophylaxis later in life than patients who had suffered their first joint bleed later or those who had never had joint bleeding (Figure 2).
Figure 2.
Percentage of patients with a history of prophylaxis according to age at first joint bleed.
Table III shows outcome in the patients divided according to age at first joint bleed: those with first joint bleed before the age of 5 years and factor activity levels of ≤2 IU/dL, compared to patients with their first joint bleed after the age of 5. Bleeding frequencies were comparable between the groups because of the high proportion of patients using prophylaxis in the former group (87% vs 15% in the other group). Without prophylaxis these patients had a much higher annual bleeding rate (median 6.1 bleed/years; maximum 23 bleeds/year). Patients with an early onset of joint bleeding and factor activity levels ≤2 IU/dL used more prophylaxis and subsequently had a higher factor consumption (median, 1,088 IU/kg/ year; IQR=533–2,427 IU/kg/year) than the other group (median, 131 IU/kg/year; IQR=39–432 IU/kg/year).
Table III.
Outcome according to age at onset of joint bleeding.
| Early onset of joint bleeding (≤5 years) and factor activity levels ≤2 IU/dL | Late onset (>5 years) or no joint bleeding | P-value | |
|---|---|---|---|
| Number of patiens | 15 (20%) | 60 (80%) | |
| Age (years) | 35 (29–40) | 39 (23–54) | 0.65 |
| Haemophilia A | 10 (77%) | 57 (95%) | 0.00 |
| Bleeding and treatment | |||
| Median annual n. of bleeds | 2.1 (1.8–7.2) | 1.9 (0.7–3.6) | 0.29 |
| Median annual n. of joint bleeds | 1.2 (1.1–2.5) | 0 (0–1.1) | 0.25 |
| No joint bleeds in last 5 years | 0 | 34 (57%) | 0.00 |
| History of prophylaxis | 13 (87%) | 9 (15%) | 0.00 |
| Annual factor consumption (IU/kg/year) | 1,088 (533–2,427) | 131 (39–462) | 0.01 |
|
| |||
| Orthopaedic outcome | |||
| HJHS (max 128 points) | 8 (0–12) | 3 (0–8) | 0.33 |
| HAL sum score (max.100 points) | 95 (82–99) | 97 (65–100) | 0.97 |
| Quality of life (EQ5D, max. 1) | 0.81 (0.69–0.92) | 0.86 (0.81–1) | 0.10 |
Values are medians (IQR) or n (%).
Although not statistically significant, there was a trend towards slightly more loss of clinical function in patients with an early onset of joint bleeding and factor activity levels ≤2 IU/dL.
Activities and quality of life
Patients with moderate haemophilia had hardly any physical limitations as measured by the HAL; the median HAL score was 96 out of 100 (IQR=83–100). The HAL score was also similar across different residual factor activity levels (P =0.98) and ages at onset of joint bleeding (P =0.96) (Tables II and III).
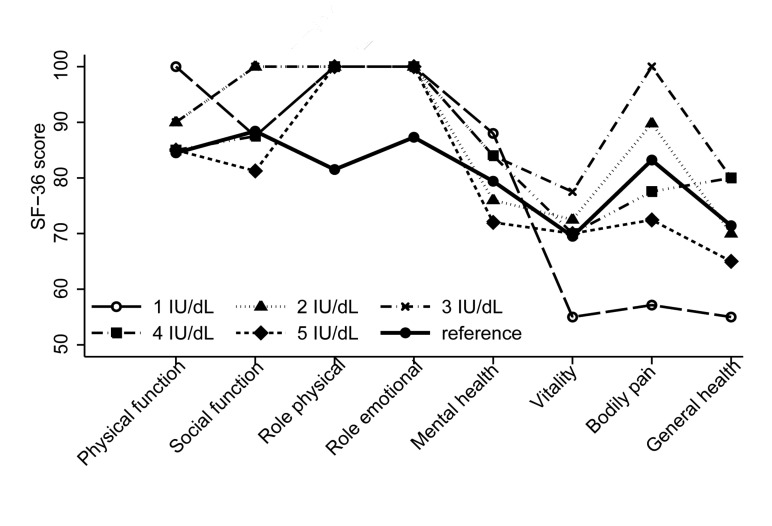
Quality of life, as measured by the SF-36 questionnaire, was similar to the general population across all domains (0.31 <P <0.97). The SF-36 domain scores were also similar across factor activity levels (0.18 <P <0.67) (Figure 3) and ages at onset of joint bleeding (0.12 <P <0.96). Utility analysis confirmed the results of the SF-36. The median utility was 0.83 (IQR=0.7–1.0) and was similar across residual factor activity levels and ages at onset of joint bleeding (P =0.33 and P =0.75 respectively).
Figure 3.
Health-related quality of life, as measured by the SF-36, according to baseline factor activity. The solid black line represents the male population in The Netherlands.
Discussion
Moderate haemophilia is rare and to our knowledge this is the largest study on outcome of patients with moderate haemophilia so far. Bleeding frequency was low with an annual bleeding frequency of 2.0 bleeds/year, with hardly any joint bleeds. Orthopaedic outcome was good; a few older patients, without access to replacement therapy in their childhood, required orthopaedic surgery. Quality of life and utility scores were comparable across all levels of residual factor activity as well as across different ages at the onset of joint bleeding.
However, a small subgroup had a high bleeding frequency requiring prophylaxis. These patients mostly suffered their first joint bleed early, before the age of 5 years. Without prophylaxis these patients bled 3–6 times more and tend to suffer more orthopaedic problems, thus being comparable to patients with severe haemophilia. We, therefore, propose a new rule that all patients with moderate haemophilia with residual factor levels <3 IU/dL should be given early prophylaxis after their first joint bleed if this occurs within the first 5 years of life. In the current study this would have meant that only two patients would have received prophylaxis they would not have needed, while in the other patients target joints and consequent problems and costs would have been prevented. In these patients a trough level of 3 IU/dL could be aimed for, because patients with moderate haemophilia and a residual factor activity level of 3 IU/ dL or more had significantly lower bleeding rates.
Although patients with moderate haemophilia showed various, different bleeding phenotypes and outcomes, a large part of this heterogeneity could be explained by the new rule. Those with a more severe phenotype could, thus, be diagnosed early.
Because moderate haemophilia is the least prevalent form of haemophilia it is difficult to collect cohorts large enough to address this heterogeneity. Additionally, patients with moderate haemophilia can increase their factor levels under stressful conditions, which could have introduced misclassification.
In this study, patients were classified according to baseline factor activity level, determined by the lowest value measured. Patients with moderate haemophilia have the ability to increase their endogenous factor activity under stress11. The assumption was made that this occurs only infrequently and the patients’ lowest activity levels were, therefore, used for classification. Very few patients had a factor activity level of 1 IU/dL, which is due to technical factors. Because of difficulty with laboratory testing of patients with factor activity in the lower ranges, patients with baseline factor activity levels of 0.5–0.99 IU/dL were classified as having severe haemophilia (<1 IU/dL)12 and were, therefore, not included.
Although joint scores, orthopaedic surgery and quality of life usually deteriorate with age, adjustment was not performed, because the age distribution was similar across subgroups. The clinical phenotype of haemophilia B is milder than that of haemophilia A13. Again, however, adjustment was unnecessary, because haemophilia B was equally distributed over all subgroups.
As all studies on rare diseases, this study was hampered by low numbers, but the variation in clinical phenotype is corroborated by other reports. Already in 1965, Ahlberg et al.2 reported that 15–20% of patients with moderate haemophilia had an unfavourable orthopaedic outcome. Surprisingly this number does not seem to have changed a lot, since in the current study 13% had a history of orthopaedic surgery and 27% of patients had clinical loss of function. A major nationwide study on self-reported outcome in all haemophilia patients in The Netherlands in 2001 also showed a large variation in bleeding frequencies among patients with moderate haemophilia3,4. Although 50% of the patients from this study originated from the Van Creveldkliniek, the reported proportion of patients who required prophylaxis was comparable at 26%.
In 2000, the lack of association between factor activity level and joint bleeding was also reported in patients from our clinic, 39 patients are included in this study as well14.
Generally, patients with moderate haemophilia seem well treated. Nevertheless, some patients had high bleeding frequencies, despite prophylactic treatment. These patients all started prophylaxis late, at a median age of 14 years, while our patients with severe haemophilia started at a median age of 2.2 years (IQR=1.2–3.0)15. From studies in severe haemophilia, it is well known that secondary prophylaxis is less effective at preventing arthropathy15,16. Factor activity level and age of first joint bleed were both associated with the need for prophylaxis. These two parameters are also the earliest signs of bleeding phenotype available to the physician.
In conclusion, although the majority of patients with moderate haemophilia had very few complications of their disease, some patients needed extra treatment. Physicians should be aware that 29% had a high bleeding frequency, with early onset of joint bleeding. Accordingly, such patients need close monitoring of their (joint) bleeding and in some cases early prophylaxis to avoid future joint damage. Primary prophylaxis should be provided for patients with moderate haemophilia (factor activity level >3 IU/dL) who have their first joint bleed before the age of 5 years.
Acknowledgements
We would like to thank all patients who contributed to this study, as well as the present and former staff of the Van Creveldkliniek for keeping complete records.
This study was sponsored by an unrestricted research grant from Pfizer and the “Strategische impuls” from UMC Utrecht.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Report on the Global Survey. World federation of Haemophilia; 2008. [Accessed on: 27/01/2011]. Available at: www1.wfh.org/publications/files/pdf-1429.pdf. [Google Scholar]
- 2.Ahlberg A. Haemophilia in Sweden. VII. Incidence, treatment and prophylaxis of arthropathy and other musculo-skeletal manifestations of haemophilia A and B. Acta Orthop Scand. 1965;77(Suppl):3–132. doi: 10.3109/ort.1965.36.suppl-77.01. [DOI] [PubMed] [Google Scholar]
- 3.Plug I, van der Bom JG, Peters M, et al. Thirty years of hemophilia treatment in the Netherlands, 1972–2001. Blood. 2004;104:3494–500. doi: 10.1182/blood-2004-05-2008. [DOI] [PubMed] [Google Scholar]
- 4.den Uijl IEM, Fischer K, van der Bom JG, et al. Clinical outcome of moderate haemophilia compared with severe and mild haemophilia. Haemophilia. 2009;15:83–90. doi: 10.1111/j.1365-2516.2008.01837.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindvall K, Colstrup L, Wollter IM, et al. Compliance with treatment and understanding of own disease in patients with severe and moderate haemophilia. Haemophilia. 2006;12:47–51. doi: 10.1111/j.1365-2516.2006.01192.x. [DOI] [PubMed] [Google Scholar]
- 6.Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12:518–25. doi: 10.1111/j.1365-2516.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 7.VanderZee KI, Sanderman R, Heyink JW, de Haes H. Psychometric qualities of the RAND 36-Item Health Survey 1.0: a multidimensional measure of general health status. Int J Behav Med. 1996;3:104–22. doi: 10.1207/s15327558ijbm0302_2. [DOI] [PubMed] [Google Scholar]
- 8.Stolk EA, Busschbach JJ. Validity and feasibility of the use of condition-specific outcome measures in economic evaluation. Qual Life Res. 2003;12:363–71. doi: 10.1023/a:1023453405252. [DOI] [PubMed] [Google Scholar]
- 9.van Genderen FR, Westers P, Heijnen L, et al. Measuring patients’ perceptions on their functional abilities: validation of the Haemophilia Activities List. Haemophilia. 2006;12:36–46. doi: 10.1111/j.1365-2516.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 10.Over J. Methodology of the one-stage assay of factor VIII (VIII:C) Scand J Haematol Suppl. 1984;41:13–24. doi: 10.1111/j.1600-0609.1984.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 11.Koch B, Luban NL, Galioto FM, et al. Changes in coagulation parameters with exercise in patients with classic hemophilia. Am J Hematol. 1984;16:227–33. doi: 10.1002/ajh.2830160304. [DOI] [PubMed] [Google Scholar]
- 12.Preston FE, Kitchen S, Jennings I, et al. SSC/ISTH classification of hemophilia A: can hemophilia center laboratories achieve the new criteria? J Thromb Haemost. 2004;2:271–4. doi: 10.1046/j.1538-7836.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- 13.Lowe GD, Ludlam CA. Less severe bleeding in hemophilia B than in hemophilia A. J Thromb Haemost. 2008;6:1982–3. doi: 10.1111/j.1538-7836.2008.03126.x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer K, van der Bom JG, Mauser-Bunschoten EP, et al. Endogenous clotting factor activity and long-term outcome in patients with moderate haemophilia. Thromb Haemost. 2000;84:977–80. [PubMed] [Google Scholar]
- 15.Fischer K, van der Bom JG, Mauser-Bunschoten EP, et al. The effects of postponing prophylactic treatment on long-term outcome in patients with severe hemophilia. Blood. 2002;99:2337–41. doi: 10.1182/blood.v99.7.2337. [DOI] [PubMed] [Google Scholar]
- 16.Astermark J, Petrini P, Tengborn L, et al. Primary prophylaxis in severe haemophilia should be started at an early age but can be individualized. Br J Haematol. 1999;105:1109–13. doi: 10.1046/j.1365-2141.1999.01463.x. [DOI] [PubMed] [Google Scholar]