Abstract
Background
As an alternative to phenotyping, large-scale DNA-based assays, which are feasible for high-throughput donor red blood cell typing, were developed for determination of blood group polymorphisms. However, high-throughput genotyping platforms based on these technologies are still expensive and the inclusion of single nucleotide polymorphisms and analysis of the alleles depend on the manufacturer’s determination. To overcome this limitation and in order to develop an assay to enable the screening of rare donors, we developed a SNaPshot assay for analysis of nine single nucleotide polymorphisms related to antigens that are difficult to assess using conventional serology.
Materials and methods
The single polymerase chain reaction multiplex SNaPshot reaction was optimized to identify nine single nucleotide polymorphisms determining 16 alleles: KEL*3/KEL*4, KEL*6/KEL*7, DI*1/DI*2, DI*3/DI*4, YT*1/YT*2, CO*1/CO*2, DO*1/DO*2, DO*4, DO*5. We designed a single multiplex PCR with primers encompassing the blood group single nucleotide polymorphisms and performed an internal reaction with probe primers able to discriminate the alleles after fragment analysis. The SNaPshot assay was validated with 140 known alleles previously determined by PCR restriction fragment length polymorphism.
Results
We were able to simultaneous detect nine single nucleotide polymorphisms defining 16 blood group alleles on an assay based on a multiplex PCR combined with a single base extension using genomic DNA.
Discussion
This study demonstrates a robust genotyping strategy for conducting rare donor screening which can be applied in blood centers and could be an important tool for identifying antigen-negative donors and, therefore, for providing rare blood.
Keywords: rare blood donors, SNaPshot, screening, genotyping
Introduction
The blood group antigens of patients and donors can be easily typed using high sensitivity haemagglutination when this is done correctly. However, as haemagglutination has certain limitations, genotyping assays are offering a good alternative for problems encountered by serology1.
Detection of rare blood donors is more complex, but can be achieved in a couple of ways, including antigen identification when a patient with a rare blood type needs a transfusion, and through a selective search by testing donors in blood banks. The detection should comprise serological and/or molecular techniques2. However, when a good commercial antibody, a potent antiserum in sufficient volume or a reagent at a reasonable price is not available, DNA-based approaches are being used as an alternative for screening donors1. The molecular bases of the majority of the blood group antigens are already known and differences between antithetical antigens within the same blood group system are associated with single nucleotide polymorphisms (SNP)3. Several molecular methods have been developed for the prediction of red blood cell (RBC) phenotypes3. Depending on the purpose of the blood centre, RBC genotyping may include polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (PCR-RFLP), allele-specific PCR (AS-PCR) or real-time-based allele-specific extension assays3,4. Although reliable, these are low or medium-throughput approaches. Commercial microarrays are an excellent option for performing high-throughput genotyping of a large panel of blood group antigens in numerous donors3,4. Although microarray platforms will bring high-throughput extended blood group typing into blood donor laboratories, the analysis of the alleles depends on the manufacturers’ determinations and high costs could be a barrier, particularly in emerging countries.
In order to enable high-throughput molecular screening to search for rare donors at lower costs, we developed a SNaPshot or mini-sequencing assay that permits analysis of several SNP from numerous donors in a short period of time4. We included SNP related to antigens that are difficult to assess using conventional serology because of the lack of commercial reagents and customised our assay in our population.
Materials and methods
Blood samples and DNA extraction
Three hundred and five blood samples were selected from volunteer blood donors at Colsan, São Paulo, Brazil. All donors signed institutional informed consent and 200 μL of blood samples were used for DNA extraction using a DNA blood mini kit (QIAamp, Qiagen, Inc., Valencia, CA, USA) following the manufacturer’s instructions. The concentration of DNA was estimated using a NanoDrop 2000 Spectrophotometer (Thermal Cycler, Uniscience Inc., São Paulo, SP, Brazil) and DNA samples were kept at −20 °C for long-term storage. We tested DNA samples with a broad range of concentrations.
Polymerase chain reaction primer and probe primer design
We selected DNA sequences encompassing nine SNP in order to determe the following alleles: KEL*3/KEL*4, KEL*6/KEL*7, DI*1/DI*2, DI*3/DI*4, YT*1/YT*2, CO*1/CO*2, DO*1/DO*2, DO*4, and DO*5. We used the ensemble database (http://www.ensembl.org/index.html) to select gene sequences and the dbRBC (http://www.ncbi.nlm.nih.gov/gv/mhc/xslcgi.cgi?cmd=bgmut/home)5 and dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/) to select the polymorphisms. Alleles and nucleotide and amino acid changes are described in Table I.
Table I.
Allele identification and PCR multiplex primers for amplification of seven PCR products to analyse 16 alleles.
| Alleles | Nucleotide/amino acid change | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product size (bp) |
|---|---|---|---|---|
| DI*1/DI*2 | 2561C>T/Pro854Leu | TATCACCCAGATGTGCCCTAC | CCCAGACTTTACCCATGACTCT | 390 |
| DI*3/DI*4 | 1972G>A/Glu658Lys | GGAACGAGCAGATGGATGTAA | TATGAGGATGAAGACCAGCAGA | 328 |
| CO*1/CO*2 | 134C>T/Ala45Val | CTCTTTGTCTTCATCAGCATCG | TACATGAGGGCACGGAAGAT | 224 |
| YT*1/YT*2 | 1057C>A/His353Asn | ACTGGTGGGAATGACACAGAG | GAGGACTTCTGGGACTTCTGG | 314 |
| DO*1/DO*2 | 793A>G/Asn265Asp | |||
| DO*4 | 323G>T/Gly108Val | GGGGAGATGAGTTTTTCAAGC | TGGTCTGTGATCCTGAGTGG | 984 |
| DO*5 | 350C>T/Thr117Ile | |||
| KEL*3/KEL*4 | 841C>T/Arg281Trp | GGGAGGTTATTTGAGGGTTTG | AGCCATGCAACTGTACTTGTGT | 428 |
| KEL*6/KEL*7 | 1790T>C/Leu597Pro | AGACCAGCCAGAGTTTGATGTT | AGGTATTAAGGGCACTAGGAGGA | 437 |
Primers used in the multiplex PCR were designed using the Primer 3 programme (http://frodo.wi.mit.edu/) with a few changes to default parameters. We established that primers should measure between 21 and 24 base pairs, have a melting temperature from 59.5 °C to 60.5 °C and a GC content from 40% to 60%. The conditions were also modified to avoid repeat and template mis-priming and self-complementarity, mainly in the 3′ region. Additionally, we avoided designing primers for any region in which any other polymorphisms have already been described, irrespective of their frequency. Hairpin and autodimer formation were evaluated using the Autodimerv1removal (http://www.cstl.nist.gov/biotech/strbase/AutoDimerHomepage/AutoDimerProgramHomepage.htm)6. Forward and reverse primers for the multiplex PCR reaction and the length of amplified products are shown in Table I.
Nine internal or probe primers from the SNaPshot reaction were designed according to the SNP locations to end exactly at 5′ of each SNP site. Probe primers were constructed to have 19 or 20 nucleotides and we also avoided regions in which other SNP have been described. To distinguish among probe primers on fragment analysis, we added poly(A) tails of different lengths to their 5′ end. Probe primers and their melting temperatures are shown in Table II.
Table II.
Internal or probe SNaPshot primers for analysis of nine SNP.
| Primer name | Sequence (5′-3′) | Size (bp) | Tm (°C) | ddNTP | Signal colour |
|---|---|---|---|---|---|
| WR | (A)15ATCATCCAGATGGGAAACT | 34 | 60.3 | C/T | Black/Red |
| DI | (A)19TGGGTGGTGAAGTCCACGC | 38 | 65.7 | C/T | Black/Red |
| CO | (A)23GGTGGGGAACAACCAGACGG | 43 | 66.7 | C/T | Black/Red |
| YT | (A)27ACTAGTTACCTGCAGGCCGT | 47 | 65.6 | G/T | Blue/Red |
| JS | (A)31AGTACTGCCTGGGGGCTGCC | 51 | 68.0 | C/T | Black/Red |
| KP | (A)35TGTCAATCTCCATCACTTCA | 55 | 63.5 | T/C | Red/Black |
| DO | (A)40TGACCTCAACTGCAACCAGT | 60 | 62,8 | T/C | Red/Black |
| JO | (A)45AATAGCCACAGCGTGTGTG | 64 | 63,1 | G/A | Blue/Green |
| HY | (A)53CTTAGCCTGGCTTAACCAAG | 73 | 63.9 | G/T | Blue/Red |
Multiplex polymerase chain reaction
Multiplex PCR was performed with 0.2 pmol of each primer and 2X QIAGEN Multiplex PCR Master Mix (Qiagen, Inc.). In an attempt to optimise our multiplex PCR adjustments, we started our reaction with a final volume of 50 μL, as the manufacturer instructed and we were able to systematise it to a final volume of 6.5 μL. PCR amplification was performed in a Veriti™ 96well Thermal Cycler (Applied Biosystems, Foster City, CA, USA) using the following conditions: denaturation at 95 °C for 15 min, followed by 35 cycles of 95 °C for 15 sec, 60 °C for 90 sec and 72 °C for 1 min, and a final extension step of 72 °C for 15 min. The multiplex PCR amplification was analysed in a 2% agarose gel stained with GelRed™ Nucleic Acid Gel Stain (Biotium, Inc, Hayward, CA, USA), 10,000X in water.
When all expected PCR products and their sizes had been confirmed, the reaction was purified with Exonuclease I and FastAP™ Thermosensitive Alkaline Phosphatase (Fermentas, Hanover, MD, USA) to remove excess dNTP and primers. For this purpose, we mixed 5 μL of multiplex PCR with 10 units of Exonuclease I and 1 unit of FastAP™ and incubated at 37 °C for 60 minutes before heat inactivation of the enzymes at 75 °C for 15 minutes. When PCR products were not used at the same day, they were saved at −20 °C; their viability up to 1 week of storage was tested.
SNaPshot reaction preparation and analysis
Purified multiplex PCR products were submitted to probe primer hybridization. The quantity, name and possibly identifiable alleles were as follows: 0.2 pmol of HY (Holley, DO*4); 0.4 pmol of DI (Diego, DI*1/DI*2), CO (Colton, CO*1/CO*2) and YT (Cartwright, YT*1/YT*2); 0.5 pmol of JS (Kell, KEL*6/KEL*7); 0.6 pmol of JO (Joseph, DO*5), DO (Dombrock, DO*1/DO*2) and WR (Wright, DI*3/DI*4); and 0.7 pmol of KP (Kell, KEL*3/KEL*4) internal primers were added to 1.5 μL of purified multiplex PCR products and 2.5 μL of the ABI Prism SNaPshot™ Multiplex Kit (Applied Biosystems). Purified water was added to a final reaction volume of 6.5 μL. The SNaPshot reaction must be carried out on ice and the reaction was placed on a Veriti™ 96 well Thermal Cycler (Applied Biosystems) with the following conditions: 25 cycles of 96 °C for 10 seconds, 50 °C for 5 second and 60 °C for 30 seconds. At the end of cycling, the SNaPshot reaction was treated with 1 unit of FastAP™ at 37 °C for 60 minutes and then at 75 °C for 15 minutes for enzyme inactivation.
Instead of having deoxynucleotides (dNTP), the ABI Prism SNaPshot™ Multiplex Kit has dideoxynucleotides (ddNTP). Each ddNTP contains a specific fluorescent dye (A = dR6G, green; C = dTAMRA, black; G = dR110, blue; T (U) = dROX, red; Applied Biosystems) and as a ddNTP is incorporated into a hybridised primer, the reaction stops because of the lack of the hydroxy-radical present in the dNTP. As the reaction stops and considering that each probe primer has an individual poly(A) tail, fragments of different lengths are generated.
The fragments were analysed in a 3500xL Genetic Analyser (Applied Biosystems) using a POP-7™ Polymer (Applied Biosystems) after denaturation, at 95 °C for 5 minutes in a Veriti™ 96well Thermal Cycler (Applied Biosystems), of a mixture containing 1 μL of SNaPshot reaction purified, 9.75 μL of formamide and 0.25 μL of GeneScan™ LIZ120™ (Applied Biosystems) which was used as a size standard. Conditions during fragment analysis were as follows: time of injection (15 seconds), pre-run (3 minutes), run time (10 minutes) and data delay (4 minutes).
The results were analysed using GeneMapper Software v4.1 (Applied Biosystems).
SNaPshot validation
To check the accuracy and reproducibility of our SNaPshot assay, we analysed 140 DNA samples previously genotyped by PCR-RFLP and compared the results7–11. When the results did not match, we repeated both the PCR-RFLP and SNaPshot analyses and also sequenced these samples.
Cost and time analyses
We also evaluated costs and required time, including hands-on time, of introducing this SNaPshot into the screening of rare donors. To do this, we first calculated reagent cost after standardisation of the protocol, since we were able to reduce volumes of the reagents after adjustments. DNA extraction was not considered in the analysis and costs were calculated first in local currency, Brazilian real (R$) and then converted to US dollars (US$) using the exchange rate US$ 1.00 = R$ 1.85, according to the monthly mean in April 201212. We also explored indispensable time for preparing the whole protocol to genotype 96 samples using 96-well plates including the final analysis. As the same nine SNP could also have been analysed using PCR-RFLP, we compared the reagent costs and time required for the whole procedure between genotyping conducted under the SNaPshot and PCR-RFLP protocols.
Results
Multiplex SNapshot assay
In this study we used the multiplex SNaPshot assay to detect nine SNP determining 16 blood group alleles difficult to identify serologically and focused on using this method to identify rare donors. Considering the costs of molecular searches in the reality of a country with an emerging economy, we decided to invest in the multiplex SNaPshot method and chose to include SNP that could distinguish alleles on KEL, DI, CO, YT and DO blood group systems in a single reaction using genomic DNA at a concentration ranging from 4.3 to 529.0 ng/μL. We studied the PCR primer sequences and found the best combination of primers to avoid any kind of undesirable formation among them that could invalidate our reaction.
Nucleotide and amino acid changes that were covered in this multiplex reaction are shown in Table I. The primer concentrations and PCR conditions were adjusted to ensure achievement of all required amplicon products (Figure 1). Moreover, we conducted additional tests to decrease the volume of reaction without losing quality and sensitivity. Following these tests we were able to standardise the multiplex PCR to a final volume of 6.5 μL using 1 μL of genomic DNA, independently of the final concentration tested. The specificity of the PCR was assessed in the next step in which we hybridised it with probe primers.
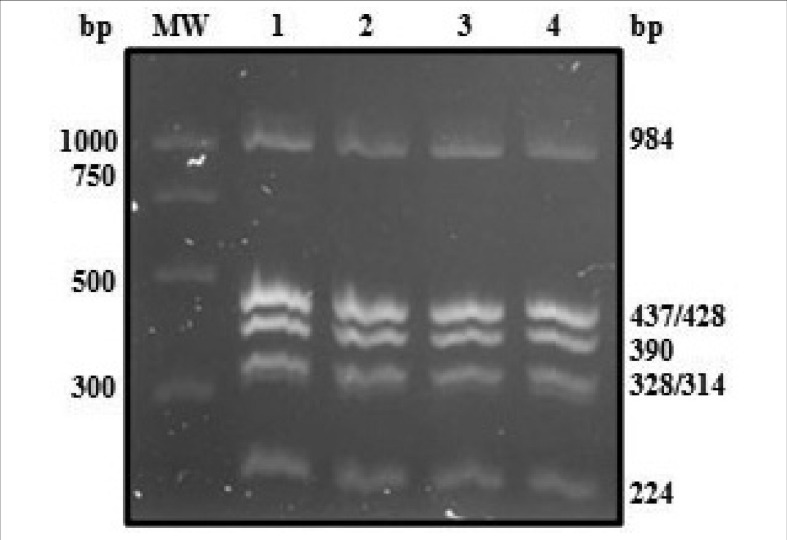
Figure 1.
Multiplex PCR amplification of seven amplicons.
A 2% agarose gel with representative PCR multiplex products (1–4) and a molecular weight marker (MW: PCR Marker Promega, Madison, WI, USA). Six bands are observed as 428 and 437bp (KEL*3/KEL*4 and KEL*6/KEL*7) bands cannot be differentiated in 2% agarose gels.
SNaPshot reaction
After purification, we performed the SNaPshot reaction by adding nine different probe primers that were hybridised at the 5′ end of the chosen SNP. By adding fluorescent labelled ddNTP, each with different colours, to the 3′ end of the internal primers, we could identify the required alleles. Since these primers differ in sizes as we added individual poly(A) tails to each one, we were able to distinguish them on fragment analysis. The specificity of the multiplex PCR and the SNaPshot reaction was confirmed as we conducted tests with each probe primer alone. Using PCR-RFLP, we selected samples that contained both alleles (heterozygous) and used them to confirm that we were analysing the designated SNP correctly.
It is important to note that we had to re-design three formerly designed probe primers, because we observed, after the first migration analysis, that the primers did not migrate exactly to the position they were supposed to, probably because of the weight of the associated ddNTP. We included adenines to the poly(A) tails from the DO, HY and JO probe primers to convert them into heavier primers, transferring their migration site to the end of the run. Using this strategy, we were able to discriminate their alleles easily without any kind of overlap.
At this point, we created our panel using GeneMapper software (Applied Biosystems, Foster City, CA, USA). With this panel, samples could be easily examined just by selecting the information regarding the exact migration of each internal primer and, consequently, the precise location of each allele. As a strategy to avoid misinterpretation, we stated that in heterozygous samples, each peak size should measure at least half of the peak size of the larger one. The expected colour and the length of the probe primers are shown in Table II. Typical GeneMapper electropherograms are shown in Figure 2.
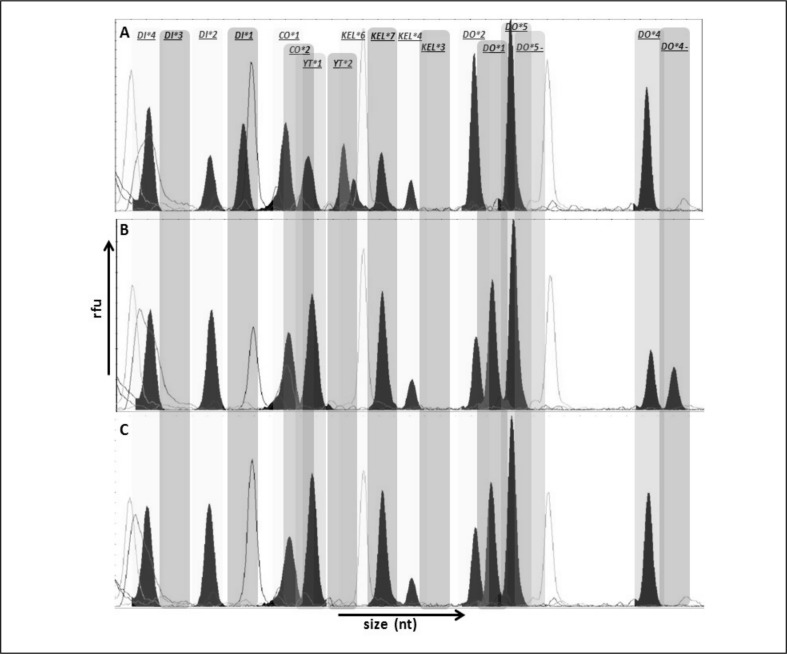
Figure 2.
GeneMapper electropherograms of representative SNaPshot fragment analysis of nine SNP. Bins are labelled at the exact place where each allele should migrate.
The X and Y axes represent size (nucleotides) and rfu (relative fluorescence units), respectively. (A) Sample genotyped as: DI*4/DI*4, DI*1/DI*2, CO*1/CO*1, YT*1/YT*2, KEL*6/KEL*7, KEL*4/KEL*4, DO*2/DO*2, DO*5/DO*5, DO*4/DO*4. (B) Sample genotyped as DI*4/DI*4, DI*2/DI*2, CO*1/CO*1, YT*1/YT*1, KEL*7/KEL*7, KEL*4/KEL*4, DO*1/DO*2, DO*5/DO*5, DO*4/DO. (C) Sample genotyped as DI*4/DI*4, DI*2/DI*2, CO*1/CO*1, YT*1/YT*1, KEL*6/KEL*6, KEL*4/KEL*4, DO*2/DO*2, DO*5/DO*5, DO*4/DO*4.
SNaPshot assay validation
After adjustment of the rare blood donor screening panel, we analysed a total of 305 DNA samples using our SNaPshot protocol and the number of samples with each genotype is presented in Table III.
Table III.
Results from SNaPshot genotyping and comparison with PCR-RFLP results.
| System | Genotypes | SNaPshot* | PCR-RFLP discrepancies† |
|---|---|---|---|
| Diego | DI*1/DI*2 | 13 | 3 |
| DI*2/DI*2 | 292 | ||
| DI*3/DI*4 | 1 | - | |
| DI*4/DI*4 | 304 | ||
|
| |||
| Colton | CO*1/CO*1 | 287 | 6 |
| CO*1/CO*2 | 18 | ||
|
| |||
| Cartwright | YT*1/YT*1 | 276 | 4 |
| YT*1/YT*2 | 28 | ||
| YT*2/YT*2 | 1 | ||
|
| |||
| Dombrock | DO*1/DO*1 | 52 | - |
| DO*1/DO*2 | 143 | ||
| DO*2/DO*2 | 110 | ||
| Presence of heterozygous DO*4 | 11 | 2 | |
| Presence of heterozygous DO*5 | 9 | 1 | |
|
| |||
| Kell | KEL*3/KEL*4 | 4 | - |
| KEL*4/KEL*4 | 301 | ||
| KEL*6/KEL*7 | 23 | 6 | |
| KEL*7/KEL*7 | 282 | ||
After PCR-RFLP repetition and sequencing, all results were in agreement with SNaPshot.
Number of samples identified with described genotype;
number of samples with discrepant results between SNaPshot and PCR-RFLP.
One hundred and forty of those samples were previously genotyped by PCR-RFLP and were used for validation. For the nine SNP analysed in these 140 samples, totalising 1,260 SNP, we observed an agreement of 98.25% with SNaPshot. There was disagreement for 22 SNP and the SNaPshot and PCR-RFLP reactions were repeated. SNaPshot showed concordant results, even when performed three times, but, after repetition, the PCR-RFLP results changed for the 22 SNP, including four in YT*1/YT*2 genotyping, two in DO*4, one in DO*5, six in KEL*6/KEL*7, six in CO*1/CO*2 and three in DI*1/DI*2. All these samples were sequenced and sequencing corroborated the SNaPshot results in all cases, confirming that our developed protocol is reliable and could be used routinely. Table III summarises the genotypes found using SNaPshot and the discrepancies between the results obtained with this technique and PCR-RFLP analysis.
Cost and time analyses
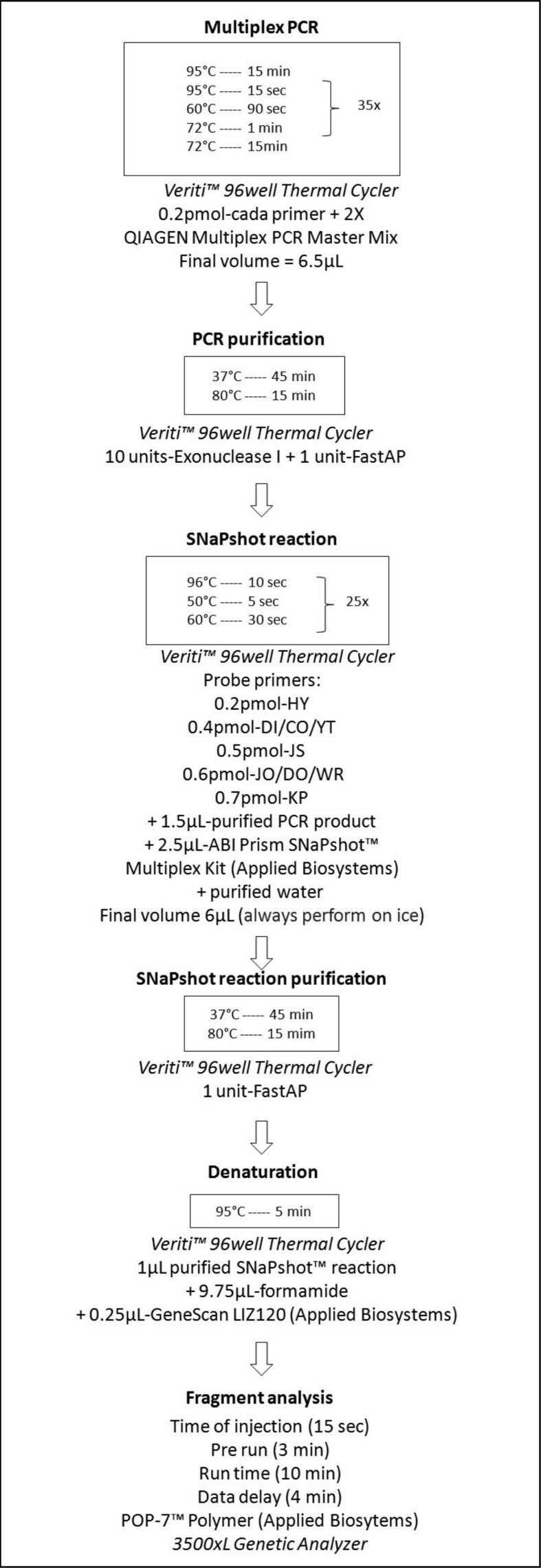
In order to provide more information about the developed SNaPshot protocol, we calculated reagent costs and time required for the whole procedure and then compared these with those for genotyping the same nine SNP using PCR-RFLP. The whole protocol is shown in Figure 3 to explain the SNaPshot steps.
Figure 3.
Schematic diagram of the whole SNaPshot protocol, including conditions, equipment and reagents.
The unit cost for SNaPshot was estimated to be US$ 0.96 per SNP or US$ 8.64 per DNA sample, while the unit cost for PCR-RFLP was US$ 1.08 per SNP or US$ 9.73 per DNA sample. For clarification, SNaPshot reagent costs included multiplex PCR and purification, SNaPshot reaction and purification, denaturation and size standard; and sequencing consumables, including buffers and capillaries. On the other hand, PCR-RFLP reagent costs included PCR, enzyme digestion and agarose gels.
Although reagent costs were similar, the time required to perform the two genotyping methods differed, as SNaPshot required 7 hours from PCR amplification to the final analysis, where, considering the same 9 SNP, the time required for the PCR-RFLP protocol was 100 hours.
Discussion
Screening for rare blood donors is a continuous search that involves several issues, including definition of the target population, selection of the method of screening and the number of donors to investigate2. Given the limitations of agglutination-based assays, the contribution of molecular techniques to immunohematology is continuously growing1. If an immunohematology laboratory decides to invest in molecular methods, there are currently some strategies that can be employed, extending from low-throughput methodologies such as PCR-RFLP to high-throughput one such as commercial microarrays4. There are numerous other options that might be used in prediction of blood group antigens4,13,14.
To the best of our knowledge, there are few studies that have explored the SNaPshot method to type RBC antigens15–19. We, therefore, concentrated our efforts on developing a multiplex SNaPshot reaction capable of identifying 16 alleles related to five blood group systems: KEL, DI, CO, YT and DO. The SNaPshot molecular assay used in this study is based on simultaneous amplification of seven exon fragments associated with single-base extensions. The whole procedure is shown in Figure 3 to provide a perspective of the final protocol.
It is essential to know that the time calculated was based on the evaluation of 96 samples in 96-well plates, that pipetting was performed by a multichannel pipette and that fragments were analysed on a 24-capillary sequencer. Additionally, we excluded the use of agarose gel electrophoresis from our final protocol in order to reduce the entire time of processing. This decision was based on the observation that, after standardisation of the multiplex PCR, all amplicons were present and that it would be more rational to repeat the reaction rather than performing agarose gels after all multiplex PCR. Interestingly, we did not have any kind of amplification problem on analysis of the 305 samples, showing that the multiplex PCR had been adjusted satisfactorily.
We believe that the success of our protocol lies mainly in the design of the multiplex PCR primers. Selecting a region in which no polymorphism has previously been described increases the chances of amplification of all amplicons. Furthermore, verification of primary and secondary structural formation among them reduces the risk of unwanted products and diminishes any kind of competition involving reagents due to formation of configurations such as hairpins and primer dimers6. As regards the design of the probe primers, although the localisation options are limited since it necessarily needs to flank the SNP, the decision of making it upstream or downstream was also based on the presence of any other defined polymorphisms. However, regardless of being located upstream or downstream, probe primers always were designed to have the SNP positioned at the 3′ end20,21. In conclusion, critical steps in developing a SNaPshot assay are the sequence analysis and primer design.
The validation performed comparing SNaPshot and PCR-RFLP analyses showed that when results were in disagreement, after repetition of the PCR-RFLP analysis and sequencing, the genotype obtained corroborated 100% with the SNaPshot results (Table III). All SNaPshot results were in agreement with sequencing. After analysis of discrepant PCR-RFLP results, we observed that the incongruities could be related to interpretation of the agarose gel and partial digestion.
As part of the considerations concerning the implementation of SNaPshot assays in routine rare donor screening, we compared reagent costs and procedural times for the the SNaPshot protocol with those of PCR-RFLP, given that PCR-RFLP is a simple technology that can be set up easily in any laboratory3. Regarding the comparison of genotyping the same nine SNP with PCR-RFLP, SNaPshot was undoubtedly a shorter procedure, the time required being decreased by more than 14-fold. When we evaluated reagent costs, both methodologies were comparable, as SNaPshot costs were remarkably reduced having successfully tested smaller volumes of reagents during the multiplex PCR and SNaPshot reaction. Since commercial antibodies for most of tested antigens are not available, we could not perform a cost comparison with phenotyping.
Summarising the benefits of the SNaPshot assays, we can highlight: the comparatively short time of preparation; the opportunity for adaptation to semiautomation since it is performed in 96-well plates, which means it can become a high-throughput strategy; convenient costs, especially when compared to low-throughput procedures; the possibility of easily including SNP genotyping for newly discovered alleles; and, the opportunity of customising a group of desired SNP according to need, for example, grouping high frequency antigen genotyping to allow identification of rare or null phenotypes.
This study demonstrates a robust genotyping strategy for “finding” rare donors that can be applied to blood centres with a sequencer analyser or facilities available. The SNaPshot assay described here can provide a solid instrument for genotyping blood group alleles that are difficult to identify by serology because specific antibodies are not available. It can help to reduce transfusion-related immunisation and haemolytic transfusion reactions by providing antigen-negative blood units to patients with rare antibodies. In our service, a blood centre responsible for approximately 4% of the blood collected in our country (according to Brazilian’s Ministry of Health, based on 2009 data)22, this assay is proving very helpful in the identification of rare blood donors and also for providing components with specific genotypes to the Brazilian population, which is characterised by a mixed ethnical origin23, a feature that contributes to the presence of different alleles24. Maintaining a database of genotyped individuals is a suitable approach to meet local transfusion needs, facilitating the provision of rare blood.
Footnotes
The Authors declare that they have no conflicts of interest relevant to this manuscript.
References
- 1.Jungbauer C. Routine use of DNA testing for red cell antigens in blood centres. Transfus Apher Sci. 2011;45:61–8. doi: 10.1016/j.transci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Nance ST. How to find, recruit and maintain rare blood donors. Curr Opin Hematol. 2009;16:503–8. doi: 10.1097/MOH.0b013e3283316bed. [DOI] [PubMed] [Google Scholar]
- 3.Veldhuisen B, van der Schoot CE, de Haas M. Blood group genotyping: from patient to high-throughput donor screening. Vox Sang. 2009;97:198–206. doi: 10.1111/j.1423-0410.2009.01209.x. [DOI] [PubMed] [Google Scholar]
- 4.Moulds JM. Future of molecular testing for red blood cell antigens. Clin Lab Med. 2010;30:419–29. doi: 10.1016/j.cll.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Patnaik SK, Helmberg W, Blumenfeld OO. BGMUT: NCBI dbRBC database of allelic variations of genes encoding antigens of blood group systems. Nucleic Acids Res. 2012;40:D1023–9. doi: 10.1093/nar/gkr958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallone PM, Butler JM. AutoDimer: a screening tool for primer-dimer and hairpin structures. Biotechniques. 2004;37:226–31. doi: 10.2144/04372ST03. [DOI] [PubMed] [Google Scholar]
- 7.Baleotti W, Jr, Rios M, Reid ME, et al. Dombrock gene analysis in Brazilian people reveals novel alleles. Vox Sang. 2006;91:81–7. doi: 10.1111/j.1423-0410.2006.00787.x. [DOI] [PubMed] [Google Scholar]
- 8.Rios M, Hue-Roye K, Oyen R, et al. Insights into the Holley- and Joseph- phenotypes. Transfusion. 2002;42:52–8. doi: 10.1046/j.1537-2995.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 9.Baleotti W, Jr, Rios M, Reid ME, et al. A novel DI*A allele without the Band 3-Memphis mutation in Amazonian Indians. Vox Sang. 2003;84:326–30. doi: 10.1046/j.1423-0410.2003.00297.x. [DOI] [PubMed] [Google Scholar]
- 10.Arnoni C, Latini FRM, Person RM, et al. Padronização das técnicas de PCR-RFLP para genotipagem dos alelos KEL*3/KEL*4 e KEL*5/KEL*6. Rev Bras Hematol Hemoter. 2011;33(Suppl 2):332–488. [Google Scholar]
- 11.Baleotti W, Jr, Suzuki RB, Ruiz M, et al. A PCR-RFLP strategy for Wright typing. Rev Bras Hematol Hemoter. 2011;33(Suppl 2):332–488. [Google Scholar]
- 12.Brazilian Real - United States Dollar Exchange rate from Central Bank of Brazil (April 1st to April 30th) [Accessed on 27/03/2013]. Available at: http://www4.bcb.gov.br/pec/taxas.
- 13.Daniels G. The molecular genetics of blood group polymorphism. Hum Genet. 2009;126:729–42. doi: 10.1007/s00439-009-0738-2. [DOI] [PubMed] [Google Scholar]
- 14.Logdberg L, Reid ME, Zelinski T. Human blood group genes 2010: chromosomal locations and cloning strategies revisited. Transfus Med Rev. 2011;25:36–46. doi: 10.1016/j.tmrv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Di Cristofaro J, Silvy M, Chiaroni J, Bailly P. Single PCR multiplex SNaPshot reaction for detection of eleven blood group nucleotide polymorphisms: optimization, validation, and one year of routine clinical use. J Mol Diagn. 2010;12:453–60. doi: 10.2353/jmoldx.2010.090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferri G, Pelotti S. Multiplex ABO genotyping by minisequencing. Methods Mol Biol. 2009;496:51–8. doi: 10.1007/978-1-59745-553-4_5. [DOI] [PubMed] [Google Scholar]
- 17.Palacajornsuk P, Halter C, Isakova V, et al. Detection of blood group genes using multiplex SNaPshot method. Transfusion. 2009;49:740–9. doi: 10.1111/j.1537-2995.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- 18.Silvy M, Simon S, Gouvitsos J, et al. Weak D and DEL alleles detected by routine SNaPshot genotyping: identification of four novel RHD alleles. Transfusion. 2011;51:401–11. doi: 10.1111/j.1537-2995.2010.02830.x. [DOI] [PubMed] [Google Scholar]
- 19.Silvy M, Di Cristofaro J, Beley S, et al. Identification of RHCE and KEL alleles in large cohorts of Afro-Caribbean and Comorian donors by multiplex SNaPshot and fragment assays: a transfusion support for sickle cell disease patients. Br J Haematol. 2011;154:260–70. doi: 10.1111/j.1365-2141.2011.08691.x. [DOI] [PubMed] [Google Scholar]
- 20.Pastinen T, Kurg A, Metspalu A, et al. Minisequencing: a specific tool for DNA analysis and diagnostics on oligonucleotide arrays. Genome Res. 1997;7:606–14. doi: 10.1101/gr.7.6.606. [DOI] [PubMed] [Google Scholar]
- 21.Syvanen AC. From gels to chips: “minisequencing” primer extension for analysis of point mutations and single nucleotide polymorphisms. Hum Mutat. 1999;13:1–10. doi: 10.1002/(SICI)1098-1004(1999)13:1<1::AID-HUMU1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Ministério da Saúde, Secretaria de Atenção à Saúde, Coordenação-Geral de Sangue e Hemoderivados. Information notebook. Blood and hemoderivates. Hemotherapy production. Unified Health System - SUS Brazil - (Public and private contractors). Private non-contracted services by Unified Health System (SUS Brazil) 4th ed. Brasília: 2011. [Google Scholar]
- 23.Santos NP, Ribeiro-Rodrigues EM, Ribeiro-Dos-Santos AK, et al. Assessing individual interethnic admixture and population substructure using a 48-insertion-deletion (INSEL) ancestry-informative marker (AIM) panel. Hum Mutat. 2010;31:184–90. doi: 10.1002/humu.21159. [DOI] [PubMed] [Google Scholar]
- 24.Storry JR. Human blood groups: inheritance and importance in transfusion medicine. J Infus Nurs. 2003;26:367–72. doi: 10.1097/00129804-200311000-00006. [DOI] [PubMed] [Google Scholar]