Abstract
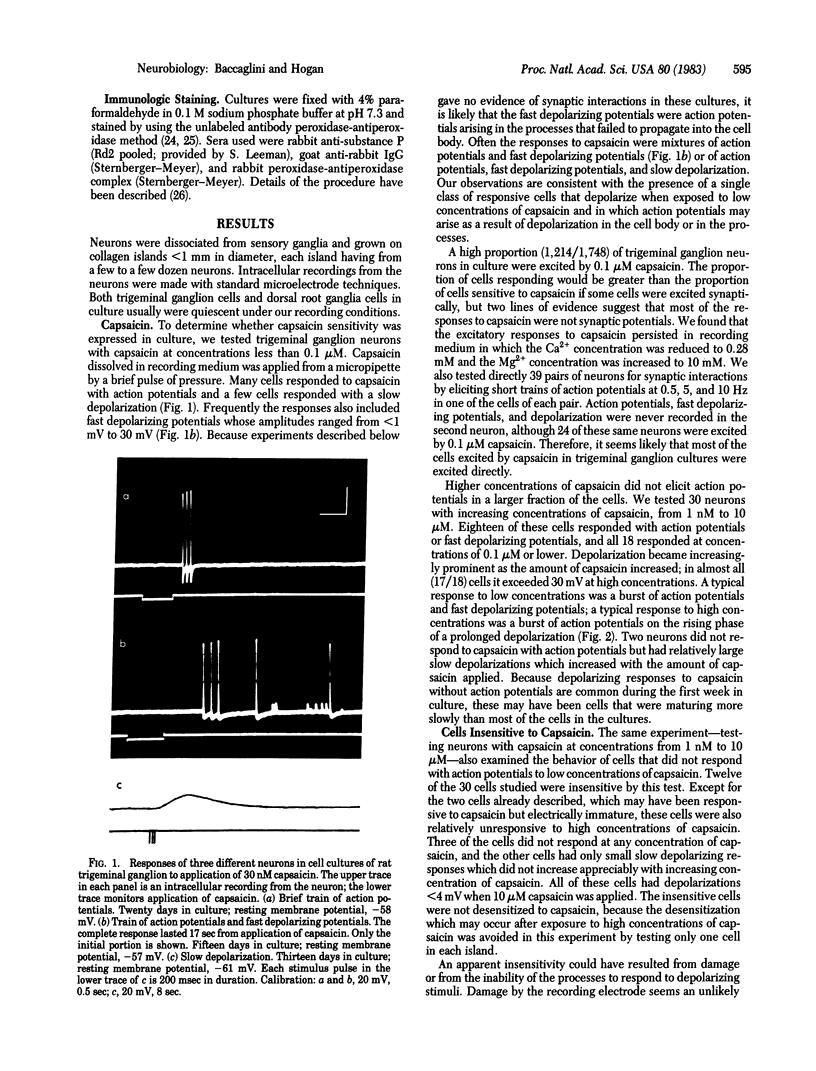
Sensory neurons were dissociated from trigeminal ganglia or from dorsal root ganglia of rats, grown in culture, and examined for expression of properties of pain sensory cells. Many sensory neurons in culture are excited by low concentrations of capsaicin, reportedly a selective stimulus for pain sensory neurons. Many are excited by bradykinin, sensitized by prostaglandin E2, or specifically stained by an antiserum against substance P. These experiments provide a basis for the study of pain mechanisms in cell culture.
Full text
PDF
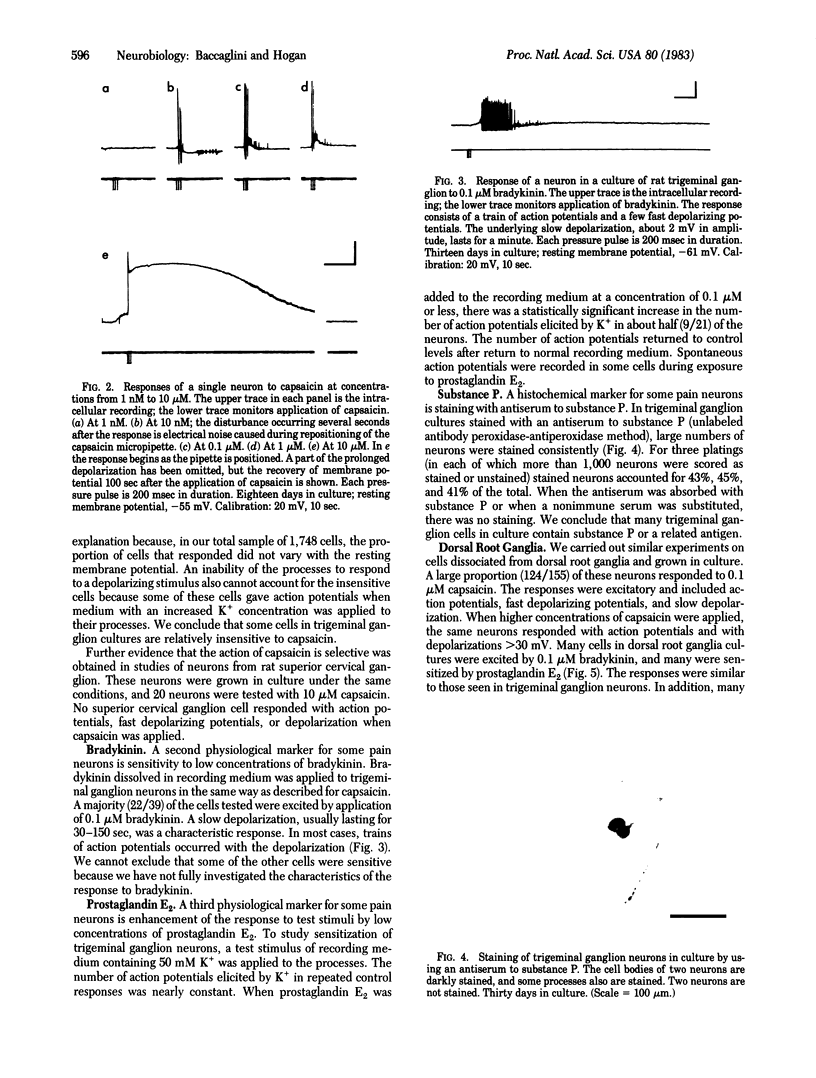


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. K., Lund J. P., Puil E. Enkephalin and substance P effects related to trigeminal pain. Can J Physiol Pharmacol. 1978 Apr;56(2):216–222. doi: 10.1139/y78-031. [DOI] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974 Mar 11;347(3):209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Chahl L. A., Iggo A. The effects of bradykinin and prostaglandin E1 on rat cutaneous afferent nerve activity. Br J Pharmacol. 1977 Feb;59(2):343–347. doi: 10.1111/j.1476-5381.1977.tb07498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. Chlordiazepoxide selectively augments GABA action in spinal cord cell cultures. Nature. 1977 Sep 22;269(5626):342–344. doi: 10.1038/269342a0. [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Del Fiacco M., Paxinos G. The central and peripheral ends of the substance P-containing sensory neurones in the rat trigeminal system. Brain Res. 1978 Sep 8;152(3):499–500. doi: 10.1016/0006-8993(78)91105-8. [DOI] [PubMed] [Google Scholar]
- FJALLBRANT N., IGGO A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961 May;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972 Dec 13;240(102):200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Foster R. W., Ramage A. G. The action of some chemical irritants on somatosensory receptors of the cat. Neuropharmacology. 1981 Feb;20(2):191–198. doi: 10.1016/0028-3908(81)90203-3. [DOI] [PubMed] [Google Scholar]
- Furshpan E. J., MacLeish P. R., O'Lague P. H., Potter D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. L. Effects of substance P on functionally identified units in cat spinal cord. Brain Res. 1976 Sep 24;114(3):439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975 Dec 19;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Kellerth J. O., Nilsson G., Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975 Nov 28;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci Lett. 1981 Sep 1;25(2):137–141. doi: 10.1016/0304-3940(81)90321-9. [DOI] [PubMed] [Google Scholar]
- Kenins P. Responses of single nerve fibres to capsaicin applied to the skin. Neurosci Lett. 1982 Mar 17;29(1):83–88. doi: 10.1016/0304-3940(82)90369-x. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. The polymodal C-fiber receptor in the muscle of the dog. Brain Res. 1976 Jan 23;101(3):589–593. doi: 10.1016/0006-8993(76)90483-2. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Primate cutaneous receptors with unmyelinated (C) fibres and their projection to the substantia gelatinosa. J Physiol (Paris) 1977 Sep;73(3):287–304. [PubMed] [Google Scholar]
- Langford L. A., Coggeshall R. E. Branching of sensory axons in the dorsal root and evidence for the absence of dorsal root efferent fibers. J Comp Neurol. 1979 Mar 1;184(1):193–204. doi: 10.1002/cne.901840111. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Folkers K., Donnerer J. Analgesic effect of antagonists of substance P. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1318–1321. doi: 10.1016/0006-291x(81)90266-7. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E2. Brain Res. 1981 Nov 23;225(1):95–105. doi: 10.1016/0006-8993(81)90320-6. [DOI] [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. I., Hunt S. P., Iversen L. L., Emson P. C. Biochemical and anatomical observations on the degeneration of peptide-containing primary afferent neurons after neonatal capsaicin. Neuroscience. 1981;6(10):1923–1934. doi: 10.1016/0306-4522(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Schenker C., Leeman S. E. Substance P as a transmitter candidate. Annu Rev Neurosci. 1980;3:227–268. doi: 10.1146/annurev.ne.03.030180.001303. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., Obata K., Claude P., Furshpan E. J., Potter D. D. Evidence for cholinergic synapses between dissociated rat sympathetic neurons in cell culture. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3602–3606. doi: 10.1073/pnas.71.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Release of substance P-like immunoreactivity from isolated spinal cord of newborn rat. Nature. 1976 Nov 4;264(5581):83–84. doi: 10.1038/264083a0. [DOI] [PubMed] [Google Scholar]
- PORSZASZ J., JANCSO N. Studies on the action potentials of sensory nerves in animals desensitized with capsaicine. Acta Physiol Acad Sci Hung. 1959;16:299–306. [PubMed] [Google Scholar]
- Pateromichelakis S., Rood J. P. Prostaglandin E1-induced sensitization of A delta moderate pressure mechanoreceptors. Brain Res. 1982 Jan 28;232(1):89–96. doi: 10.1016/0006-8993(82)90612-6. [DOI] [PubMed] [Google Scholar]
- Randić M., Miletić V. Effect of substance P in cat dorsal horn neurones activated by noxious stimuli. Brain Res. 1977 Jun 3;128(1):164–169. doi: 10.1016/0006-8993(77)90245-1. [DOI] [PubMed] [Google Scholar]
- Rosell S., Olgart L., Gazelius B., Panopoulos P., Folkers K., Hörig J. Inhibition of antidromic and substance P-induced vasodilatation by a substance P antagonist. Acta Physiol Scand. 1981 Mar;111(3):381–382. doi: 10.1111/j.1748-1716.1981.tb06752.x. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol (Paris) 1977 Sep;73(3):251–259. [PubMed] [Google Scholar]
- Takahashi T., Otsuka M. Regional distribution of substance P in the spinal cord and nerve roots of the cat and the effect of dorsal root section. Brain Res. 1975 Apr 4;87(1):1–11. doi: 10.1016/0006-8993(75)90774-x. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E. Afferent C units responding to mechanical, thermal and chemical stimuli in human non-glabrous skin. Acta Physiol Scand. 1974 Nov;92(3):374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Jessell T. M., Gamse R., Mudge A. W., Leeman S. E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980 Jul 10;286(5769):155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]



