Abstract
Background
An intra-operative cell salvage machine, commonly known as a “cell saver”, aspirates, washes, and filters patient’s blood during an operation so that the blood can be returned to the patient’s circulation instead of being discarded. This procedure could significantly reduce the risks related to the use of allogeneic blood and blood products in surgery. The aim of this study was to analyse the influence of intra-operative cell salvage on reducing the need for allogeneic blood in patients with asymptomatic infrarenal abdominal aortic aneurysm undergoing elective repair of the aneurysm.
Material and methods
We retrospectively collected data from the clinical records of patients who underwent elective infrarenal abdominal aortic aneurysm repair. Two groups were formed: the “cell saver” group, in which intra-operative cell salvage was used, and the control group, in which a cell saver was not used.
Results
Thirty patients underwent abdominal aortic aneurysm repair with the use of a cell saver, while 32 underwent the same operation without cell salvage. We found a significant association between use of the cell saver and a reduced need for allogeneic blood in these patients. Operations performed with the use of a cell saver lasted, on average, less time than those performed without it. The difference between pre-operative and post-operative haemoglobin levels was significantly greater in the group of patients who underwent repair with the use of a cell saver than in the control group.
Conclusion
The use of a cell saver in elective abdominal aortic aneurysm repair significantly reduces the need for intra-operative use of allogeneic blood.
Keywords: cell saver, abdominal aortic aneurysm, allogeneic blood
Introduction
Aneurysm of the infrarenal segment of the abdominal aorta is a permanent local dilatation of the infrarenal aorta, where the widest outer diameter of the artery is over 29 mm. In developed countries, abdominal aortic aneurysm (AAA) is the cause of 1–3% of deaths of men aged 65 to 85 years1. An early diagnosis of AAA is important to enable elimination of the aneurysm and, thereby, the prevention of fatal complications. About 80% of AAA are located between the renal arteries and the aortic bifurcation2. Enlargement of the aneurysmatic diameter is followed by an exponentially increased risk of rupture (Laplace’s law). In cases of rupture, the mortality rate is 45–80%3.
Only surgical treatment can eliminate an AAA. Elective treatment is indicated when the risk of rupture exceeds the risk of the operative procedure itself. The most important predictor of rupture is the maximal outer diameter. If the outer diameter of an aneurysm is greater than 5.5 cm, it is suggested that operative risk is assessed and a decision made about possible surgical treatment. Elective open surgery is associated with an operative mortality rate of 2 to 5% in specialised institutions. Although endovascular treatment significantly reduces peri-operative morbidity and shortens patient’s recovery time, it requires long follow up by computed tomography, use of endografts and highly sophisticated radiological technology4,5.
Open surgery of the abdominal aorta requires transfusion of blood and blood products, as this type of surgery is inevitably associated with loss of intravascular volume of blood. The use of allogeneic blood is associated with a real risk of immunological complications. The price of allogeneic blood components is rising because of the increasingly sophisticated tests and processes used routinely with the aim of reducing the risks of infective complications of the transfusions.
A machine for intra-operative blood salvage, known as a “cell saver” (CS), aspirates, washes, and filters a patient’s blood during an operation, so that the blood can be returned to the patient’s circulation. The machine has the capacity to save up to 15% of blood, which would otherwise be lost6. This method is also a good alternative for patients who refuse to accept donated blood because of religious beliefs. Other advantages of using cell salvage are the lack of viral disease transmission, a reduced risk of alloimmunisation, normal potassium concentration, the fact that the infused cells are at room temperature and have normal red blood cell oxygen carrying capacity and 2,3-diphosphoglycerate (2,3 DPG) levels7,8.
There are recommendations for pre-operative procedures that can increase the level of red blood cells (usage of B12, iron, folic acid). Erythropoetin can also be used several weeks prior the surgery (optimally 6 weeks)9. A pre-operative increase of red blood cells allows better and easier tolerance of intra-operative blood losses. Whether a CS should be used is a clinical decision. Each case should, therefore, be considered individually. The indication for the use of intra-operative cell salvage is an expected blood loss greater than 20% of the overall volume, or approximately 1 L in an adult10. Autotransfusion is used in order to decrease the need for allogeneic blood transfusion, thereby avoiding the complications associated with the administration of this type of blood11.
The aim of this study was to analyse the influence of intra-operative blood salvage and autologous transfusion on the need for allogeneic transfusion in asymptomatic patients undergoing elective repair of an infarenal AAA.
Material and methods
Patient’s individual clinical records from the Vascular and Transplant Surgery Clinic, Vojvodina Clinical Centre in Novi Sad (Serbia) were used. We analysed only patients who underwent elective surgical treatment of infrarenal AAA in the period from January 1st to December 31st, 2009. The retrospectively collected data were drawn from the patients’ clinical documentation (medical history, operative protocol, computer database, archive of images and photo documentation, additional clinical and laboratory documentation, medical death reports). Criteria for inclusion in the study were low/medium risk patients, aneurysmal diameter greater than 55 mm, elective surgery, intravenous antibiotic prophylaxis with cefuroxime 750 mg and metronidazole 500 mg, transperitoneal access, heparin 5,000 units before aortic cross-clamping, aneurysmectomy with implantation of a Dacron® tube graft (Intervascular, Datascope Intervascular GmbH, Bensheim, Germany) and no pre-operative anaemia (haemoglobin level above 120 g/L).
Two homogeneous groups of patients were formed, differing only according to whether a CS (autoLog® Autotransfusion System, Medtronic, Inc. Minneapolis, United States of America) machine for intra-operative blood salvage was used or not. During the study period, 62 patients were analysed, of whom 30 were operated with the use of a machine for intra-operative blood salvage (CS group) and 32 patients without it (NCS or control group).
The patients’ sex distribution and distribution of risk factors for cardiovascular diseases are presented in Table I. There were no statistically significant differences between the patients in the CS and NCS groups with regards to these parameters.
Table I.
Demographic data, risk factors and aneurysm size of the patients studied.
| CS group | NCS group | |
|---|---|---|
| N. of patients | 30 | 32 |
| Males (n.) | 28 | 26 |
| Mean age (years) | 67.34 | 66.73 |
| Mean size of aneurysm (cm) | 6.62 | 6.75 |
| Hypertension (n.) | 29 | 21 |
| Smokers (n.) | 18 | 17 |
| Heritage* | 25 | 22 |
Heritage: family history of death due to cardiovascular disease.
The following information was collected and analysed individually for all patients:
- risk assessment data: age, sex, atherosclerotic risk factors (smoking, high blood pressure), pre-operative haemoglobin level;
- intra-operative data: diameter of the aneurysm, duration of the operation, clamp time, blood loss, amount of allogeneic blood transfused;
- post-operative data: first post-operative level of haemoglobin in the Intensive Care Unit (ICU), amount of allogeneic blood transfusion during intensive care treatment, number of days spent in the ICU, occurrence of post-operative complications related to massive blood transfusions, occurrence of transfusion reactions in intensive care, lung complications (acute respiratory distress syndrome, ARDS), early infections of the surgical wound during intensive care.
All data were analysed statistically and are shown in Table II for each group individually.
Table II.
Comparative overview of the parameters examined.
| Variables (values are median) | CS group | NCS group | p |
|---|---|---|---|
| Length of operation (min) | 113.28 | 171 | <0.000001 |
| Aortic cross-clamping time (min) | 45.6 | 59.2 | 0.23 |
| Intra-operative blood loss (mL) | 1,483 | 1170 | 0.11 |
| Intra-operative administration of allogeneic erythrocytes (mL) | 25.31 | 632.83 | <0.000001 |
| Post-operative transfusions (mL) | 118.48 | 240.17 | 0.15 |
| Δ Haemoglobin* | 20 | 13.73 | 0.03 |
| N. of days in ICU | 2.75 | 2.97 | 0.47 |
The difference between haemoglobin concentration before and after surgery.
Using descriptive statistics, we determined the mean value, standard deviation, median, minimum and maximum and the frequency of certain categories. Concerning comparative statistics, for the analysis of differences between mean values of attributes between examined groups, we used Student’s t-test. P values <0.05 were considered statistically significant.
For comparisons of the differences in intensity of appearance between observed groups Pearson’s χ2-test was used with a level of significance at 95%. The calculations were performed with the StatSoft, Inc. package, Tulsa, Oklahoma, United States of America (http://www.statsoft.com/).
Results
Aortic cross-clamping time was defined as the time from application of the aortic cross-clamp to the time of clamp release to a distal anastomosis. The median duration of the aortic cross-clamping was similar in the two groups (Table II). A trend towards an increased recorded blood loss in the CS group was not statistically significant (P =0.11). After statistical analysis, we concluded there was a statistically significant difference in average duration of surgery between the study (CS) group and the control (NCS) group (P <0.01).
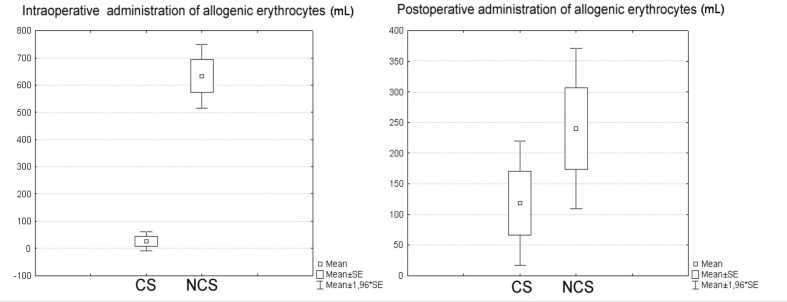
Patients in whom the CS was used were given an average of 25.3 mL of allogeneic red blood cells (Table II). In fact, 93.8% of patients from this group did not receive any allogeneic blood intra-operatively. Patients in the control group were given an average of 632.83 mL of allogeneic red blood cells intra-operatively and 93.3% of patients in this group received allogeneic blood intra-operatively. There was a statistically significant difference (P <0.01) in intra-operative administration of allogeneic erythrocytes between the two groups (Figure 1).
Figure 1.
Intra-operative and post-operative administration of allogeneic erythrocytes.
On average, patients in the CS group received 118.47 mL of blood in the ICU, whereas patients in the control group received an average of 240.17 mL of blood in this period. The amount of allogeneic blood transfused postoperatively during ICU treatment was not statistically different between the two groups (P =0.15) (Table II).
The difference between the haemoglobin concentration before and after surgery was statistically significant different between the study group and the control group (P =0.034) (Table II). Interestingly, however, there was no statistically significant difference (P =0.61) between the median haemoglobin concentration in the CS (114.1 g/L) and NCS (109.6 g/L) groups of patients at the time of hospital discharge.
Although the number of days spent in the ICU was higher in the control group than in the study group, we found that the difference was not statistically significant (P =0.47).
A total of 10 postoperative complications occurred, four in the CS group and six in the NCS group: this difference was not statistically significant (P =0.648) and complications could not be directly related to blood transfusion.
Discussion
There are numerous studies11–16 about usage of intra-operative blood salvage during elective surgical repair of AAA. Most of them analysed the average amount of allogeneic administered intra-operatively and overall to patients who were operated with the use of a CS. These studies showed that there is a statistically significant difference in the amount of transfused donor blood between such patients and patients who are operated upon without intraoperative cell salvage. It is, therefore, obvious that the use of CS is the safer strategy. The results of our study led us to the same conclusion. However, CS is not always able to eliminate the need for allogeneic transfusion. As a result of this fact and the costs of machine maintenance, there is no consensus about the routine use of CS in open surgery of an AAA.
In a single centre, randomised clinical trial conducted by Marcer et al.13, information was collected about 81 patients who underwent elective surgery of AAA: a CS was used intra-operatively in 40 of these patients, while in the other 41 patients it was not. There was a significant correlation (P =0.02) between the use of a CS and reduced need for intra-operative allogeneic blood transfusion. We obtained similar results (average of 25 mL allogeneic blood administered intra-operatively in the CS group compared to 633 mL in the control group). Our results show that the difference between pre-operative and postoperative levels of haemoglobin was significantly greater in the CS group (20 g/L) than in the NCS group (13.73 g/L) (P <0.05). The average amount of total allogeneic blood administered to patients was 0.9 units in the CS group and 3.5 units in the control group (P <0.0001). In our study, there was no statistical difference regarding post-operative transfusion of allogeneic blood between the two groups, although patients in the NCS group did, on average, receive a larger volume of allogeneic blood. Patients from the control group were on average older, but again the difference was not statistically significant (P >0.05). The same applied to the size of aneurysm, which is consistent with the results from our study.
Ouriel et al.17 studied 200 patients who underwent AAA surgery. Those in whom a CS machine was used intra-operatively were given an average of 0.6 units of allogeneic blood during the operation compared to the average of 3.4 units given to patients in the NCS group (P <0.01). Only 34% of patients in the CS group required allogeneic blood, compared to 92% in the NCS group. In our study, we found an even more pronounced difference between the groups, as only 6.25% of patients in the CS group received allogeneic blood, compared to 93.33% of the patients in the control (NCS) group. Operative mortality and morbidity in the two groups were similar, as were the number of days spent in the ICU; these findings are consistent with those of our study.
Spark et al.12 presented the results of a prospective, randomised study of 50 patients who were operated on for AAA: a CS machine was used intra-operatively in 23 patients (CS group) whereas it was not used in the other 27 patients (NCS group). Only 14% of patients in the CS group were given allogeneic blood during the operation, whereas 96.2% of patients operated without the use of a CS received donor blood cells, which is very similar to the results we obtained. In this study, three out of 27 patients in the NCS group had post-operative sepsis caused by pneumonia, while such complications were not recorded in the CS group. Then complications did not occur in our study. It was concluded that the use of CS significantly reduces exposure to homologous blood. The levels of post-operative haemoglobin were similar in the two groups (11.0 and 10.8 g/dL) and there was no difference in post-operative mortality.
Huber et al.18 analysed 168 patients who underwent elective treatment of AAA. The use of a CS machine reduced the need for further allogeneic transfusions, although 73% of the patients were exposed to allogeneic blood. Huber et al. concluded that the method was not profitable, as more than 1,250 mL of saved blood was needed in order to gain a financial benefit (5.2 units of blood). A “cost-benefit” was observed in only 16% of cases, which led the authors to conclude that intra-operative cell salvage should be used only in selected cases.
In a study by Hallett et al.19, who analysed the use of a CS in elective surgery of AAA, it was found that 34 of 50 patients (68%) operated upon with the use of CS received only their own blood, without administration of autologous red blood cells. In this study the level of post-operative haemoglobin was similar in the CS and control groups (11.91 g/dL and 11.90 g/dL, respectively), which differs from the results of our study.
In a study conducted by Healy et al.15 no postoperative complications which could be directly associated with the use of the CS were detected. It was concluded that the use of intra-operative CS is safe. Complications such as haemolysis, intravascular coagulation, decreased haematocrit level and microembolisation, which used to occur more often, are attributed to the characteristics of older machines, which had rotating heads and damaged red blood cells, thus releasing free haemoglobin and causing complications.
In conclusion, according to the results of our study, the use of intra-operative blood salvage and autologous transfusion in elective surgery of AAA significantly reduces the need for allogeneic blood during this operation.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–89. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146:735–41. doi: 10.7326/0003-4819-146-10-200705150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Adam DJ, Mohan IV, Stuart WP, et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30:922–8. doi: 10.1016/s0741-5214(99)70018-2. [DOI] [PubMed] [Google Scholar]
- 4.Greenhalgh RM, Allison DJ, Bell PR, et al. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm: randomized controlled trial. Lancet. 2005;365:2179–86. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 5.Blankensteijn JD, de Jong SE, Prinssen M, et al. Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398–405. doi: 10.1056/NEJMoa051255. [DOI] [PubMed] [Google Scholar]
- 6.Thomas D, Wee M, Clyburn P, et al. Association of Anaesthetists of Great Britain and Ireland. Blood transfusion and the anaesthetist: management of massive haemorrhage. Anaesthesia. 2010;65:1153–61. doi: 10.1111/j.1365-2044.2010.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bélisle S, Hardy JF. Haemorrhage and the use of blood products after adult cardiac operations: myths and realities. Ann Thorac Surg. 1996;62:1908–17. doi: 10.1016/s0003-4975(96)00944-7. [DOI] [PubMed] [Google Scholar]
- 8.Munoz GM, Sanchez AY, Garcia VJ, et al. Pre and postoperative autotransfusion. A comparative study of hematology, biochemistry and red cell metabolism in pre-donated blood and blood from post-operative surgical drainage. Sangre. 1999;44:443–50. [PubMed] [Google Scholar]
- 9.Posacioglu H, Apaydin AZ, Islamoglu F. Adverse effects of cell saver in patients undergoing ruptured abdominal aortic aneurysm repair. Ann Vasc Surg. 2002;16:450–5. doi: 10.1007/s10016-001-0123-7. [DOI] [PubMed] [Google Scholar]
- 10.Napier JA, Bruce M, Chapman J, et al. Guidelines for autologous transfusion II: perioperative haemodilution and cell salvage. Br J Anaesth. 1997;78:768–71. doi: 10.1093/bja/78.6.768. [DOI] [PubMed] [Google Scholar]
- 11.Markovic M, Davidovic L, Savic N, et al. Intraoperative cell salvage versus allogenic transfusion during abdominal aortic surgery: clinical and financial outcomes. Vascular. 2009;17:83–92. doi: 10.2310/6670.2009.00009. [DOI] [PubMed] [Google Scholar]
- 12.Spark JI, Chetter IC, Kester RC, Scott DJ. Allogeneic versus autologous blood during abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 1997;14:482–6. doi: 10.1016/s1078-5884(97)80128-1. [DOI] [PubMed] [Google Scholar]
- 13.Mercer KG, Spark JI, Berridge DC, et al. Randomized clinical trial of intraoperative autotransfusion in surgery for abdominal aortic aneurysm. Br J Surg. 2004;91:1443–8. doi: 10.1002/bjs.4793. [DOI] [PubMed] [Google Scholar]
- 14.Wong JC, Torella F, Haynes SL, et al. ATIS Investigators. Autologous versus allogeneic transfusion in aortic surgery: a multicenter randomized clinical trial. Ann Surg. 2002;235:145–51. doi: 10.1097/00000658-200201000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy CF, Doyle M, Egan B, et al. Transfusion requirements and outcomes in patients undergoing abdominal aortic surgery using intra-operative cell salvage. Royal Academy of Medicine in Ireland. 2007;176:33–6. doi: 10.1007/s11845-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 16.Tawfick WA, O’Connor M, Hynes N, Sultan S. Implementation of the Continuous AutoTransfusion System (C.A.T.S) in open abdominal aortic aneurysm repair: an observational comparative cohort study. Vasc Endovasc Surg. 2008;42:32–9. doi: 10.1177/1538574407309316. [DOI] [PubMed] [Google Scholar]
- 17.Ouriel K, Shortell CK, Green RM, De Weese JA. Intraoperative autotransfusion in aortic surgery. J Vasc Surg. 1993;18:16–22. doi: 10.1067/mva.1993.41709. [DOI] [PubMed] [Google Scholar]
- 18.Huber TS, McGorray SP, Carlton LC, et al. Intraoperative autologous transfusion during elective infrarenal aortic reconstruction. J Surg Res. 1997;67:14–20. doi: 10.1006/jsre.1996.4971. [DOI] [PubMed] [Google Scholar]
- 19.Hallett JW, Jr, Popovsky M, Ilstrup D. Minimizing blood transfusions during abdominal aortic surgery: recent advances in rapid autotransfusion. J Vasc Surg. 1987;5:601–6. [PubMed] [Google Scholar]