Abstract
Background
Hereditary haemochromatosis may result in severe organ damage which can be prevented by therapy. We studied the possible advantages and disadvantages of erythrocytapheresis as compared with phlebotomy in patients with hereditary haemochromatosis.
Materials and methods
In a prospective, randomised, open-label study, patients with hereditary haemochromatosis were randomised to bi-weekly apheresis or weekly whole blood phlebotomy. Primary end-points were decrease in ferritin levels and transferrin saturation. Secondary endpoints were decrease in haemoglobin levels, discomfort during the therapeutic procedure, costs and technicians’ working time.
Results
Sixty-two patients were included. Thirty patients were randomised to apheresis and 32 to whole blood phlebotomy. Initially, ferritin levels declined more rapidly in the apheresis group, and the difference became statistically highly significant at 11 weeks; however, time to normalisation of ferritin level was equal in the two groups. We observed no significant differences in decline of transferrin saturation, haemoglobin levels or discomfort. The mean cumulative technician time consumption until the ferritin level reached 50 μg/L was longer in the apheresis group, but the difference was not statistically significant. The cumulative costs for materials until achievement of the desired ferritin levels were three-fold higher in the apheresis group.
Conclusion
Treatment of hereditary haemochromatosis with erythrocytapheresis instead of whole blood phlebotomy results in a more rapid initial decline in ferritin levels and a reduced number of procedures per patient, but not in earlier achievement of target ferritin level. The frequency of discomfort was equally low with the two methods. The costs and, probably, technician time consumption were higher in the apheresis group.
Keywords: haemochromatosis, phlebotomy, erythrocytapheresis, erythrocyte apheresis, therapy
Introduction
Hereditary (primary) haemochromatosis (HH) is a frequent hereditary metabolic disturbance in Europe and among Americans of European ancestry, although worldwide there are large racial and geographical variations1–4. The condition is characterised by increased iron absorption and hence a slow accumulation of superfluous iron5. Pathological iron overload may result in severe organ damage which can be efficiently prevented by iron-depleting therapy5,6. Only a small proportion of patients, however, develop such organ damage1,7 and, as a result of screening, most affected individuals are diagnosed before the onset of clinical disease7–9. The preclinical phase can be divided into a pure genetic disposition with normal iron stores and biochemical haemochromatosis with a genetic disposition and increased iron stores, but no organ damage6. Clinical benefit from treatment has not been documented in individuals with pure genetic disposition or slight biochemical haemochromatosis1,7.
The optimal treatment method is still a matter of discussion. Prevention of organ damage has traditionally been accomplished by whole blood phlebotomy, which has to be repeated frequently during the initial phase and then continued indefinitely as a maintenance treatment5,6. The amount of iron removed can be increased two- or three-fold for each procedure by using modern technology (erythrocytapheresis) for selective withdrawal of red blood cells10,11. Possible drawbacks associated with this technique may be prolonged time for each therapeutic procedure, higher costs and some patient-related limitations because the procedure involves an extracorporeal blood volume. The potential advantages are the reduced number of therapeutic procedures and, possibly, a more rapid normalisation of iron stores and less strain for the patients12.
Before this trial was initiated, no larger, randomised study comparing the two methods had been published. In the original description of erythrocytapheresis, only 14 of 65 patients had hemochromatosis10, and some subsequently published reports described very small series11,13–17. Results from these studies indicate that red cell apheresis may be a more efficient method than phlebotomy, but the results are partly conflicting and the number of patients included is small. It is still not possible, therefore, to determine which method should be preferred.
We wanted to investigate whether more rapid decreases in ferritin levels and transferrin saturation can be achieved by erythrocytapheresis as compared with whole blood phlebotomy, without significant disadvantages, in patients with iron overload due to HH.
Materials and methods
Study design
From 2006 through 2010, we conducted a prospective, randomised, multicentre trial of erythrocytapheresis vs whole blood phlebotomy in patients with HH. Patients were included from three participating hospitals in Norway. The study was posted at www.ClinicalTrials.gov as NCT00509652.
Eligible patients were randomised to bi-weekly apheresis or weekly whole blood phlebotomy. The randomisation procedure was centralised to one of the participating centres, using a randomly generated sequence. Prolongation of the treatment interval based on clinical assessment was allowed. Data were assessed according to an intention-to-treat principle.
The Regional Ethics Committee of Western Norway and Norwegian Social Science Data Services approved the protocol. The trial was carried out in accordance with the Helsinki Declaration, and written informed consent was obtained from all participating patients.
Inclusion and exclusion criteria
To be eligible for the trial, patients had to be diagnosed with HH. The diagnosis of HH was defined as confirmed in the presence of a homozygous genotype for C282Y or H63D or compound heterozygous genotype for C282Y and H63D, combined with increased ferritin levels or a transferrin saturation higher than 50%. Given the lack of exact knowledge of which individuals should be treated, we based our criteria mainly on the 2003 version of the guidelines established by the Norwegian Society of Haematology18. The ferritin cut-off level for inclusion, however, was set at 300 μg/L in patients homozygous for C282Y or H63D or compound heterozygous for these mutations. A haemochromatosis phenotype can also be found in a limited number of individuals heterozygous for C282Y, probably due in part to additional chromosome 6 mutations19. Therefore, patients with a heterozygous C282Y genotype were included if they had ferritin levels higher than 500 μg/L or transferrin saturation higher than 50%. Individuals with atypical HH without any documented genetic aberration or exclusively due to mutations other than C282Y and H63D were not eligible for the study.
The patients had to be 18 years or older and to have received no previous treatment for HH. Patients were excluded if they had a body weight less than 65 kg or initial haemoglobin level less than 13.5 g/dL. All patients underwent a thorough initial clinical assessment and individuals were excluded if diagnosed with alcoholism, active liver disease, chronic inflammatory disorders, chronic infection or active malignant disease. Liver biopsy was not performed in any patient.
Laboratory assessments
Genotyping was performed at the Centre for Medical Genetics, Haukeland University Hospital, Bergen, using standard polymerase chain reaction-based analysis in blood collected into EDTA. Haematological and biochemical parameters were measured at the local hospital laboratories according to standard methods. Transferrin saturation was calculated using the formula:
Therapeutic procedures
Whole blood phlebotomy
Per single treatment procedure, 450 mL of whole blood (equivalent to 200–220 mL of red blood cells) were removed once weekly using a collection bag from Fenwal Europe, SPRL, Belgium.
Erythrocytapheresis
Per single treatment, 400 mL of red blood cells were withdrawn every second week. We used Haemonetics MCS+ (Haemonetics Scandinavia AB, Lund, Sweden) to collect red blood cells using citrate-phosphate-dextrose anticoagulant at a ratio of 16 volumes of blood to 1 volume of anticoagulant. After the removal of the red blood cells, the autologous platelet-rich plasma was re-infused together with 400 mL of 0.9% NaCl solution.
Assessment of response and tolerance
Primary end-points were reduction of ferritin levels and transferrin saturation. These end-points were defined by the rate of decline in each variable as well as the time to achievement of ferritin levels of 50 μg/L. Secondary end-points were decrease in haemoglobin levels, discomfort during the therapeutic procedure, material costs and technicians’ working time. Discomfort was categorised into grade 0 (none or negligible discomfort) and grade 1 (significant discomfort). Patients who fainted at any time or withdrew from the study because of discomfort were classified as having grade 1 discomfort.
Statistics
Median values were calculated for variables with a skewed distribution and mean values for normally distributed data. For each time point when apheresis and whole blood phlebotomy were applied in the respective treatment arms, i.e. every second week, we tested the difference in ferritin levels (μg/L) and transferrin saturation (%) between the two groups using the Wilcoxon test. The general significance level was set at 0.05. Taking into account multiple effects, we adjusted the significance level to 0.01. This adjusted P-value is somewhat higher than it would have been using Bonferroni’s procedure20 which is too conservative, given that the tests for the several time points cannot be considered independent.
An estimation of statistical power was based on the standard deviations for decline in ferritin levels and transferrin saturation found in a small pilot study of apheresis vs whole blood phlebotomy in HH (B. I. V. Telset. Master thesis. University of Bergen, 2004; unpublished). Based on t-test and using the “Power and Precision” program (Biostat Inc., Englewood, NJ, USA), we found that 67 patients were needed in each arm in order to achieve 80% probability of detecting a statistically significant difference at the 5% level. The relevance of this power analysis for the results and interpretation is further evaluated in the Discussion section.
Results
Baseline data
Sixty-two patients were included, six women (10%) and 56 men (90%). Their median age was 42 years (range, 20–67 years). The median ferritin level was 671 μg/L (range, 283–4,179 μg/L), and the median transferrin saturation was 66% (range, 13–97 %). Thirty patients were randomised to apheresis and 32 patients to whole blood phlebotomy. Additional baseline data are shown in Table I. The percentage of C282Y homozygotes was 63% in the apheresis arm and 53% among those treated with phlebotomy (not significant; P=0.77). Age and sex distribution, ferritin levels and transferrin saturation were nearly identical in the two treatment groups (Table I).
Table I.
Baseline data.
| Total | Whole blood phlebotomy | Apheresis | Difference/significance2 | |
|---|---|---|---|---|
| Number of patients, n (%) | 62 (100) | 32 (52) | 30 (48) | n.s. |
| Age, years (SD) | 43 (11.6) | 43 (12.6) | 43 (10.6) | 0 |
| Male, N (%) | 56 (90) | 29 (911) | 26 (871) | n.s. |
| Female, N (%) | 6 (10) | 3 (91) | 3 (131) | n.s. |
| C282Y homozygous, N (%) | 36 (58) | 17 (531) | 19 (631) | n.s. (P=0.77) |
| Compound heterozygous, N (%) | 19 (31) | 10 (311) | 9 (301) | 0 |
| Haemoglobin, g/dL, mean (SD) | 15.4 (1.0) | 15.3 (1.1) | 15.4 (1.1) | 0 |
| Ferritin, μg/L, median (range) | 671 (283–4179) | 678 (283–4179) | 664 (331–2558) | n.s. |
| Transferrin saturation, %, median (range) | 66 (13–97) | 66 (25–97) | 66 (13–95) | 0 |
Percentages of the whole blood phlebotomy group and the apheresis group, respectively;
n.s.: not significant.
None of the patients presented with cardiomyopathy or liver cirrhosis. Alanine aminotransferase (ALT) levels above the reference range (10–50 U/L) were found in 29 individuals (47%); 14 patients in the apheresis group (upper range, 183 U/L) and 15 in the phlebotomy group (upper range, 140 U/L). These small differences were not statistically significant.
Normalisation of ferritin levels and transferrin saturation
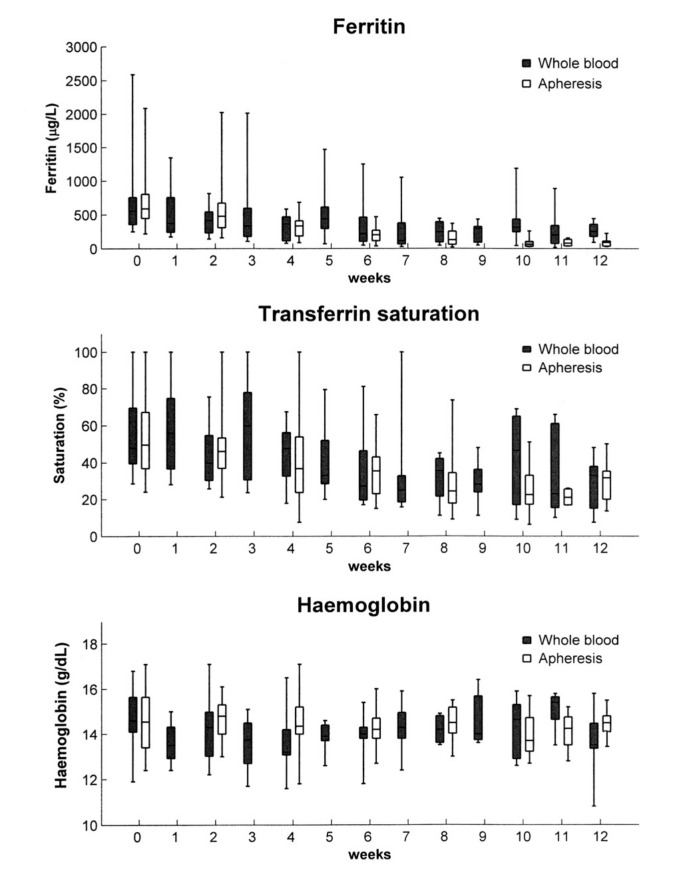
The initial decline in ferritin levels was more rapid in the apheresis group than in the whole blood phlebotomy group. The difference appeared at 6 weeks and became statistically significant at 10 weeks (P<0.01). These data are shown in Figure 1 and Table II. The median time for ferritin levels to be reduced to 50 μg/L was equal in the two groups; 96 days (range, 42–393 days) in the apheresis arm and 98 days (range, 42–396 days) in the phlebotomy arm. There was no difference in decline in transferrin saturation between the groups.
Figure 1.
Decline in ferritin levels, transferrin saturation and haemoglobin levels.
Table II.
Initial decrease in ferritin levels and transferrin saturation.
| Time (week) | Ferritin (μg/L) | Transferrin saturation (%) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Whole blood | Apheresis | Whole blood | Apheresis | |||
|
|
|
|||||
| Mean (SD) | Mean (SD) | P1 | Mean (SD) | Mean (SD) | P1 | |
| 0 | 678 (531) | 664 (368) | 0.41 | 66.4 (20.9) | 66.0 (20.1) | 0.87 |
| 2 | 429 (221) | 559 (394) | 0.30 | 42.8 (15.3) | 49.4 (20.5) | 0.37 |
| 4 | 304 (200) | 338 (160) | 0.66 | 45.1 (15.5) | 42.6 (25.7) | 0.42 |
| 6 | 321 (318) | 209 (114) | 0.64 | 34.7 (18.8) | 36.2 (14.2) | 0.59 |
| 8 | 244 (181) | 159 (112) | 0.46 | 31.8 (14.8) | 30.4 (19.0) | 0.54 |
| 10 | 426 (395) | 86.8 (72) | 0.009 | 42.1 (25.6) | 25.7 (13.0) | 0.24 |
| 12 | 260 (122) | 86.5 (60) | 0.002 | 29.1 (15.1) | 29.4 (11.7) | 0.94 |
Wilcoxon’s test
Tolerance
Figure 1 illustrates the reduction in haemoglobin levels, showing no difference between the groups. No patient in either treatment group developed iron-deficiency anaemia. Albumin and immunoglobulin G (IgG) levels did not decline during treatment. After 12 weeks, the mean albumin level was identical in the two groups at 44g/L, while the mean IgG levels were 10.5 g/L in the apheresis group and 10.2 g/L in the phlebotomy group (no significant difference). Grade 1 discomfort was recorded in 13 patients (21%); seven patients in the apheresis group and six in the phlebotomy group. Altogether, six patients withdrew or dropped out from the study; four patients in the whole blood phlebotomy arm and two patients in the apheresis arm. Three patients dropped out after inclusion because one facility withdrew from the cooperation due to organisational problems, one patient withdrew from the apheresis group because of grade 1 discomfort, and two patients dropped out for reasons related to employment or relocation.
Time consumption and costs
The mean cumulative technician time consumption until decline of ferritin levels to 50 μg/L was calculated to be 424 minutes in the apheresis group and 343 minutes in the phlebotomy group. This difference was not statistically significant (P=0.11). The cumulative costs for materials per patient until achievement of the same target ferritin level were NOK 5,186 (€ 691) in the apheresis group and NOK 1,739 (€ 232) in the phlebotomy group (P<0.001).
Discussion
In this prospective, randomised trial, the initial decrease in ferritin levels was considerably faster in the apheresis arm than in the whole blood phlebotomy arm, and the difference was statistically significant. The target ferritin level of 50 μg/L, however, was reached after the same time in both groups. Furthermore, there was no difference in decrease of transferrin saturation. As expected, the number of technical procedures per patient during the initial phase was lower in the apheresis arm.
The numbers of patients were nearly identical in the two arms. The baseline data (Table I) show that the homozygous C282Y genotype was slightly more frequent in the apheresis group. This minor difference was not statistically significant and is very unlikely to have influenced the results. For all other baseline parameters, the two groups matched perfectly.
The frequency of discomfort during the procedures was low in each group and there was no statistically significant difference. Because of the relatively low numbers, however, this study would not have been able to detect small differences in discomfort. Only one patient in the apheresis group requested discontinuation of treatment. The tendency to decreased haemoglobin levels was low and equal in the two groups (Figure 1). Both treatment modalities were, therefore, well tolerated. It may be argued that substantial amounts of plasma proteins are removed in patients treated with whole blood phlebotomy, while apheresis treatment allows much more selective removal of superfluous iron. We examined such aspects only to a limited extent. We found, however, no decline in either albumin or IgG levels and no difference in these variables between the two groups.
The cumulative material costs for apheresis until a ferritin level of 50 μg/L was achieved were three-fold higher as compared to those for phlebotomy, which was a highly significant difference. The difference in the cumulative time consumption of technicians between the two arms was not statistically significant.
During the study period, it was impossible to reach the number of patients (67 in each arm) estimated to be required for an 80% probability of detecting statistically significant differences in the primary end-points. For the following reasons, however, this turned out to be unimportant for the results and interpretation. First, some significant differences were, after all, detected. Second, the time to achievement of the target ferritin level was actually equal in the two arms and, therefore, there was no difference to be statistically tested.
More recent literature tends to propose a higher ferritin level as an indication for treatment than that used in this trial, based on previous recommendations6. Nevertheless, the baseline ferritin levels found in the study seem representative of those in patients with HH treated in clinical practice at the time of the study5,21,22. Furthermore, the finding that no study participant developed iron-deficiency anaemia following therapy shows that all patients had an overload of iron that could be mobilised. Thus, the changing criteria for therapy in clinical practice will hardly affect our conclusions.
After the data collection had been completed, Rombout-Sestrienkova and colleagues published the final results of a Dutch controlled trial in which 38 patients altogether were randomised to apheresis or whole blood phlebotomy: this study indicated the non-inferiority and, in some respects, superiority of erythrocytapheresis23. Like us, they found a more rapid decline in ferritin levels in the apheresis group than in the whole blood phlebotomy group. In their somewhat smaller cohort of patients, they found a cost difference in the opposite direction as compared with our findings23. The explanation for this difference between the two studies is probably that we did not take into account the patients’ productivity loss and travel costs, since calculation of these variables would be uncertain and the figures would not be comparable between different countries.
In conclusion, treatment of HH with erythrocytapheresis instead of whole blood phlebotomy results in a more rapid initial decrease in ferritin levels and fewer procedures per patient are required. These advantages should, however, be balanced against the findings of equal time to normalisation of ferritin levels in both groups, no difference in the reduction of transferrin saturation and equally low frequencies of discomfort. Furthermore, according to our findings, apheresis is more expensive for the hospital and, probably, more time-consuming for the technicians. It should also be borne in mind that apheresis is not necessarily available at every facility in all countries. Overall, our data do not provide evidence for a general recommendation of erythrocytapheresis instead of whole blood phlebotomy. Some findings may support apheresis in patients requiring a rapid initial reduction of ferritin, but the number of patients with very high serum ferritin level does not permit a relevant subgroup analysis.
Acknowledgements
We thank technicians at the participating hospitals. Thanks to Helge Børresen for recording the patients’ data and Tore Wentzel Larsen for statistical advice. This study was made possible by grants from Helse Vest RHF and Helse Fonna HF (public hospital trusts).
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Asberg A, Hveem K, Thorstensen K, et al. Screening for hemochromatosis: high prevalence and low morbidity in an unselected population of 65,238 persons. Scand J Gastroenterol. 2001;36:1108–15. doi: 10.1080/003655201750422747. [DOI] [PubMed] [Google Scholar]
- 2.Adams PC, Barton JC. Haemochromatosis. Lancet. 2007;370:1855–60. doi: 10.1016/S0140-6736(07)61782-6. [DOI] [PubMed] [Google Scholar]
- 3.Yen AW, Fancher TL, Bowlus CL. Revisiting hereditary hemochromatosis: current concepts and progress. Am J Med. 2006;119:391–9. doi: 10.1016/j.amjmed.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–78. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 5.Brittenham GM. Disorders of iron metabolism: Iron deficiency and iron overload. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, et al., editors. Hematology: Basic Principles and Practice. Philadelphia: Churchill Livingstone Elsevier; 2008. pp. 453–68. [Google Scholar]
- 6.Adams PC, Barton JC. How I treat hemochromatosis. Blood. 2010;116:317–25. doi: 10.1182/blood-2010-01-261875. [DOI] [PubMed] [Google Scholar]
- 7.Asberg A, Hveem K, Kruger O, et al. Persons with screening-detected haemochromatosis: as healthy as the general population? Scand J Gastroenterol. 2002;37:719–24. doi: 10.1080/00365520212510. [DOI] [PubMed] [Google Scholar]
- 8.Ulvik RJ. Screening for haemochromatosis. Scand J Clin Lab Invest Suppl. 1990;202:90–3. [PubMed] [Google Scholar]
- 9.Adams PC. Haemochromatosis: find them or forget about them? Eur J Gastroenterol Hepatol. 2004;16:857–8. doi: 10.1097/00042737-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Zoller WG, Kellner H, Spengel FA. Erythrocytapheresis. A method for rapid extracorporeal elimination of erythrocytes. Results in 65 patients. Klin Wochenschr. 1988;66:404–9. doi: 10.1007/BF01737944. [DOI] [PubMed] [Google Scholar]
- 11.Muncunill J, Vaquer P, Galmes A, et al. In hereditary hemochromatosis, red cell apheresis removes excess iron twice as fast as manual whole blood phlebotomy. J Clin Apheresis. 2002;17:88–92. doi: 10.1002/jca.10024. [DOI] [PubMed] [Google Scholar]
- 12.Hicken BL, Tucker DC, Barton JC. Patient compliance with phlebotomy therapy for iron overload associated with hemochromatosis. Am J Gastroenterol. 2003;98:2072–7. doi: 10.1111/j.1572-0241.2003.07292.x. [DOI] [PubMed] [Google Scholar]
- 13.Conte D, Mandelli C, Cesana M, et al. Effectiveness of erythrocytapheresis in idiopathic hemochromatosis. Report of 14 cases. Int J Artif Organs. 1989;12:59–62. [PubMed] [Google Scholar]
- 14.Kellner H, Zoller WG. Repeated isovolemic large-volume erythrocytapheresis in the treatment of idiopathic hemochromatosis. Z Gastroenterol. 1992;30:779–83. [PubMed] [Google Scholar]
- 15.Kohan A, Niborski R, Daruich J, et al. Erythrocytapheresis with recombinant human erythropoietin in hereditary hemochromatosis therapy: a new alternative. Vox Sang. 2000;79:40–5. doi: 10.1159/000031204. [DOI] [PubMed] [Google Scholar]
- 16.Mariani R, Pelucchi S, Perseghin P, et al. Erythrocytapheresis plus erythropoietin: an alternative therapy for selected patients with hemochromatosis and severe organ damage. Haematologica. 2005;90:717–8. [PubMed] [Google Scholar]
- 17.Rombout-Sestrienkova E, van Noord PA, van Deursen CT, et al. Therapeutic erythrocytapheresis versus phlebotomy in the initial treatment of hereditary hemochromatosis - a pilot study. Transfus Apher Sci. 2007;36:261–7. doi: 10.1016/j.transci.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Knutsen H, Hammerstrom J. Norwegian national guidelines for treatment of haemochromatosis. [In Norwegian] Norwegian Society of Haematology. 2003. [Accessed on 9 June 2013]. Available at: http://legeforeningen.no/PageFiles/5783/Handlingsprogram%20for%20Hemokromatose.pdf.
- 19.Aguilar-Martinez P, Grandchamp B, Cunat S, et al. Iron overload in HFE C282Y heterozygotes at first genetic testing: a strategy for identifying rare HFE variants. Haematologica. 2011;96:507–14. doi: 10.3324/haematol.2010.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman DG. Comparing groups - continuous data. In: Altman DG, editor. Practical Statistics for Medical Research. Washington DC: Chapman & Hall/CRC; 1999. pp. 179–228. [Google Scholar]
- 21.Brissot P, De Bels F. Management of hemochromatosis linked to HFE gene. [In French; English abstract] Presse Med. 2007;36:1295–300. doi: 10.1016/j.lpm.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Hagve TA, Asberg A, Ulvik R, et al. Hemochromatosis--from an underdiagnosed curiosity to a common disease. [In Norwegian; English abstract] Tidsskr Nor Laegeforen. 2009;129:863–6. doi: 10.4045/tidsskr.08.0084. [DOI] [PubMed] [Google Scholar]
- 23.Rombout-Sestrienkova E, Nieman FH, Essers BA, et al. Erythrocytapheresis versus phlebotomy in the initial treatment of HFE hemochromatosis patients: results from a randomized trial. Transfusion. 2012;52:470–7. doi: 10.1111/j.1537-2995.2011.03292.x. [DOI] [PubMed] [Google Scholar]