Abstract
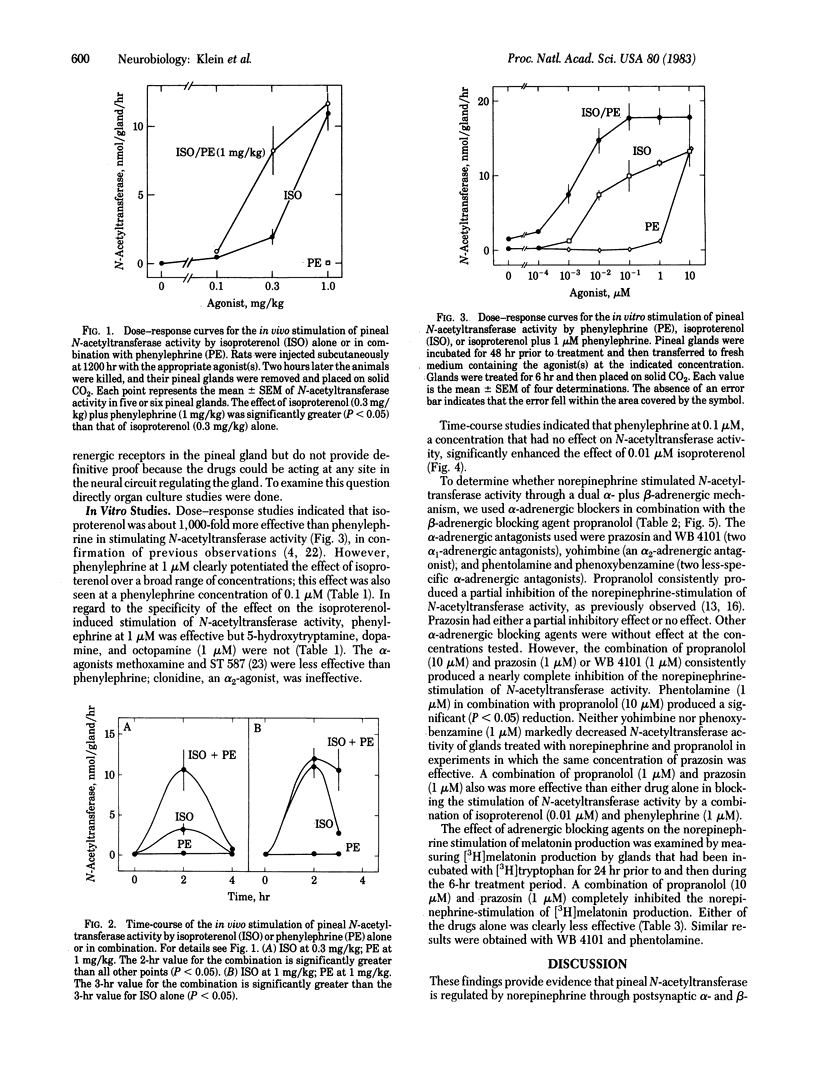
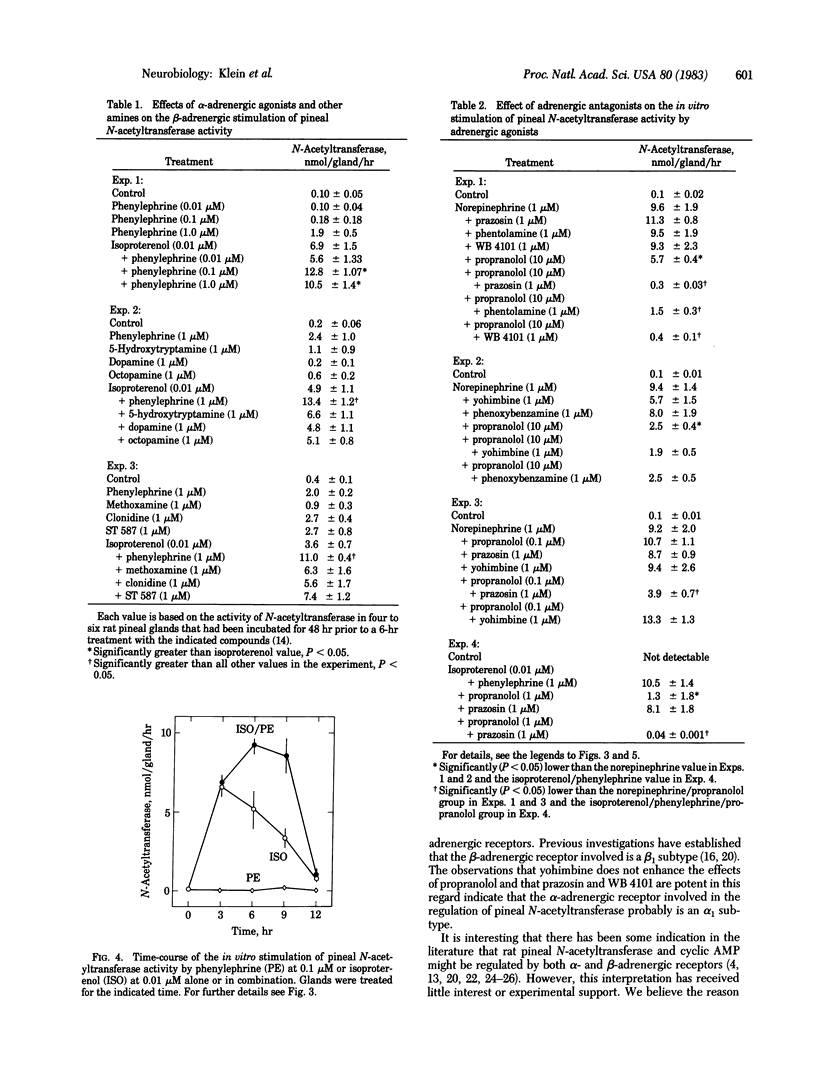
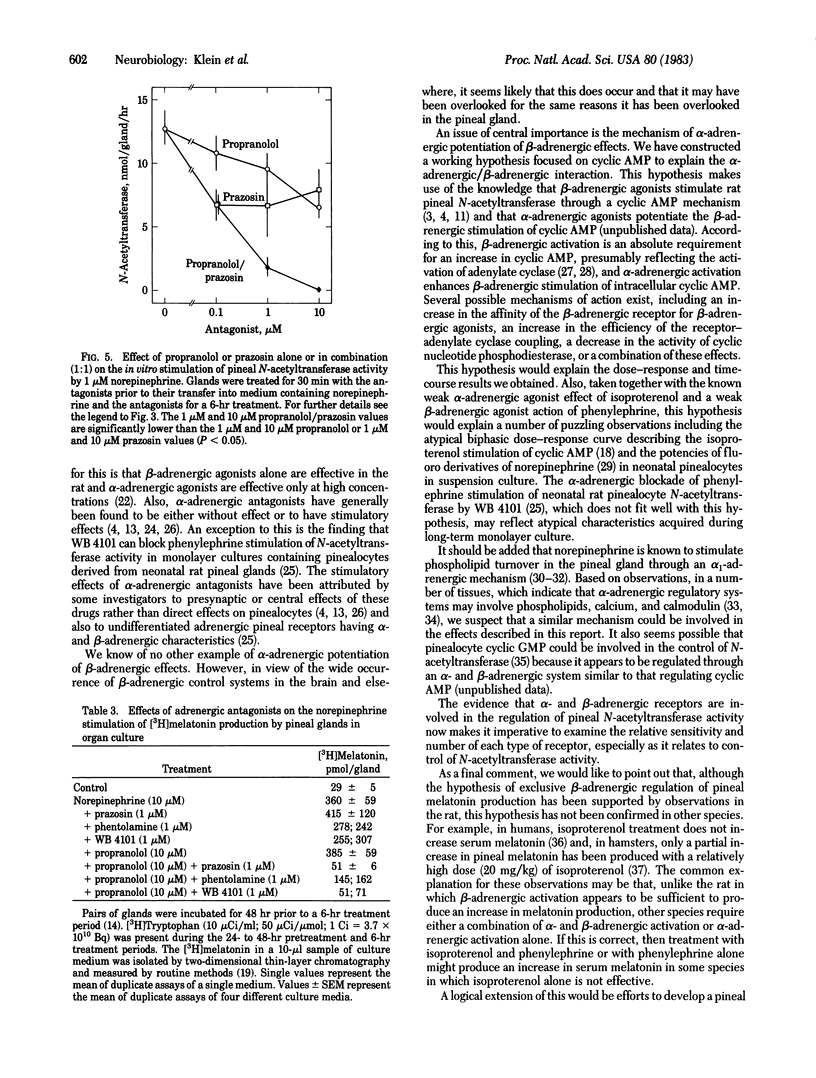
The role played by postsynaptic alpha-adrenergic receptors in the stimulation of pineal N-acetyltransferase (EC 2.3.1.5) and [3H]melatonin production was investigated in the rat. In vivo studies indicated that phenylephrine, an alpha-adrenergic agonist, potentiated and prolonged the effects of isoproterenol, a beta-adrenergic agonist. Similar observations were made in organ culture with glands devoid of functional nerve endings. In addition, a combination of 1 microM prazosin, an alpha 1-adrenergic blocking agent, and 1 microM propranolol, a beta-adrenergic blocking agent, was many times more potent then either agent alone in blocking the stimulatory effects of norepinephrine on N-acetyltransferase activity and [3H]melatonin production. These findings establish that norepinephrine acting through alpha- and beta-adrenergic receptors stimulates rat pineal N-acetyltransferase activity and, as a result, the production of melatonin. Apparently, beta-adrenergic activation is an absolute requirement, and an alpha-adrenergic receptor mechanism potentiates beta-adrenergic activation. These findings are significant because they demonstrate alpha-adrenergic potentiation of beta-adrenergic effects. In addition, they indicate that the widely held belief that melatonin production is regulated exclusively by a postsynaptic beta-adrenergic mechanism must be revised.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphs L., Heller A., Lovenberg W. Adrenergic regulation of the reduction in acetyl coenzyme A:arylamine N-acetyltransferase activity in the rat pineal. J Neurochem. 1980 Jan;34(1):83–90. doi: 10.1111/j.1471-4159.1980.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Auerbach D. A., Klein D. C., Kirk K. L., Cantacuzene D., Creveling C. R. Effects on fluorine analogs of norepinephrine on stimulation of cyclic adenosine 3',5'-monophosphate and binding to beta-adrenergic receptors in intact pinealocytes. Biochem Pharmacol. 1981 May 15;30(10):1085–1089. doi: 10.1016/0006-2952(81)90446-9. [DOI] [PubMed] [Google Scholar]
- Auerbach D. A., Klein D. C., Woodard C., Aurbach G. D. Neonatal rat pinealocytes: typical and atypical characteristics of [125I]iodohydroxybenzylpindolol binding and adenosine 3',5'-monophosphate accumulation. Endocrinology. 1981 Feb;108(2):559–567. doi: 10.1210/endo-108-2-559. [DOI] [PubMed] [Google Scholar]
- Berg G. R., Klein D. C. Norepinephrine increases the (32P)labelling of a specific phospholipid frac tion of post-synaptic pineal membranes. J Neurochem. 1972 Nov;19(11):2519–2532. doi: 10.1111/j.1471-4159.1972.tb01311.x. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Saavedra J. M., Axelrod J. Control of pineal N-acetylserotonin by a beta adrenergic receptor. Mol Pharmacol. 1973 Sep;9(5):605–611. [PubMed] [Google Scholar]
- Buda M., Klein D. C. A suspension culture of pinealocytes: regulation of N-acetyltransferase activity. Endocrinology. 1978 Oct;103(4):1483–1493. doi: 10.1210/endo-103-4-1483. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin. Sci Am. 1982 Jun;246(6):62–70. doi: 10.1038/scientificamerican0682-62. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Control of circadian change of serotonin N-acetyltransferase activity in the pineal organ by the beta--adrenergic receptor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2547–2550. doi: 10.1073/pnas.69.9.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Induction and superinduction of serotonin N-acetyltransferase by adrenergic drugs and denervation in rat pineal organ. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2208–2211. doi: 10.1073/pnas.69.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Superinduction of serotonin N-acetyltransferase and supersensitivity of adenyl cyclase to catecholamines in denervated pineal gland. Mol Pharmacol. 1973 Sep;9(5):612–618. [PubMed] [Google Scholar]
- Deguchi T. Role of the beta adrenergic receptor in the elevation of adenosine cyclic 3',5'-monophosphate and induction of serotonin N-acetyltransferase in rat pineal glands. Mol Pharmacol. 1973 Mar;9(2):184–190. [PubMed] [Google Scholar]
- Fain J. N., García-Sáinz J. A. Role of phosphatidylinositol turnover in alpha 1 and of adenylate cyclase inhibition in alpha 2 effects of catecholamines. Life Sci. 1980 Apr 14;26(15):1183–1194. doi: 10.1016/0024-3205(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Jonge A. D., van Meel J. C., Timmermans P. B., van Zwieten P. A. A lipophilic, selective alpha1 -adrenoceptor agonist: 2-(2-chloro-5-trifluoromethylphenylimino)imida-zolidine (St 587). Life Sci. 1981 May 4;28(18):2009–2016. doi: 10.1016/0024-3205(81)90648-2. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Auerbach D. A., Weller J. L. Seesaw signal processing in pineal cells: homologous sensitization of adrenergic stimulation of cyclic GMP accompanies homologous desensitization of beta-adrenergic stimulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4625–4629. doi: 10.1073/pnas.78.7.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. C., Berg G. R. Pineal gland: stimulation of melatonin production by norepinephrine involves cyclic AMP-mediated stimulation of N-acetyltransferase. Adv Biochem Psychopharmacol. 1970;3:241–263. [PubMed] [Google Scholar]
- Klein D. C., Berg G. R., Weller J. Melatonin synthesis: adenosine 3',5'-monophosphate and norepinephrine stimulate N-acetyltransferase. Science. 1970 May 22;168(3934):979–980. doi: 10.1126/science.168.3934.979. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Moore R. Y. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979 Oct 5;174(2):245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Ntides A. Thin-layer chromatographic separation of pineal gland derivatives of serotonin-14C. Anal Biochem. 1969 Oct 1;31(1):480–483. doi: 10.1016/0003-2697(69)90290-5. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science. 1970 Sep 11;169(3950):1093–1095. doi: 10.1126/science.169.3950.1093. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Weller J. L., Moore R. Y. Melatonin metabolism: neural regulation of pineal serotonin: acetyl coenzyme A N-acetyltransferase activity. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3107–3110. doi: 10.1073/pnas.68.12.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D., Weller J. L. Adrenergic-adenosine 3',5'-monophosphate regulation of serotonin N-acetyltransferase activity and the temporal relationship of serotonin N-acetyltransferase activity synthesis of 3H-N-acetylserotonin and 3H-melatonin in the cultured rat pineal gland. J Pharmacol Exp Ther. 1973 Sep;186(3):516–527. [PubMed] [Google Scholar]
- Moore R. Y., Klein D. C. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974 May 10;71(1):17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- Parfitt A., Weller J. L., Klein D. C. Beta adrenergic-blockers decrease adrenergically stimulated N-acetyltransferase activity in pineal glands in organ culture. Neuropharmacology. 1976 Jun;15(6):353–358. doi: 10.1016/0028-3908(76)90083-6. [DOI] [PubMed] [Google Scholar]
- Parfitt A., Weller J. L., Klein D. C., Sakai K. K., Marks B. H. Blockade by ouabain or elevated potassium ion concentration of the adrenergic and adenosine cyclic 3',5'-monophosphate-induced stimulation of pineal serotonin N-acetyltransferase activity. Mol Pharmacol. 1975 May;11(3):241–255. [PubMed] [Google Scholar]
- Rowe V., Parr J. Developmental changes in the stimulation of serotonin N-acetyltransferase activity and melatonin synthesis in the rat pineal in monolayer culture. J Pharmacol Exp Ther. 1981 Jul;218(1):97–102. [PubMed] [Google Scholar]
- Shein H. M., Wurtman R. J. Cyclic adenosine monophosphate: stimulation of melatonin and serotonin synthesis in cultured rat pineals. Science. 1969 Oct 24;166(3904):519–520. doi: 10.1126/science.166.3904.519. [DOI] [PubMed] [Google Scholar]
- Smith T. L., Eichberg J., Hauser G. Postsynaptic localization of the alpha receptor-mediated stimulation of phosphatidylinositol turnover in pineal gland. Life Sci. 1979 Jun 4;24(23):2179–2184. doi: 10.1016/0024-3205(79)90116-4. [DOI] [PubMed] [Google Scholar]
- Strada S. J., Klein D. C., Weller J., Weiss B. Effect of norepinephrine on the concentration of adenosine 3',5'-monophosphate of rat pineal gland in organ culture. Endocrinology. 1972 Jun;90(6):1470–1475. doi: 10.1210/endo-90-6-1470. [DOI] [PubMed] [Google Scholar]
- Tamarkin L., Reppert S. M., Klein D. C. Regulation of pineal melatonin in the Syrian hamster. Endocrinology. 1979 Feb;104(2):385–389. doi: 10.1210/endo-104-2-385. [DOI] [PubMed] [Google Scholar]
- Vaughan G. M., Pelham R. W., Pang S. F., Loughlin L. L., Wilson K. M., Sandock K. L., Vaughan M. K., Koslow S. H., Reiter R. J. Nocturnal elevation of plasma melatonin and urinary 5-hydroxyindoleacetic acid in young men: attempts at modification by brief changes in environmental lighting and sleep and by autonomic drugs. J Clin Endocrinol Metab. 1976 Apr;42(4):752–764. doi: 10.1210/jcem-42-4-752. [DOI] [PubMed] [Google Scholar]
- Zatz M., Kebabian J. W., Romero J. A., Lefkowitz R. J., Axelrod J. Pineal beta adrenergic receptor: correlation of binding of 3H-l-alprenolol with stimulation of adenylate cyclase. J Pharmacol Exp Ther. 1976 Mar;196(3):714–722. [PubMed] [Google Scholar]


