Abstract
Background
Cord blood serum (CBS)-based eye drops are successfully used in corneal epithelial wound healing and are prepared to supply a known amount of epidermal growth factor (EGF). Product standardisation includes expensive EGF dosage in all cord blood (CB) units. The influence of donor obstetric and haematological characteristics on EGF content was evaluated, to exclude unsuitable CBS and pre-select those CB units able to provide the correct EGF supply for healing corneal wounds.
Materials and methods
Data were retrospectively collected from 135 donors included in the Emilia Romagna Cord Blood Bank records. Obstetric characteristics, parity and gestational age of the mother, sex, birth weight and Apgar score of the neonate, placental weight, duration of labour and mode of delivery were considered. Haematological characteristics, CD34+ cell number, and total nucleated cell, white blood cell and platelet counts were recorded. EGF content in CB units was estimated by enzyme-linked immunosorbent assay. Statistical evaluation was performed by Mann-Whitney unpaired and Student’s t tests. Correlations between variables were evaluated by using Pearson’s (r) or Spearman’s (ρ) correlation coefficients.
Results
EGF content was significantly higher in CBS from donors aged <30 years and after vaginal deliveries as compared with scheduled Caesarean sections (1,386±580 vs 1,106±391 pg/mL; P=0.002). EGF content was significantly correlated with duration of labour (r=0.45; P=0.0001), number of CD34+ cells/mL (r=0.3; P=0.002) particularly in vaginal deliveries (r=0.36; P=0.003), mother’s age (−0.25; P=0.005), neonate’s birth weight (r=0.27; P=0.005), and total nucleated cell (r=0.25; P=0.006), white cell (r=0.29; P=0.001) and platelet (r=0.24; P=0.009) counts. No significant correlations were found between EGF content and parity, gestational age, placental weight, neonate’s sex or Apgar scores.
Discussion
EGF levels are higher in CB units from younger mothers (<30 years), with longer labour duration (>6 hours), and higher CD34+ cell content (>0.05×106/mL). In order to optimise the preparation and costs of CBS–based eye drops, pre-selection of CB units is recommended.
Keywords: cord blood serum, eye drops, epidermal growth factor, CD34+ cells, child delivery
Introduction
Cord blood (CB) is now a recognised and accepted source of haematopoietic stem cells for the treatment of malignant and non-malignant haematological and immunological diseases and a significant increase in its use was recorded over the last decade1. The advantages of CB, as compared with bone marrow or mobilised peripheral blood progenitor cells, include ease of collection, rapid availability, low risk of transmission of infections, absence of donor risk and the relatively lower risk of graft-vs-host disease with preserved graft-vs-malignancy effects2. However, the relatively small volume and low number of haematopoietic stem cells (HSC) present in a single CB unit have limited the use of CB transplants in adults, unless an intra-bone protocol or double cord transplant is carried out3.
Several studies have been performed in animal models suggesting that CB can repair tissues other than blood, in diseases ranging from heart attacks to strokes4,5 but these findings are controversial due to poor repeatability of experiments and it is also not clear how CB may produce such effects.
The pluripotent nature of CB as a biological tear substitute was evidenced in the healing of severe epithelial corneal defects. Several authors have demonstrated the safety and efficacy of CB serum (CBS), topically applied as eye drops, in the treatment of severe dry eye with or without Sjogren’s syndrome6,7, ocular graft-vs-host disease8, persistent corneal epithelial defects9, recurrent corneal erosions10, chemical burns11 and neurotrophic keratitis12. The rationale for the use of CBS eye drops is their content of biologically active components and in particular of growth factors essential in corneal homeostasis and wound healing, by regulating cellular proliferation, differentiation and migration. Epidermal growth factor (EGF), in particular, plays a central role in corneal wound healing13. In vitro experiments have shown the corneal epitheliotropic capacity of different blood-derived preparations14 and in a previous study7 we demonstrated the healing efficacy of CBS eyedrops prepared according to a standardised protocol focused on obtaining a daily supply of 0.15 ng/mL EGF, similar to the physiological human tear content. A certain variability in EGF levels in CBS may be present7 and a preliminary dosage of EGF content in CB units from all donors is performed to pursue the aim of a controlled amount of EGF in the final eye drops. Assaying EGF levels is a time-consuming and expensive procedure and a method for pre-selecting CB units with possibly higher EGF levels, before dosage, is desirable.
Obstetric factors, including gestational age, parity of the mother, sex and birth weight of the neonate, weight of the placenta, duration of labour, and the mode of delivery are known to influence the stem cell content of CB15,16. It has also been shown that haematological characteristics such as the number of CD34+ stem cells is increased in CB units from Caesarean sections compared to vaginal deliveries17,18.
The purpose of the present study was to analyse the influence of obstetric and haematological characteristics on the variability of EGF concentration in CB units. This information could aid successful pre-selection of CB units containing higher levels of EGF and, thereby, reduce the number of CB units that need to assayed.
Materials and methods
Data analysis
This is a retrospective study with data from CBS samples obtained from the Emilia Romagna Cord Blood Bank records between 2010 and 2012. Data were collected from 135 donors (median age: 33.1±4.7 years; range, 18 to 42 years).
The following obstetric factors were considered: parity and gestational age of the mother, sex, birth weight and Apgar score of the neonate, placental weight, duration of labour and the mode of delivery (physiological childbirth with vaginal delivery or primary planned Caesarean delivery prior to labour initiation).
Collection method
All CB units were collected after a donor selection questionnaire based on international criteria for CB banking. All steps from the recruitment to the processing and registration of CB were performed according to standard operating procedures and guidelines edited by the Foundation for the Accreditation of Hematopoietic Cellular Therapy (FACT). CB was collected when the placenta was still in utero and the umbilical vein was punctured with a sterile system. The blood was collected under gravitational force into a bag containing 20 mL citrate-phosphate-dextrose (CPD) (Fresenius-Kabi AG, Bad Homburg, Germany). Moreover blood samples were collected from ex utero placental vessels with a sterile syringe and transferred into Vacutest tubes (Kima srl, Padua, Italy) without any anticoagulant. CB units and the related samples were sent for further steps to the processing facility laboratory.
Assessment of cord blood units
The CB units were processed within 48 hours according to CB bank procedures. The presence of markers of maternal infections was determined.
Serum samples
Blood from the ex utero placenta was centrifuged at 3,380 g for 10 minutes and the serum samples obtained were transferred into sterile tubes under a laminar flow hood and stored at −80 °C.
Haematological characteristics
The numbers of total nucleated cells before (TNCb) and after volume reduction (TNCa) were counted with an autoanalyser (Act5/5dif, Beckman Coulter, Cassina de’ Pecchi, Milan, Italy). Erythroblasts were not excluded from the count as it had been estimated that they accounted for less than 3% of the total cells. The number of CD34+ cells was measured by flow cytometry with a single-platform technique (Stem Kit, Beckman Coulter Immunotech, Marseille, France).
Epidermal growth factor dosage
The concentration of EGF in CB units was determined using a Quantikine Human EGF Immunoassay kit (R&D Systems, Inc. Minneapolis, MN, USA). The procedure was carried out according to the manufacturer’s instructions (website: http://www.rndsystems.com/pdf/deg00.pdf) and the absorbance was read at 450 nm using a spectrophotometer (BioRad 680, Hercules, CA, USA).
Statistical analysis
The patients’ characteristics and haematopoietic variables are expressed as mean and standard deviation (SD). Categorical variables (such as sex and parity) were tested by a chi-square test, and continuous variables (such as haematopoietic variables) by the t test. A P value of less than 0.05 was considered to be statistically significant. The statistical analysis was performed with SPSS software, version 14.0 (SPSS Inc., Chicago, IL, USA) and MedCalc 5.0 (MedCalc Software, Ostend, Belgium). Descriptive statistics for tests and variables analysed in subjects are reported as the mean+ SD. Pearson’s (r) or Spearman’s (ρ) correlation coefficients were applied when appropriate; correlations were considered statistically significant for P values <0.05.
Results
The 135 CB units were divided into two groups on the basis of type of delivery: 100 CBS units were derived from physiological vaginal deliveries (group 1) while 35 were derived from planned Caesarean sections (group 2). Maternal (donor) and neonatal characteristics for each group are summarised in Table I. The distribution of mothers’ age, gestational age, child parity and neonatal sex was comparable between the two groups. Similarly, no significant differences were found in neonatal weight and Apgar scores I and II between the two groups.
Table I.
Characteristics of the donors (mothers) and neonates.
| Characteristics | Group 1: PC | Group 2: CS | P value | |
|---|---|---|---|---|
|
|
||||
| Donor | Number | 100 | 35 | not done |
|
| ||||
| Maternal age | 32.7±4.6 [18–41] | 34.6±4.6 [26–42] | 0.04 | |
|
| ||||
| Gestational age (days) | 280±7 | 276±8 | 0.003 | |
|
| ||||
| Labour duration (h) | 7.62±5.26 | 0 | not applicable | |
|
| ||||
| Parity * | ||||
| P1 | 72 | 22 | not done | |
| P>1 | 28 | 13 | ||
|
| ||||
| Newborn | Sex | |||
| Female | 46 | 18 | not done | |
| Male | 54 | 17 | ||
|
| ||||
| Weight (g) | 3,599±416 | 3,556±370 | ||
| Female | 3,623±412 | 3,550±315 | not significant | |
| Male | 3,574±427 | 3,561±422 | ||
|
| ||||
| Apgar | ||||
| Apgar I | 9.13±0.71 [9 (5–10)] | 8.97±0.89 [9 (6–10)] | not significant | |
| Apgar II | 9.85±0.42 [10 (8–10)] | 9.67±0.58 [10 (8–10)] | ||
Demographic characteristics of the study population (total subjects =135), divided according to the two methods of delivery: group 1: PC = physiological childbirth, vaginal delivery; and group 2: CS = Caesarean section.
P1 tot = 94 (nulliparous); P>1 tot =41 (primiparous and multiparous).
Haematological variables in the CB were analysed within the 48 hours following collection of the blood. No statistically significant difference was found in the volume of CB collected after the two ways of delivery, nor was the TNC count different between the two groups. Higher numbers of CD34+ cells, TNCb and platelets were found in CB derived from physiological deliveries than from deliveries by Caesarean section, with the difference being statistically significant (P<0.05).
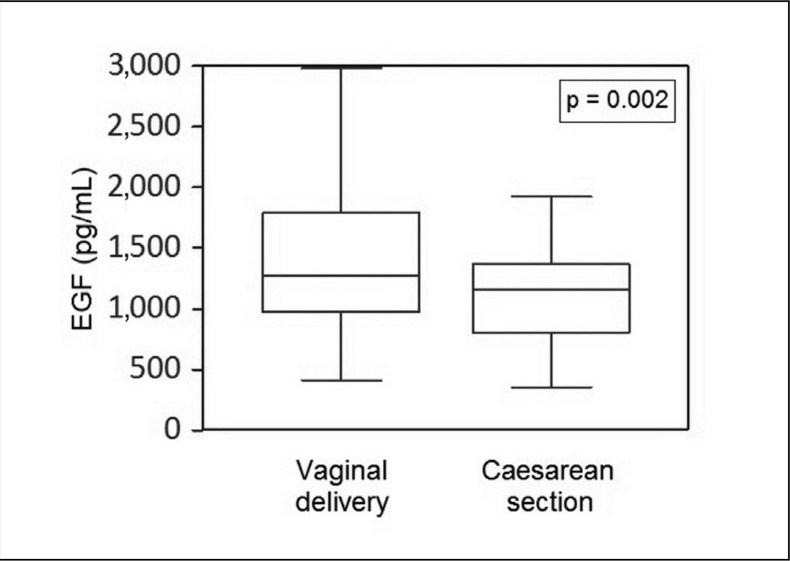
EGF content was statistically significant different between the two groups, with the higher content found in CB derived from physiological delivery (Table II). A certain variability in EGF content was found among donors (Figure 1), with the range being 200–2,000 pg/mL in CB from Caesarean section deliveries and 300–3,000 pg/mL in CB from physiological deliveries.
Table II.
Maternal and neonatal haematological variables (means ± SD).
| Variable | Group 1: PC | Group 2: CS | P value |
|---|---|---|---|
| Volume/CB unit* | 108.7±37.76 | 112.07±30.56 | NS |
| TNCb (×103/μL) | 15.73±3.97 | 13.75±3.63 | 0.01 |
| TNCa (×106)/CB unit | 1,742±516 | 1,598±360 | NS |
| TNCa (×106)/mL | 21.72±14.6 | 16.98±11.88 | NS |
| CD34+ (×106)/CB unit | 4.94±3.3 | 3.96±1.47 | 0.02 |
| CD34+ (×106)/mL | 0.06±0.05 | 0.05±0.03 | 0.03 |
| Platelets (×103/μL) | 267.45±58 | 254.2±52.61 | NS |
| EGF (pg/mL) | 1,386±580 | 1,106±391 | 0.002 |
Haematological parameters of the study population divided according to the two methods of delivery: group 1: PC: physiological childbirth, vaginal delivery; and group 2: CS: Caesarean section; CB: cord blood; TNCb: total nucleated cells before volume reduction; TNCa: total nucleated cells after volume reduction; EGF: epidermal growth factor;
excluding CPD anticoagulant; NS: not significant.
Figure 1.
EGF concentration in CBS obtained after different modes of delivery.
A great variability in EGF content was found among the samples obtained after both vaginal delivery and Caesarean section. A statistically significant difference was demonstrated in EGF content between the two groups, with the higher content found in CBS from vaginal deliveries.
Significant differences (Student’s t test) and correlations (Pearson’s r and Spearman’s ρ) were evaluated between EGF content and patients’ characteristics and between EGF content and haematological parameters.
There was a significant inverse correlation between EGF levels and maternal age (r=−0.25, P=0.005), with a positive predictive value of 91 for age <30 years and of 100 for age <26 years as thresholds for selecting a CB unit with an EGF content higher than 1,000 pg/mL.
A significant inverse correlation was also found between maternal age and both CD34+ cell content/mL (r=−0.36, P<0.0001) and TNC/mL (r=−0.31; P=0.0004).
No correlation was found between EGF content and either gestational age or placental weight.
No differences were found between the groups regarding the neonates’ sex (males vs females), Apgar scores (Apagar I vs Apagar II) and parity (primiparae vs pluriparae).
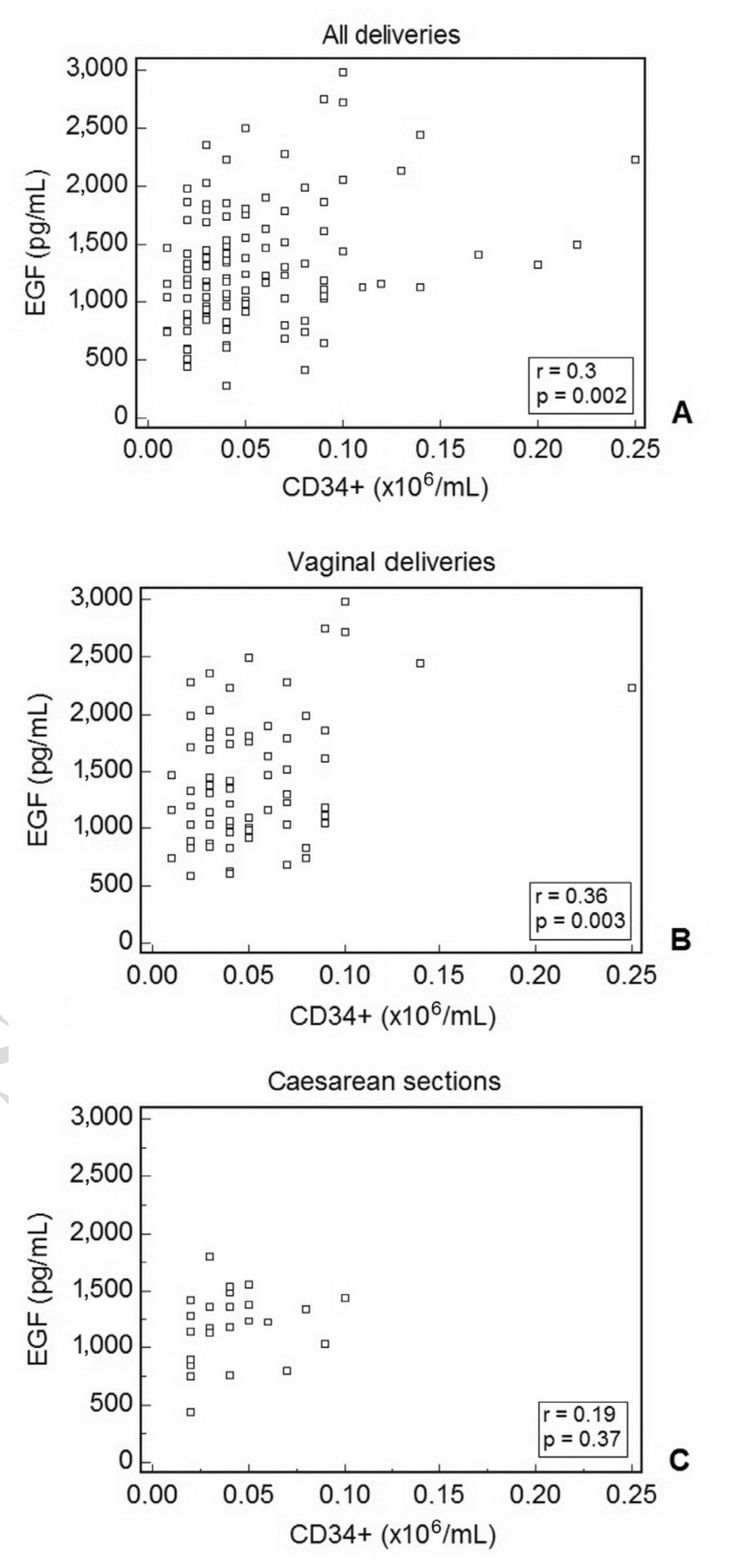
A direct correlation between EGF levels and CD34+ cells/mL was found when analysing all the CB units included in the study (Figure 2A). A threshold CD34+ cell count of >0.05×106/mL was used in order to identify CB units with an EGF content higher than 1,000 pg/mL (positive predictive value=80). When the data were split into the two groups, the strength of correlation increased in the physiological childbirth group (Figure 2B) while the correlation in the group of CB units from Caesarean section delivery was not statistically significant (Figure 2C).
Figure 2.
Correlation between EGF and CD34+ cell content in CBS samples.
A statistically significant correlation was found between the two parameters when all the samples were analysed together (A). When analysing the correlation in samples divided according the method of delivery of the neonate, significance increased in the vaginal delivery group (B) but decreased in the Caesarean section group (C).
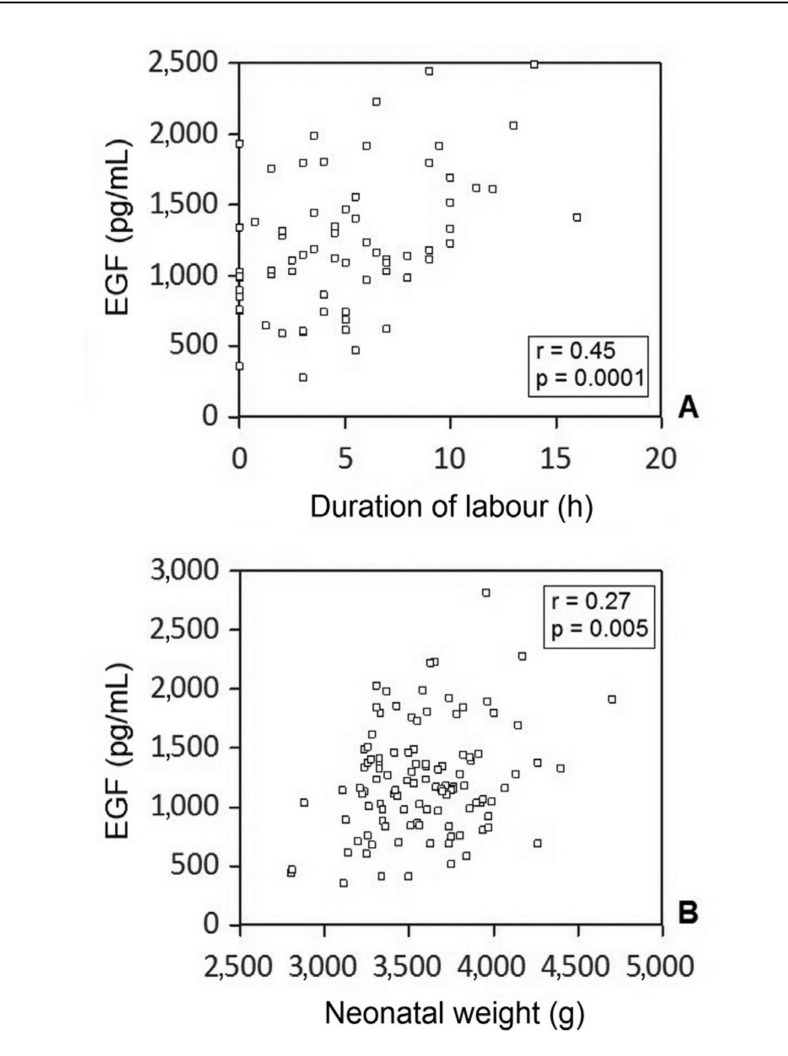
EGF content was strongly correlated with the duration of labour in the physiological vaginal delivery group (Figure 3A) as well as with neonatal weight (Figure 3B).
Figure 3.
Correlation between EGF content in CBS and duration of labour in hours (A) and between EGF content in CBS and neonatal weight in grams (B).
A strong, statistically significant correlation was found in both cases.
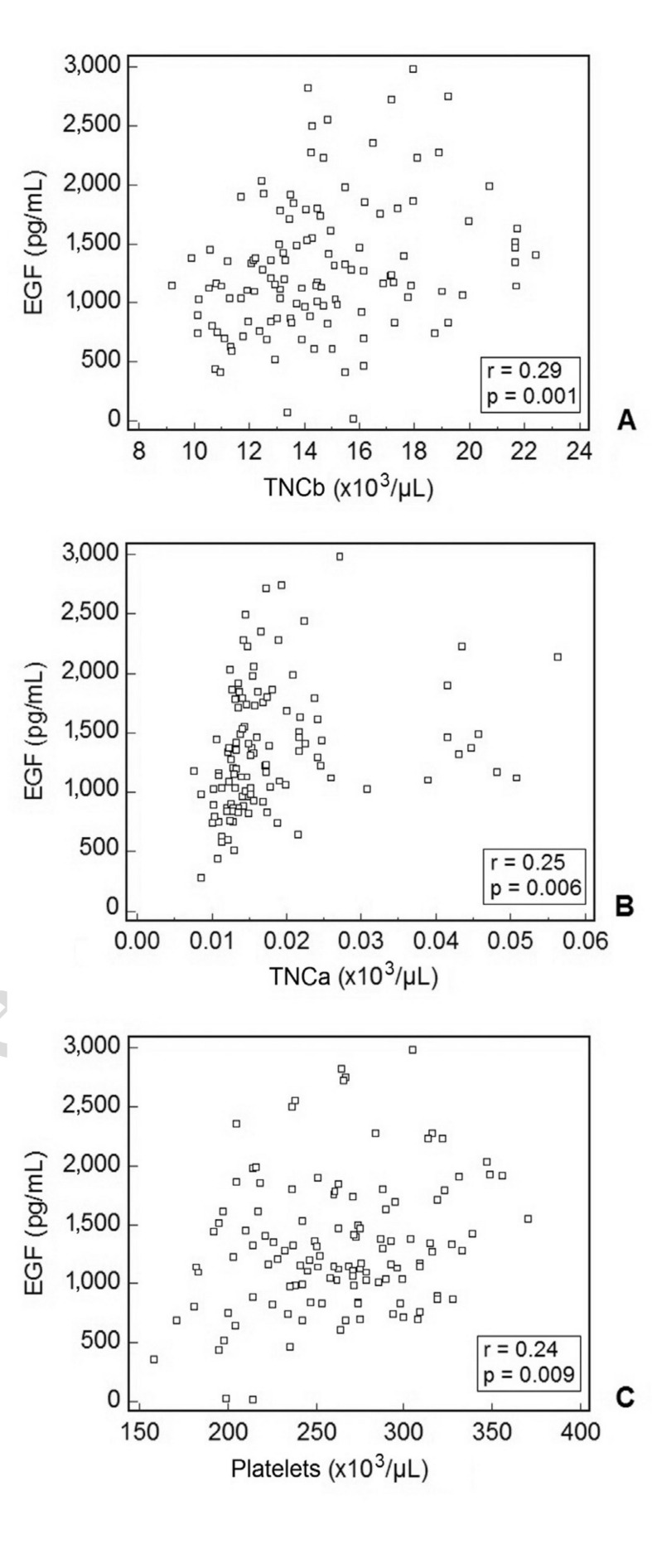
EGF content was also significantly correlated with some haematological parameters such as TNCb/μL (r=0.29, Figure 4A), TNCa/μL (r=0.25, Figure 4B) and platelets/μL (r=0.24, Figure 4C).
Figure 4.
Correlation between EGF content in CBS and some haematological parameters: total nucleated cells before volume reduction/μL CB sample (TNCb) (A), total nucleated cells after volume reduction/μL CB sample (TNCa) (B), platelets/mL CB sample (C).
A statistically significant correlation was found in all cases.
Discussion
Blood derivatives are being currently used as therapeutic agents for several ocular surface diseases and are generally prepared upon request from peripheral blood samples from each single patient. Heterologous blood sources have been recently proposed, with the advantage that they can be routinely obtained as quality/microbiologically-controlled products from blood banks, where a supply may be kept and stored in advance to be readily dispensed.
In a previous study7 we demonstrated the healing efficacy of CBS-based eye drops obtained from pooled CB units assayed for EGF content, a biologically variable parameter that has to be estimated during the standardised preparation process.
The role of mode of delivery and the effect of foetal distress on the levels of various growth factors and cytokines in CB have been investigated in the past, to understand whether these factors may have an impact on developmental and functional changes in the neonate. Neural growth factor level was found to be significantly lower in preterm infants than in term ones19 and it was postulated that this growth factor has a role in brain development in later life20. In fact, reduced levels of neural growth factor in cord blood may affect foetal growth in preterm deliveries and have implications for neurodevelopmental disorders21. Transforming growth factor (TGF)-α and TGF-β1 contribute substantially to normal pregnancy outcomes; higher cord blood TGF-β1 concentrations could be due to increased shear stress in vaginal deliveries22, but further studies are needed to evaluate the immunomodulatory role of the labour-associated increase in TGF-β1 in CB23.
EGF is detectable in the CB at 23 weeks gestation and its levels rise gradually with increasing gestational age, suggesting that EGF may play a role in foetal growth24,25.
In the present study a strong relationship was demonstrated between EGF levels and neonatal weight, further suggesting the role of EGF in neonatal development. EGF levels were also strongly related to haematopoietic CD34+ stem cell content and TNC count, and these relationships were statistically significant in CB derived from vaginal deliveries.
We also found that vaginal delivery and labour duration strongly influence EGF content in CB, in agreement with what was reported for haematopoietic CD34+ stem cell content26. Our data demonstrated that labour-related foetal distress increases the levels of EGF in CB, in particular when labour lasted longer than 6 hours.
Maternal age also influenced EGF levels: CB from mothers aged less than 26 years contained the highest EGF levels, in agreement with previous findings27 on the relationship between maternal age and stem cell content.
This study was mainly focused on a search for obstetric and haematological parameters related to both the mother and child that could have an impact on EGF levels in CB, with the goal of pre-selecting CB units appropriate for the preparation of CBS-based eye drops prior to laboratory dosage of EGF. A proper supply of EGF is fundamental for corneal epithelial cell growth in wound healing as shown in in vitro experiments: EGF enhanced corneal epithelial cell growth in a dose-dependent manner at concentrations from 0.1 to 5 ng/mL; conversely increasing concentrations of EGF from 5 ng/mL to 10 and 100 ng/mL resulted in the down-regulation of clonal growth28.
Following the discovery of this dose-dependent response in vitro, in a previous study by our group7 we standardised a CBS-based eye drop product containing a supply of 0.15 ng/mL EGF per day, to be administered topically for the treatment of severe permanent corneal epithelial defects. A great variability in growth factor content had already been demonstrated7; indeed, in order to meet the requirements regarding EGF content, a preliminary phase of assaying and pre-selection of CB units had to be performed so that the final pool of sera included only those containing appropriate EGF levels and excluded those containing too little EGF. This is both time-consuming and expensive and so we investigated alternative ways of making a preliminary selection of CB units.
In the present study we demonstrated that many different obstetric factors must be taken into account when processing CB units for preparation of this particular type of blood component for non-transplantation use. In particular, efforts should be made to select CB units from young mothers, after a labour that lasted longer than 6 hours, and with a haematopoietic CD34+ stem cell content higher than 0.05×106/mL. By applying these three criteria as a whole, we could have been able to pre-select 37 out of 135 CB units, possibly avoiding EGF dosage.
Acknowledgements
The authors thank Mrs Chiara Coslovi for her excellent technical support. The work was supported in part by a grant from the Fondazione Cassa di Risparmio in Bologna to Prof EC Campos and by a grant from the Region of Emilia Romagna to the Cord Blood Bank.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Newcomb JD, Willing AE, Sanberg PR. Umbilical cord blood cells. Methods Mol Biol. 2009;549:119–36. doi: 10.1007/978-1-60327-931-4_9. [DOI] [PubMed] [Google Scholar]
- 2.Brunstein CG. Umbilical cord blood transplantation for the treatment of hematologic malignancies. Cancer Control. 2011;18:222–36. doi: 10.1177/107327481101800403. [DOI] [PubMed] [Google Scholar]
- 3.Rocha V, Labopin M, Ruggeri A, et al. Unrelated cord blood transplantation: outcomes after single-unit intrabone injection compared with double-unit intravenous injection in patients with hematological malignancies. Transplantation. 2013;95:1284–91. doi: 10.1097/TP.0b013e318288ca4d. [DOI] [PubMed] [Google Scholar]
- 4.Körbling M, Robinson S, Estrov Z, et al. Umbilical cord blood-derived cells for tissue repair. Cytotherapy. 2005;7:258–61. doi: 10.1080/14653240510027145. [DOI] [PubMed] [Google Scholar]
- 5.Harris DT. Non-hematological uses of cord blood stem cells. Br J Haematol. 2009;147:177–84. doi: 10.1111/j.1365-2141.2009.07767.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoon KC, Heo H, Im SK, et al. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144:86–92. doi: 10.1016/j.ajo.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Versura P, Profazio V, Buzzi M, et al. Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea. 2013;32:412–8. doi: 10.1097/ICO.0b013e3182580762. [DOI] [PubMed] [Google Scholar]
- 8.Yoon KC, Jeong IY, Im SK, et al. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007;39:231–5. doi: 10.1038/sj.bmt.1705566. [DOI] [PubMed] [Google Scholar]
- 9.Yoon KC, Im SK, Park YG, et al. Application of umbilical cord blood serum eyedrops for the treatment of dry eye syndrome. Cornea. 2006;25:268–72. doi: 10.1097/01.ico.0000183484.85636.b6. [DOI] [PubMed] [Google Scholar]
- 10.Yoon KC, Choi W, You IC, Choi J. Application of umbilical cord serum eyedrops for recurrent corneal erosions. Cornea. 2011;30:744–8. doi: 10.1097/ICO.0b013e31820d850f. [DOI] [PubMed] [Google Scholar]
- 11.Sharma N, Goel M, Velpandian T, et al. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52:1087–92. doi: 10.1167/iovs.09-4170. [DOI] [PubMed] [Google Scholar]
- 12.Yoon KC, You IC, Im SK, et al. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114:1637–42. doi: 10.1016/j.ophtha.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Lou-Bonafonte JM, Bonafonte-Marquez E, Bonafonte-Royo S, Martinez-Carpio A. Posology, efficacy, and safety of epidermal growth factor eye drops in 305 patients: logistic regression and group-wise odds of published data. J Ocul Pharmacol Ther. 2012;28(5):467–72. doi: 10.1089/jop.2011.0236. [DOI] [PubMed] [Google Scholar]
- 14.Shen EP, Hu FR, Lo SC, et al. Comparison of corneal epitheliotrophic capacity among different human blood–derived preparations. Cornea. 2011;30:208–14. doi: 10.1097/ICO.0b013e3181eadb67. [DOI] [PubMed] [Google Scholar]
- 15.Shlebak AA, Roberts IA, Stevens TA, et al. The impact of antenatal and perinatal variables on cord blood haemopoietic stem/progenitor cell yield available for transplantation. Br J Haematol. 1998;103:1167–71. doi: 10.1046/j.1365-2141.1998.01093.x. [DOI] [PubMed] [Google Scholar]
- 16.Mancinelli F, Tamburini A, Soagnoli A, et al. Optimizing umbilical cord blood collection: impact of obstetric factors versus quality of cord blood units. Transplant Proc. 2006;38:1174–6. doi: 10.1016/j.transproceed.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 17.Aufderhaar U, Holzgreve W, Danzer E, et al. The impact of intrapartum factors on umbilical cord blood stem cell banking. J Perinat Med. 2003;31:317–22. doi: 10.1515/JPM.2003.045. [DOI] [PubMed] [Google Scholar]
- 18.Kurtzberg JK, Cairo MS, Fraser JK. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–55. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 19.Haddad J, Vilge V, Juif JG, et al. Beta-nerve growth factor levels in newborn cord sera. Pediatr Res. 1994;35:637–9. doi: 10.1203/00006450-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kilari A, Mehendale S, Pisal H, et al. Nerve growth factor, birth outcome and pre-eclampsia. Int J Dev Neurosci. 2011;29:71–5. doi: 10.1016/j.ijdevneu.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Dhobale M, Mehendale S, Pisal H, et al. Reduced maternal and cord nerve growth factor levels in preterm deliveries. Int J Dev Neurosci. 2012;30:99–103. doi: 10.1016/j.ijdevneu.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Briana DD, Liosi S, Gourgiotis D, et al. Fetal concentrations of the growth factors TGF-α and TGF-β1 in relation to normal and restricted fetal growth at term. Cytokine. 2012;60:157–61. doi: 10.1016/j.cyto.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Tutdibi E, Hunecke A, Lindner U, et al. Levels of cytokines in umbilical cord blood in relation to spontaneous term labor. J Perinat Med. 2012;40:527–32. doi: 10.1515/jpm-2011-0204. [DOI] [PubMed] [Google Scholar]
- 24.Ichiba H, Fujimura M, Takeuchi T. Levels of epidermal growth factor in human cord blood. Biol Neonate. 1992;61:302–7. doi: 10.1159/000243758. [DOI] [PubMed] [Google Scholar]
- 25.Shigeta K, Hiramatsu Y, Eguchi K, Sekiba K. Urinary and plasma epidermal growth factor levels are decreased in neonates with intrauterine growth retardation and in their mothers. Biol Neonate. 1992;62:76–82. doi: 10.1159/000243857. [DOI] [PubMed] [Google Scholar]
- 26.Manegold G, Meyer-Monard S, Tichelli A, et al. Cesarean section due to fetal distress increases the number of stem cells in umbilical cord blood. Transfusion. 2008;48:871–6. doi: 10.1111/j.1537-2995.2007.01617.x. [DOI] [PubMed] [Google Scholar]
- 27.McGuckin CP, Basford C, Hanger K, et al. Cord blood revelations: the importance of being a first born girl, big, on time and to a young mother! Early Hum Dev. 2007;83:733–41. doi: 10.1016/j.earlhumdev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Kruse FE, Tseng SC. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34:1963–76. [PubMed] [Google Scholar]