Abstract
Background
Prolonged air leak is the major cause of morbidity after pulmonary resection. In this study we used in vitro and in vivo experiments to investigate an innovative approach based on the use of human umbilical cord blood platelet gel.
Materials and methods
In vitro, a scratch assay was performed to test the tissue repair capability mediated by cord blood platelet gel compared to the standard culture conditions using human primary mesothelial cells. In vivo, an iatrogenic injury was made to the left lung of 54 Wistar rats. Cord blood platelet gel was placed on the injured area only in treated animals and at different times histological changes and the presence of pleural adhesions were evaluated. In addition, changes in the pattern of soluble inflammatory factors were investigated using a multiplex proteome array.
Results
In vitro, mesothelial cell damage was repaired in a shorter time by cord blood platelet gel than in the control condition (24 versus 35 hours, respectively). In vivo, formation of new mesothelial tissue and complete tissue recovery were observed at 45±1 and 75±1 hours in treated animals and at 130±2.5 and 160±6 hours in controls, respectively. Pleural adhesions were evident in 43% of treated animals compared to 17% of controls. No complications were observed. Interestingly, some crucial soluble factors involved in inflammation were significantly reduced in treated animals.
Discussion
Cord blood platelet gel accelerates the repair of pleural damage and stimulates the development of pleural adhesions. Both properties could be particularly useful in the management of prolonged air leak, and to reduce inflammation.
Keywords: platelet gel, human cord blood, growth factors, pleural tissue, mesothelial cells
Introduction
Prolonged air leak (PAL), defined as an air leak lasting longer than 7 days, is considered the most prevalent postoperative complication in thoracic surgery. Indeed, PAL is a common problem after pulmonary resection, occurring in 3–26% of such patients1. Relevant consequences are maintenance of chest drainages, prolonged hospital stay and increased postoperative morbidity (e.g. infections, emphysema). Consequently, this leads to higher hospital costs and major risks for the patient2.
Many studies have investigated what risk factors could predispose to the development of PAL: incomplete lobar fissures, pleural adhesions, lung diseases (emphysema, chronic obstructive lung disease, tuberculosis and fibrosis), patient’s age (>75 years) and low diffusing capacity of the lung for carbon monoxide all seem strongly associated with PAL3,4.
The use of staplers as well as the introduction of new surgical techniques and proper management of chest drainage have contributed to a reduction in PAL5,6. Nevertheless, the pronounced clinical and economic impact of PAL promoted the development of a large variety of materials, such as natural/synthetic glues7 and fibrin sealants obtained from platelet-poor plasma and rich in fibrinogen8; similarly, postoperative techniques, such as blood patches, have been proposed9. Surgical sealants are now widely used in cardiovascular and thoracic surgery, as well as in neurosurgery, mainly with one of the following objectives: to facilitate tissue adhesion, to support the surgical sutures and to promote haemostasis. Moreover, it has been proven that the use of surgical sealants reduces PAL, even though such sealants are not always able to shorten hospital stay. Platelet gel (PG) has been found to be an interesting and useful product, rich in growth factors, supporting tissue regeneration in dentistry, oral-maxillofacial and bone surgery, wound healing and soft tissue injuries10–12 and a relevant added value is that the costs associated with PG treatment amount to approximately one tenth of those associated with the use of advanced medications13. PG is composed of platelet-rich plasma mixed with thrombin and other activators such as calcium which promote both platelet degranulation and the coagulation cascade14. This process enriches PG in growth factors and bioactive substances that have been found to be effective in accelerating significant tissue repair and wound healing15.
Usually PG is obtained from platelets derived from peripheral blood. Recently, some of us developed a patented procedure to obtain PG from umbilical cord blood (PCT WO/2010/007502) and showed that cord blood platelet gel (CBPG) contains factors more prone to support soft tissue repair compared to the PG from peripheral blood16.
In this study, in vitro and in vivo experimental approaches were used to test the efficacy of CBPG in the repair of pleural damage.
Materials and methods
In vitro experiments
Sample collection
Having obtained parental informed consent, cord blood (CB) units were collected into plastic bags containing 29 mL of citrate-phosphate-dextrose anticoagulant by trained midwives, before and after delivery of the placenta in cases of vaginal births and Caesarean sections, respectively. The units were stored at 2–22 °C and processed to obtain platelets within 24±2 hours of collection.
Platelet gel preparation
The CB units were centrifuged at 550 g to sediment red and white cells and to concentrate most of the platelets in the supernatant plasma. They were then centrifuged at slow spin (100 g) in order to discard all the nucleated cells. The resulting platelet fraction was centrifuged at 2,000 g to obtain a platelet pellet and was then diluted in an appropriate volume of supernatant platelet-poor plasma to obtain a final concentration of 2×106 platelets/μL17(called platelet-rich plasma). Finally, the sample was let to stand for 30 minutes at room temperature and split into aliquots of 250 μL, which were stored at −20 °C until use. Before in vivo application, a single platelet-rich plasma aliquot was thawed at 37 °C and activated inside the same plastic device with a rounded shape of 1 cm diameter. In this way, the shape and the dimension of the gel were always the same within the whole experiment with a defined volume of 292 μL (250 μL of platelet-rich protein with 42 μL of calcium gluconate and batroxobin; Plateltex-Act, Plateltex, Bratislava, Slovakia). The gel consolidation was completed in approximately 10 minutes.
Platelet releasate preparation
The CBPG was centrifuged at 2,000 g to obtain the platelet releasate.
Mesothelial cell isolation
Samples of normal human pleural mesothelium (n =5) were obtained after informed consent from patients undergoing thoracic surgery procedures for non-oncological diseases.
Each tissue was washed with sterile phosphate-buffered saline (PBS; EuroClone S.p.A., Pavia, Italy) + 20% foetal bovine serum (FBS; Biochrom, AG, Berlin, Germany) and was then exposed to 1 mg/mL collagenase A (Roche Applied Science, Mannheim, Germany) for 1 hour at 37 °C. The digestion was stopped using PBS +20% FBS and then the product was centrifuged at 400 g for 10 minutes to collect the cells. The cells were cultured in M199 (Gibco Life Technologies, Carlsbad, CA, USA) supplemented with 20% FBS and 1% penicillin/streptomycin (P/S; Sigma Aldrich, St Louis, MO, USA) at 37 °C in a humidified atmosphere, at 5% CO2.
After 10 days of primary culture, when the attached cells reached confluence, they were harvested by treatment with TryPLE Select (Gibco Life Technologies), analysed by flow cytometry and used for the scratch assay (see below).
Flow cytometric analysis
Primary mesothelial cells were extensively characterised by flow cytometry to analyse their purity. Cells (5×105) were washed in PBS for 20 minutes at room temperature and incubated in the dark with the following conjugated mouse-anti human antibodies: CD24 FITC (StemCell Technologies Inc., Vancouver, Canada), CD49f FITC (StemCell Technologies Inc.), Cytokeratin 18 PE (Santa Cruz Biotechnology, Santa Cruz, CA, USA), EpCAM FITC (StemCell Technologies Inc.), CD34 PE (Becton Dickinson, BD, San Josè, CA, USA), CD45 PC7 (Beckman Coulter, Fullerton, CA, USA), CD146 PE (Beckman Coulter) and von Willebrand factor FITC (Abcam, Cambridge, UK). The isotype immunoglobulins IgG1 PE-FITC (EMD Millipore Corporation, Billerica, MA, USA) and IgG1 PC7 (Beckman Coulter) were used as negative controls under the same conditions. After staining, the cells were washed once with PBS. At least 50,000 events were acquired with a Cytomics FC500 flow cytometer (Beckman Coulter) and plots were generated using the CXP analysis software.
Scratch assay
Mesothelial cells were grown in six-well plates to a confluent monolayer. The cell monolayer was scraped in a straight line with a 200 μL pipette tip. Cell debris was removed by washing the cells once with PBS. Soon after the mesothelial cells were cultured with M199 plus 20% CB platelet releasate or 20% FBS as control. Dishes were placed under a phase-contrast microscope and the first (baseline T0) image of the scratch was acquired. The cells were incubated at 37 °C for 35 hours and observed under a phase-contrast microscope (matching the reference point) at 8, 16, 24 and 35 hours.
In vivo experiments
Animals and treatments
Fifty-four male Wistar outbred rats (Charles River, Calco, Italy) weighing 200–250 g were used in the study. Rats were housed individually in a ventilated cage system (Tecniplast, Buguggiate, Italy), at 22±1 °C, with 55±5% humidity, and a 12 hour dark/light cycle, with free access to food and water. All animals received care in compliance with the principles of laboratory animal care, and the guide for the care and use of laboratory animals (NIH publication n. 86-23, revised 1985); the Institutional Review Board approved the protocol. The same surgeons performed all the procedures, aseptically and under anaesthesia, using a combination of 100 mg/kg ketamine and 6 mg/kg xylazine injected intraperitoneally.
The intubation technique described by Kastl et al.18 was partially modified: rats were positioned on an inclined cork board, suspended by a sling hooked to the upper incisors. After using lidocaine (Ecocain 10 g/100 mL spray), the vocal cords were reached by an otoscope (Heine mini 3000®, Heine, Herrsching, Germany); under vision a 22 cm wire guide was inserted and then an intravenous catheter (Abbocath 16 G × 1–1/4, Progressive Medical International, Vista, CA, USA) was introduced following Seldinger’s technique. During surgery, rats were connected to a ventilator for small animals (SAR 830-p, CWE Inc., Ardmore, PA, USA) programmed at 55 breaths/min.
Experimental design and surgery
Fifty-four animals were divided into two groups, a treated group and a control group, and a standardised single lesion was created on the left lung. In detail, after the operative field had been prepared and left hemithorax shaved, the animal was placed in right lateral decubitus. A thoracotomy incision of about 2.5 cm was performed in the third/fourth left intercostal space. Using a Beckmann’s autostatic retractor, the pleural cavity was reached using a muscle sparing technique. Under direct vision a standardised lesion was created on the left lung parenchyma with a biopsy forcep (FB-56D-1, Olympus America Inc., Center Valley, USA) and scissors.
In the control group (23 rats) after the injury a 16 G chest tube was inserted and the incision was sutured with absorbable simple interrupted stitches 3/0 (Safil Quick 3/0, B. Braun, Melsungen, Germany). Only in the treatment group (31 rats) was CBPG placed on the injured area using a simple anatomical forceps. The appropriate position was double checked for 1 minute and then the animal was sutured as previously described for the control group. In each animal, the chest tube was connected to an underwater seal bottle at −2 cm H2O to allow correct lung expansion.
The time taken for the entire procedure was approximately 15–20 minutes per rat; no antibiotic was administered. The animals were extubated after regaining full ability to breathe spontaneously and then moved to the recovery room for the resumption of vital functions.
Sampling
The animals were sacrificed at different times. The rats were anaesthetised and put in the supine position. A laparotomic incision was performed and major abdominal vessels were severed. By a median sternotomy, the entire pleural cavity was carefully inspected, checking the hypothetical presence of anomalies. Free from any contact with the parietal pleura, pulmonary ligament and the hilum, the left lung was then completely excised and placed in formalin for the histological examination.
Histological evaluation
Before collecting the samples, a scale for the mesothelial cell recovery and pleural healing was defined a priori based on our previous experience in other animal models. This was the anatomopathological scale: grade A: surgical injury, grade B: strongly damaged (more than 80% of denuded mesothelium), grade C: still evident damage (more than 50% of denuded mesothelium), grade D: initial lining (from 20% to 50% of denuded mesothelium), grade E: evident lining (less the 20% of denuded mesothelium), grade F: complete tissue repair (presence of mesothelial cells in all the damaged area). To avoid any influence by the operator, the blind histological assessment of the samples was performed twice by two independent observers. The damage was observed first at low magnification to identify the presence or absence of mesothelial lining. In a second step, the lesion was analysed at higher magnification (10X and 20X) and a score of injury was assigned based on the defined scale. All specimens were formalin-fixed and paraffin-embedded. Three sections of 4-micron thickness were obtained from each paraffin block and stained with haematoxylin-eosin, Pass and Alcian blue.
Rat inflammatory profile
Before the rats were sacrificed, blood samples were collected 75 hours after the pleural damage and after CBPG treatment and then centrifuged at 900 g to collect sera.
The inflammatory profile was analysed in serum samples taken after pleural damage (n =4) and after CBPG treatment (n =6). The inflammatory cytokines in serum samples were detected by a multiplexed sandwich enzyme-linked immunosorbent assay (ELISA) that allows quantitative chemiluminescent measurement of nine proteins per well: interleukin-1alpha (IL-1α), interleukin-1beta (IL-1β), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF). The lower detection limits for these cytokines were 1.6, 6, 6, 1.6, 6, 0.8, 6, 12 and 1.6 pg/mL, respectively (SearchLight Rat Cytokine 9-Plex Array, Aushon Biosystems, Billerica, MA, USA).
Each well of the microplate was pre-spotted with target protein-specific antibodies and 50 μL of standards and samples were added to the plate for 1 hour at room temperature with shaking at 200 rpm. After washing the wells to remove unbound proteins, 50 μL of biotinylated antibodies were added to the plate for 30 min at room temperature with shaking at 200 rpm. Excess biotinylated antibody was washed away, and 50 μL of streptavidin-horseradish peroxidase were added to the plate for 30 minutes at room temperature with shaking at 200 rpm.
In order to identify the signal, we used SuperSignal ELISA femto chemiluminescent substrate (Pierce, Rockford, IL, USA). The luminescent signal was recorded within 10 minutes using a cooled CCD camera and the amount of each target protein was analysed using Microsoft Excel 2000 as recommended by the manufacturers.
Statistical analysis
Results were analysed with Fisher’s exact test, an unpaired t-test and the Cox test utilising JMP® software (SAS Institute Inc., SAS Campus Drive, Cary, NC, USA).
Results
In vitro experiments
Mesothelial cell characterisation
Mesothelial cells were characterised by the presence of classic cobblestone morphology, absence of CD24, epithelial/endothelial markers such as EpCAM, CD146 and von Willebrand factor. They resulted negative for haematopoietic markers such as CD34 and CD45, and positive for cytokeratin 18 and CD49f.
Evaluation of platelet releasate in the scratch assay
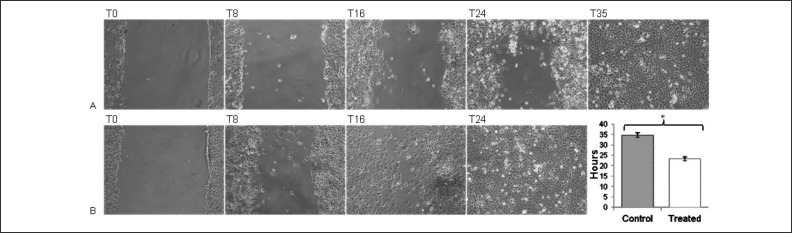
As previously reported16, CB platelet releasate contained the following factors: platelet-derived growth factors (PDGF-AB, PDGF-BB), transforming growth factor-beta1 (TGF-β1), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF). A scratch assay was used to assess the effects of CB platelet releasate on mesothelial cell repair. The extent of regrowth to close the scratch wound was measured after 8, 16, 24, and 35 hours of incubation in medium containing 20% CB platelet releasate or 20% FBS. The restoration of the full cellular density of mesothelium was faster with CB platelet releasate than with FBS. Indeed, wound closure was completed at 24 hours with CB platelet releasate, while it was repaired at 35 hours in the presence of FBS (P <0.0001) (Figure 1).
Figure 1.
The effects of FBS and CB platelet releasate on mesothelial repair in a scratch assay.
(A) Reconstitution of mesothelial lining in the presence of FBS (standard condition, n =5) at T0, T8, T16, T24, T35; (B) Reconstitution of mesothelial lining in the presence of CB platelet releasate (n =5) at T0, T8, T16, T24; (C) Data are represented as mean ± SD. The P value was obtained performing an unpaired t-test. *P <0.0001.
Inflammatory profile
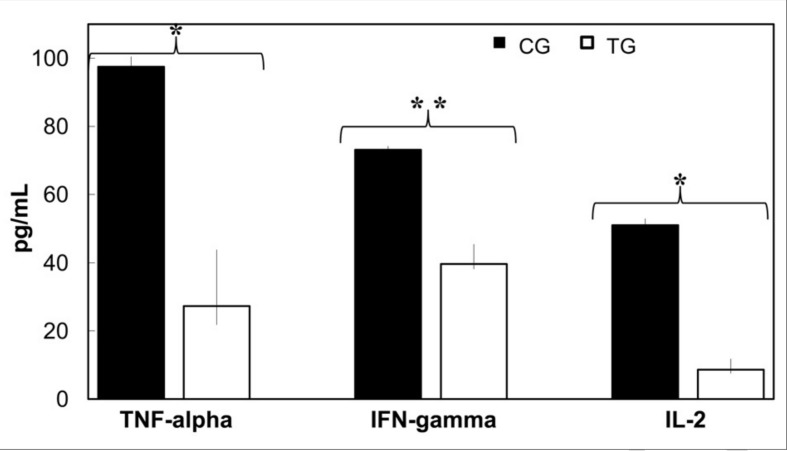
The concentrations of some of inflammatory cytokines, including IL-2, IFN-γ and TNF-α, were statistically significantly different between the treatment group and the control group as shown in Figure 2, while the values of the remaining factors were either not statistically significant different or below the lower detection limit.
Figure 2.
TNF-α, IFN-γ and IL-2 concentrations in rat sera at 75 hours after pleural damage (control group, CG) and after CBPG treatment (treatment group, TG). Inflammatory factors were measured by proteome assay (pg/mL; CG n =4, TG n =6; median and range). The P value was obtained performing an unpaired t-test. *P <0.0001; **P <0.0003.
In vivo experiments
Pleural cavity
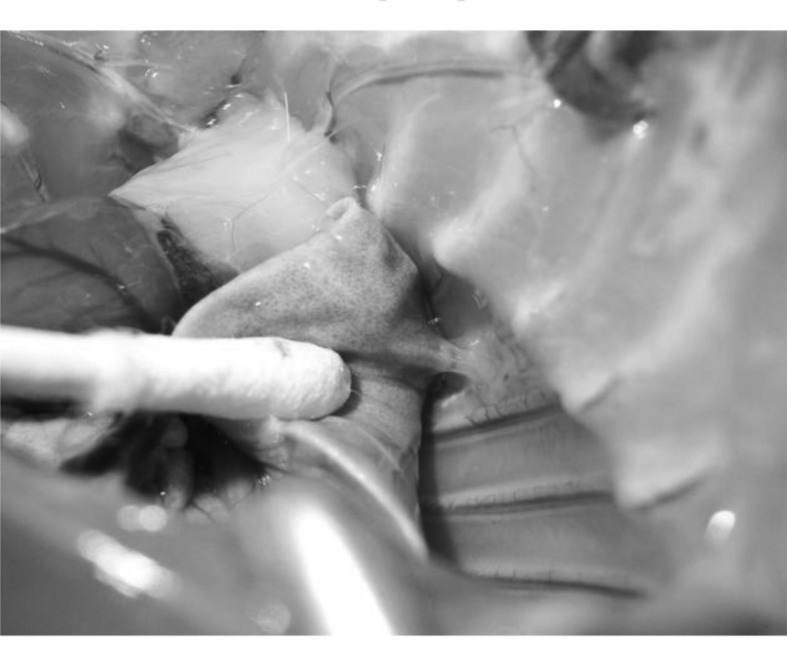
Neither infections nor other complications were observed during the experiment. The chest x-rays performed before the sacrifices showed correct parenchymal expansion, with no sign of pneumothorax or haemothorax in either groups. The pleural cavity was observed (Figure 3): pleural adhesions were found in 13 treated rats versus 4 control rats (Fisher’s exact test, P =0.0505).
Figure 3.
Presence of a pleural adhesion on the thoracotomy.
Histological examination
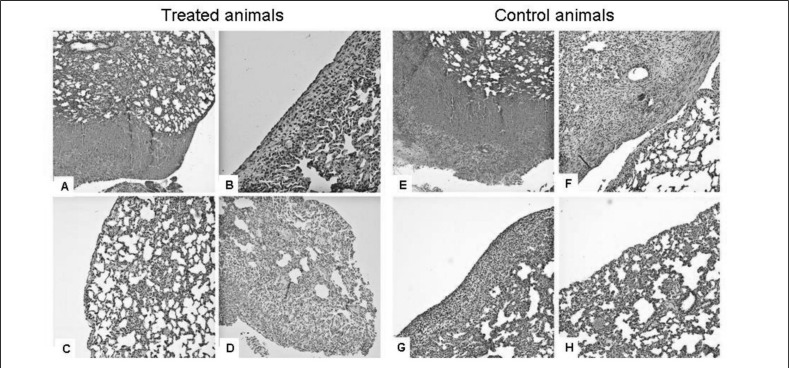
Figure 4 shows histological sections for the animals in the treated and control groups at different times. Some crucial features related to tissue damage and repair were analysed including subpleural damage characterised by marked haemorrhagic extravasation (Figures 4A and 4E), subpleural damage with scar-like component (Figure 4F), subpleural damage with visible new mesothelial lining (Figures 4B and 4G) and subpleural damage no longer visible with complete mesothelial repair (Figures 4C and 4H).
Figure 4.
Histological sections of rat lung in the two different groups.
On the left side the treated animals: (A) recent subpleural damage characterised by marked haemorrhagic extravasation, fibrin-rich clot bleeding associated with acute and chronic inflammation and fibroblast hyperplasia; (B) subpleural damage with a visible new mesothelial lining; (C) subpleural damage no longer visible. Complete mesothelial repair; (D) late subpleural damage in presence of weakly basophilic material due to Alcian blue 8GX staining. On the right side the controls: (E) recent subpleural damage characterised by marked haemorrhagic extravasation, fibrin-rich clot bleeding associated with acute inflammation; (F) subpleural damage with leucocyte-fibrin clot associated with scattered scar-like components; (G) subpleural damage in an advanced stage of mesothelial tissue repair; (H) subpleural damage no longer visible. Complete mesothelial repair.
The correct application of the PG on the injured lung was verified by the anatomopathologist and using Alcian blue staining the gel matrix was visible outside the subpleural connective tissue corresponding to the injured area (Figure 4D).
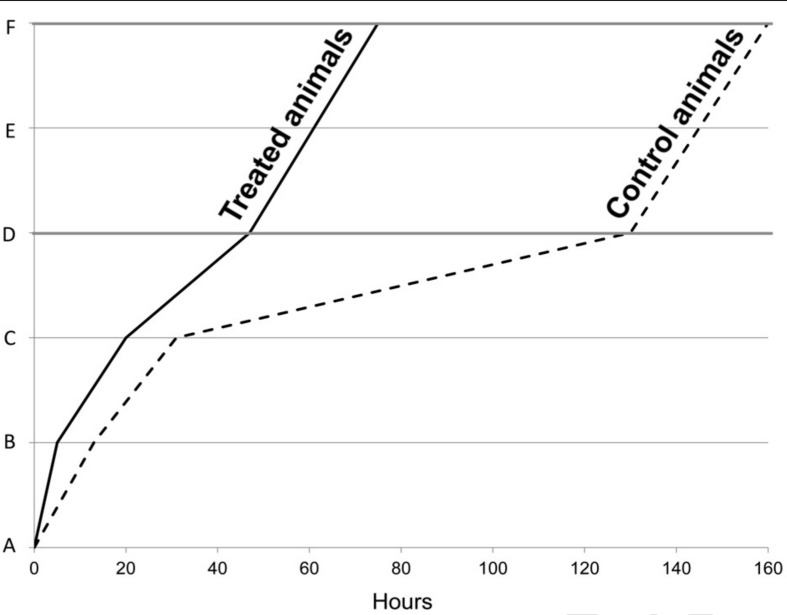
A thin lining of mesothelial cells was observed on the injured area after 45±1 hours in the treatment group and after 130±2.5 hours in the control group (HR 0.468; P =0.0017). Complete tissue repair was observed after 75±1 hours in the treatment group and after 160±6 hours in the control group.
Discussion
The development of PAL is a common complication that the thoracic surgeon has to face every day1. The persistence of parenchymal air leaks and residual pleural space may promote the development of several serious complications: an increase in the maintenance of pleural drainage associated with pain and immobilisation, the risk of developing pneumothorax, infection, bronchopleural fistula and, consequently, a prolongation of time spent in hospital2.
The debate regarding the need for and appropriateness of new techniques to minimise intraoperative air leaks after lung surgery is still ongoing even though many experimental attempts have been made and several studies have been published.
The routine use of surgical staplers has strongly decreased the incidence of this complication as have fissureless techniques for lobectomy and pleural tenting. In addition, reinforcement of the staple line with bovine pericardium or synthetic materials has been prophylactically used with success in patients with emphysematous lungs5,6. Moreover, alternative methods, such as surgical sealants of various origins, can be used effectively7,8. A recent review evaluated 16 randomised controlled trials that had investigated the role of surgical sealants for preventing air leaks after pulmonary resections: fibrin glue and an autologous fibrin sealant (Vivostat®, A/S, Alleroed, Denmark) were used in six and one trials, respectively; different synthetic biodegradable sealants based on polyethylenglycol were tested in five trials; absorbable patches coated with human fibrinogen and human thrombin (TachoSil®, Takeda Pharmaceuticals International GmbH, Zurich, Switzerland) were used in four studies19. Interestingly, in 12 of these trials there was a statistically significant difference in reduction of air leaks in treated patients but only in three of them was there an advantage in terms of reduction of hospital stay. However, most of the studies present involved small cohorts of patients and did not, therefore, allow definitive conclusions.
In this context, new biological or synthetic materials, able to obtain better control of air leaks, are still needed. In recent years, the autologous blood patch has been demonstrated to be an effective, cheap, readily available and well-tolerated treatment for PAL. Its effect seems to be achieved by direct clot formation and subsequently the fibrogenic activity of the blood creates a pleurodesis via pleural irritation and inflammation. However, the most controversial issue is the amount of blood required for successful pleurodesis. Several authors are concerned about using large volumes of blood, which is considered an ideal culture medium for bacteria; nevertheless a recent review reported high rates of success with large volumes of blood applied during the procedure (about 150 mL)8.
Analysing these available but still controversial results, a first consideration that can be made is that the expected effects of these treatments are largely based on their mechanical properties and generally no growth support is given to the damaged tissue to rapidly and efficiently reconstitute the mesothelial lining. In fact, growth factors, which help and support tissue regeneration and could be crucial in pleural healing, are missing from all these treatments. It is exactly in this context that an innovative approach such as platelet gel can be envisioned to combine mechanical properties and growth factor release. In fact, it has been extensively demonstrated that platelets can release several growth factors contained in their granules such as PDGF-AB, PDGF-BB, TGF-b1, bFGF, HGF and VEGF12,15,20.
In this study on mesothelial repair we tested an innovative product, CBPG, which not only provides optimal mechanical support and growth factors but which also contains a combination of growth factors/cytokines more appropriate for tissue repair than the PG obtained from peripheral blood16.
Our in vitro results clearly show that CBPG efficiently restores PAL damage and in a shorter time than that taken for spontaneous repair.
Another effect of CBPG, and by no means the least important one, is the reduction of some crucial inflammatory factors such as IL-2, TNF-α and IFN-γ, which are well-known to be highly involved in inflammation processes including wound healing21,22. The success of wound healing depends on a balance between growth factors for tissue repair and a reduction of inflammatory factors.
All of these properties were well demonstrated in our in vivo animal model. Although there are already some studies in the literature about parenchymal lung damage in rats23, we developed a new experimental approach: after anaesthesia and intubation, a standardised injury was made on the left lung with biopsy forceps in order to obtain better defined and more reproducible pleural damage.
According to the histological analysis, the results were definitely better with CBPG than in the control group not only regarding the formation of the first mesothelial lining (−60%; P =0.0017), but also in complete tissue repair (−57%). The representative trend of tissue repair is clearly shown in Figure 5.
Figure 5.
Representative trends of tissue damage and repair in treated and control animals over time (hours): (A) surgical injury, (B) strongly damaged, (C) still evident damage, (D, bold grey line) initial lining, (E) evident lining, (F, bold grey line) complete tissue repair.
Another important aspect was the presence of adhesions on the thoracotomy, which was found in 43% of treated animals versus 17% of the controls (P =0.0505). Adhesions are able to seal the pleural cavity, acting in a positive way on the control of PAL and decreasing the risk of residual pleural space.
No animals developed pneumothorax, haemothorax or any other complications, including infections.
In conclusion, our in vitro and in vivo findings suggest that CBPG could be a useful therapeutic tool in the pulmonary field and pave the way to the development of a large clinical trial in humans aimed at comparing its effectiveness with that of currently available therapeutic approaches to pleural tissue repair.
Footnotes
Funding
This study was supported in part by a “Young Researchers” grant from the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy [2010/2011 code 325/02] and by the EC FP7 through the REBORNE project, Grant Agreement Number 241879.
Conflict of interests disclosures
Valentina Parazzi, Lorenza Lazzari and Paolo Rebulla declare their role as inventors in patent n. PCT WO/2010/007502 “Platelet fraction deriving from placental blood”. The other Authors have no conflicts of interest.
References
- 1.Abolhoda A, Liu D, Brooks A, Burt M. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest. 1998;113:1507–10. doi: 10.1378/chest.113.6.1507. [DOI] [PubMed] [Google Scholar]
- 2.Varela G, Jiménez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27:329–33. doi: 10.1016/j.ejcts.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thoracic Surg. 2004;77:1205–10. doi: 10.1016/j.athoracsur.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 4.Cerfolio RJ, Bass CS, Pask AH, Kartholi CR. Predictors and treatment of persistent air leaks. Ann Thorac Surg. 2002;73:1727–30. doi: 10.1016/s0003-4975(02)03531-2. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Caro A, Calvo MJ, Lanzas JT, et al. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg. 2007;31:203–8. doi: 10.1016/j.ejcts.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Refai M, Brunelli A, Salati M, et al. Efficacy of anterior fissureless technique for right upper lobectomies: a case-matched analysis. Eur J Cardiothoracic Surg. 2011;39:1043–6. doi: 10.1016/j.ejcts.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Montana M, Tabélé C, Curti C, et al. Organic glues or fibrin glues from pooled plasma: efficacy, safety and potential as scaffold delivery systems. J Pharm Pharm Sci. 2012;15:124–40. doi: 10.18433/j39k5h. [DOI] [PubMed] [Google Scholar]
- 8.Jackson MR. Fibrin sealants in surgical practice: An overview. Am J Surg. 2001;182:1–7S. doi: 10.1016/s0002-9610(01)00770-x. [DOI] [PubMed] [Google Scholar]
- 9.Manley K, Coonar A, Wells F, Scarci M. Blood patch for persistent air leak: a review of the current literature. Curr Opin Pulm Med. 2012;18:333–8. doi: 10.1097/MCP.0b013e32835358ca. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Driggs J, Elgharably H, et al. Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Repair Regen. 2011;19:753–66. doi: 10.1111/j.1524-475X.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 12.Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:156–65. [PubMed] [Google Scholar]
- 13.Greppi N, Mazzucco L, Galetti G, et al. Treatment of recalcitrant ulcers with allogeneic platelet gel from pooled platelets in aged hypomobile patients. Biologicals. 2011;39:73–80. doi: 10.1016/j.biologicals.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Mazzucco L, Balbo V, Cattana E, Borzini P. Platelet-rich plasma and platelet gel preparation using Plateltex. Vox Sang. 2008;94:202–8. doi: 10.1111/j.1423-0410.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 15.Boswell SG, Cole BJ, Sundman EA, et al. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–39. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Parazzi V, Lazzari L, Rebulla P. Platelet gel from cord blood: a novel tool for tissue engineering. Platelets. 2010;21:549–54. doi: 10.3109/09537104.2010.514626. [DOI] [PubMed] [Google Scholar]
- 17.Borzini P, Mazzucco L, Giampaolo A, et al. Platelet gel - the Italian way: a call for procedure standardization and quality control. Transfus Med. 2006;16:303–4. doi: 10.1111/j.1365-3148.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 18.Kastl S, Kotschenreuther U, Hille B, et al. Simplification of rat intubation on inclined metal plate. Adv Physiol Educ. 2004;28:29–32. doi: 10.1152/advan.00008.2003. [DOI] [PubMed] [Google Scholar]
- 19.Belda-Sanchís J, Serra-Mitjans M, Iglesias Sentis M, Rami R. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database of Systematic Reviews. 2010;20:CD003051. doi: 10.1002/14651858.CD003051.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirz S, Dietrich M, Flanagan TC, et al. Influence of platelet-derived growth factor-AB on tissue development in autologous platelet-rich plasma gels. Tissue Eng. 2011;17:1891–9. doi: 10.1089/ten.TEA.2010.0610. [DOI] [PubMed] [Google Scholar]
- 21.Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. doi: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunetti ND, Munno I, Pellegrino PL, et al. Inflammatory cytokines imbalance in the very early phase of acute coronary syndrome: correlations with angiographic findings and inhospital events. Inflammation. 2011;34:58–66. doi: 10.1007/s10753-010-9208-1. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, Sekine T, Nakamura T, Shimizu Y. In vivo evaluation of a new sealant material on a rat lung air leak model. J Biomed Mater Res. 2001;58:658–65. doi: 10.1002/jbm.1066. [DOI] [PubMed] [Google Scholar]